Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#176 Science HQ » Melting Point » 2025-11-05 21:40:16
- Jai Ganesh
- Replies: 0
Melting Point
Gist
The melting point is the temperature at which a solid substance changes into a liquid under normal atmospheric pressure. At this temperature, the solid and liquid phases of the substance exist together in equilibrium. Melting occurs when added heat provides enough energy for the particles in the solid to break free from the rigid structure and move more freely as a liquid. For example, ice melts at 0 degrees Celsius (32 degrees Fahrenheit), which is also the freezing point for water.
Melting point is the temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. As heat is applied to a solid, its temperature will increase until the melting point is reached. More heat then will convert the solid into a liquid with no temperature change.
Summary
Melting point is the temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. As heat is applied to a solid, its temperature will increase until the melting point is reached. More heat then will convert the solid into a liquid with no temperature change. When all the solid has melted, additional heat will raise the temperature of the liquid. The melting temperature of crystalline solids is a characteristic figure and is used to identify pure compounds and elements. Most mixtures and amorphous solids melt over a range of temperatures.
The melting temperature of a solid is generally considered to be the same as the freezing point of the corresponding liquid; because a liquid may freeze in different crystal systems and because impurities lower the freezing point, however, the actual freezing point may not be the same as the melting point. Thus, for characterizing a substance, the melting point is preferred.
Details
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.
When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point."
Examples
For most substances, melting and freezing points are approximately equal. For example, the melting and freezing points of mercury is 234.32 kelvins (−38.83 °C; −37.89 °F). However, certain substances possess differing solid-liquid transition temperatures. For example, agar melts at 85 °C (185 °F; 358 K) and solidifies from 31 °C (88 °F; 304 K); such direction dependence is known as hysteresis. The melting point of ice at 1 atmosphere of pressure is very close to 0 °C (32 °F; 273 K); this is also known as the ice point. In the presence of nucleating substances, the freezing point of water is not always the same as the melting point. In the absence of nucleators water can exist as a supercooled liquid down to −48.3 °C (−54.9 °F; 224.8 K) before freezing.
The metal with the highest melting point is tungsten, at 3,414 °C (6,177 °F; 3,687 K); this property makes tungsten excellent for use as electrical filaments in incandescent lamps. The often-cited carbon does not melt at ambient pressure but sublimes at about 3,700 °C (6,700 °F; 4,000 K); a liquid phase only exists above pressures of 10 MPa (99 atm) and estimated 4,030–4,430 °C (7,290–8,010 °F; 4,300–4,700 K). Hafnium carbonitride (HfCN) is a refractory compound with the highest known melting point of any substance to date and the only one confirmed to have a melting point above 4,273 K (4,000 °C; 7,232 °F) at ambient pressure. Quantum mechanical computer simulations predicted that this alloy (HfN0.38C0.51) would have a melting point of about 4,400 K. This prediction was later confirmed by experiment, though a precise measurement of its exact melting point has yet to be confirmed. At the other end of the scale, helium does not freeze at all at normal pressure even at temperatures arbitrarily close to absolute zero; a pressure of more than twenty times normal atmospheric pressure is necessary.
Melting point measurements
Many laboratory techniques exist for the determination of melting points. A Kofler bench is a metal strip with a temperature gradient (range from room temperature to 300 °C). Any substance can be placed on a section of the strip, revealing its thermal behaviour at the temperature at that point. Differential scanning calorimetry gives information on melting point together with its enthalpy of fusion.
A basic melting point apparatus for the analysis of crystalline solids consists of an oil bath with a transparent window (most basic design: a Thiele tube) and a simple magnifier. Several grains of a solid are placed in a thin glass tube and partially immersed in the oil bath. The oil bath is heated (and stirred) and with the aid of the magnifier (and external light source) melting of the individual crystals at a certain temperature can be observed. A metal block might be used instead of an oil bath. Some modern instruments have automatic optical detection.
The measurement can also be made continuously with an operating process. For instance, oil refineries measure the freeze point of diesel fuel "online", meaning that the sample is taken from the process and measured automatically. This allows for more frequent measurements as the sample does not have to be manually collected and taken to a remote laboratory.[citation needed]
Techniques for refractory materials
For refractory materials (e.g. platinum, tungsten, tantalum, some carbides and nitrides, etc.) the extremely high melting point (typically considered to be above, say, 1,800 °C) may be determined by heating the material in a black body furnace and measuring the black-body temperature with an optical pyrometer. For the highest melting materials, this may require extrapolation by several hundred degrees. The spectral radiance from an incandescent body is known to be a function of its temperature. An optical pyrometer matches the radiance of a body under study to the radiance of a source that has been previously calibrated as a function of temperature. In this way, the measurement of the absolute magnitude of the intensity of radiation is unnecessary. However, known temperatures must be used to determine the calibration of the pyrometer. For temperatures above the calibration range of the source, an extrapolation technique must be employed. This extrapolation is accomplished by using Planck's law of radiation. The constants in this equation are not known with sufficient accuracy, causing errors in the extrapolation to become larger at higher temperatures. However, standard techniques have been developed to perform this extrapolation.
Consider the case of using gold as the source (mp = 1,063 °C). In this technique, the current through the filament of the pyrometer is adjusted until the light intensity of the filament matches that of a black-body at the melting point of gold. This establishes the primary calibration temperature and can be expressed in terms of current through the pyrometer lamp. With the same current setting, the pyrometer is sighted on another black-body at a higher temperature. An absorbing medium of known transmission is inserted between the pyrometer and this black-body. The temperature of the black-body is then adjusted until a match exists between its intensity and that of the pyrometer filament. The true higher temperature of the black-body is then determined from Planck's Law. The absorbing medium is then removed and the current through the filament is adjusted to match the filament intensity to that of the black-body. This establishes a second calibration point for the pyrometer. This step is repeated to carry the calibration to higher temperatures. Now, temperatures and their corresponding pyrometer filament currents are known and a curve of temperature versus current can be drawn. This curve can then be extrapolated to very high temperatures.
In determining melting points of a refractory substance by this method, it is necessary to either have black body conditions or to know the emissivity of the material being measured. The containment of the high melting material in the liquid state may introduce experimental difficulties. Melting temperatures of some refractory metals have thus been measured by observing the radiation from a black body cavity in solid metal specimens that were much longer than they were wide. To form such a cavity, a hole is drilled perpendicular to the long axis at the center of a rod of the material. These rods are then heated by passing a very large current through them, and the radiation emitted from the hole is observed with an optical pyrometer. The point of melting is indicated by the darkening of the hole when the liquid phase appears, destroying the black body conditions. Today, containerless laser heating techniques, combined with fast pyrometers and spectro-pyrometers, are employed to allow for precise control of the time for which the sample is kept at extreme temperatures. Such experiments of sub-second duration address several of the challenges associated with more traditional melting point measurements made at very high temperatures, such as sample vaporization and reaction with the container.
Additional Information
1. What Is Melting Point?
Melting point is a characteristic property of solid crystalline substances. It is the temperature at which the solid phase changes to the liquid phase. Melting point determination is the thermal analysis most frequently used to characterize solid crystalline materials. It is used in research and development as well as in quality control in various industry segments to identify solid crystalline substances and to check their purity.
Melting point is a characteristic property of solid crystalline substance. It is the temperature at which the solid phase changes to the liquid phase. This phenomenon occurs when the substance is heated. During the melting process, all of the energy added to the substance is consumed as heat of fusion, and the temperature remains constant (see diagram below). During the phase transition, the two physical phases of the material exist side-by-side.
Crystalline materials consist of fine particles that for a regular, 3-dimensional arrangement – a crystalline lattice. The particles within the lattice are held together by lattice forces. When the solid crystalline material is heated, the particles become more energetic and start to move more strongly, until finally the forces of attraction between them are no longer strong enough to hold them together. The crystalline structure is destroyed and the solid material melts.
The stronger the forces of attraction between the particles, the more energy is needed to overcome them. The more energy is needed, the higher the melting point. The melting temperature of a crystalline solid is thus an indicator for the stability of its lattice.
At the melting point not only the aggregate state changes; quite a lot of other physical characteristics also change significantly. Amongst these are the thermodynamic values, specific heat capacity, enthalpy, and rheological properties such as volume or viscosity. Last but not least, the optical properties birefringence reflection and light transmission change. Compared to other physical values the change in light transmission can easily be determined and can therefore be used for melting point detection.
2. Why Measure Melting Points?
Melting points are often used to characterize organic and inorganic crystalline compounds and to ascertain their purity. Pure substances melt at a sharp, highly-defined temperature (very small temperature range of 0.5 – 1 °C) whereas impure, contaminated substances generally exhibit a large melting interval. The temperature at which all material of a contaminated substance is molten is usually lower than that of a pure substance. This behavior is known as melting point depression and can be used to obtain qualitative information about the purity of a substance.
In general, melting point determination is used in the lab in research and development as well as in quality control in various industry segments to identify and check the purity of different substances.
3. Melting Point Determination Principle
At the melting point, there is a change in light transmission. Compared to other physical values the change in light transmission can easily be determined and can therefore be used for melting point detection. Powdered crystalline materials are opaque in the crystalline state and transparent in the liquid state. This distinct difference in optical properties can be measured in order to determine the melting point by recording the percentage of light intensity shining through the substance in the capillary, the transmittance, in relation to the measured furnace temperature.
There are different stages of the melting point process of a solid crystalline substance: At the collapse point, the substance is mostly solid and comprises only a small amount of molten material. At the meniscus point, most of the substance has melted but some solid material is still present. At the clear point, the substance has completely melted.
#177 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-11-05 17:26:53
2385) Ragnar Granit
Gist:
Work
Our vision works by the light around us being captured by a large number of light-sensitive cells located in the retinas at the back of our eyes. After a series of nerve switches and conversions of chemical and electrical signals, this results in visual impressions. Using very sophisticated electrodes, Ragnar Granit was able to study the electrical impulses from the retina’s cells. In studies conducted from the 1930s to the 1950s, he demonstrated that there are different types of cones (the cells that enable color vision) and that these are sensitive to light of three different wavelengths.
Summary
Ragnar Arthur Granit (born October 30, 1900, Helsinki, Finland—died March 12, 1991, Stockholm, Sweden) was a Finnish-born Swedish physiologist who was a corecipient (with George Wald and Haldan Hartline) of the 1967 Nobel Prize for Physiology or Medicine for his analysis of the internal electrical changes that take place when the eye is exposed to light.
Granit received an M.D. degree from the University of Helsinki in 1927, after which he conducted research at the University of Pennsylvania and at the laboratory of Sir Charles Scott Sherrington at Oxford, England. He was appointed professor of physiology at the University of Helsinki in 1937. A naturalized Swede, Granit joined the medical school of the Karolinska Institute, Stockholm, in 1940; he was named chairman of the institute’s department of neurophysiology in 1946. A year earlier he had also become the director of the Nobel Institute for Neurophysiology in Stockholm. In the 20 years from 1956 to 1976 Granit also served as a visiting professor or researcher at numerous institutions.
From studies of the action potentials in single fibres of the optic nerve, Granit formed his “dominator-modulator” theory of colour vision. In this theory he proposed that in addition to the three kinds of photosensitive cones—the colour receptors in the retina—which respond to different portions of the light spectrum, some optic nerve fibres (dominators) are sensitive to the whole spectrum while others (modulators) respond to a narrow band of light wavelengths and are thus colour-specific. Granit also proved that light could inhibit as well as stimulate impulses along the optic nerve. His book Sensory Mechanisms of the Retina (1947) is a classic work in the field of retinal electrophysiology.
Granit then turned his attention to the study of the control of movement, specifically the role of muscle sense-organs called muscle spindles and tendon organs. He helped to determine the neural pathways and processes by which these internal receptors regulate and coordinate muscle action.
Details
Ragnar Arthur Granit (30 October 1900 – 12 March 1991) was a Finnish-Swedish scientist who was awarded the Nobel Prize in Physiology or Medicine in 1967 along with Haldan Keffer Hartline and George Wald "for their discoveries concerning the primary physiological and chemical visual processes in the eye"
Early life and education
Ragnar Arthur Granit was born on 30 October 1900 in Riihimäki, Finland, at the time part of the Russian Empire, into a Swedish-speaking Finnish family. Granit was raised in Oulunkylä, a suburb of the Finnish capital of Helsinki, and attended the Svenska normallyceum in Helsinki.
Granit graduated from the Faculty of Medicine at the University of Helsinki in 1927.
Career and research
In 1940, when Finland became the target of a massive Soviet attack during the Winter War, Granit sought refuge – and peaceful surroundings for his studies and research work – in Stockholm, the capital of neighbouring Sweden, at the age of 40. In 1941, Granit received Swedish citizenship, which made it possible for him to live and continue with his work without having to worry about the Continuation War, which lasted in Finland until 1944. Granit was proud of his Finnish-Swedish roots and remained a patriotic Finnish-Swede throughout his life, maintaining homes in both Finland and Sweden after the Moscow Armistice ended the Continuation War and secured Finnish independence.
Granit was professor of neurophysiology at the Karolinska Institute from 1946 to his retirement in 1967.
Awards and honors
Granit was elected an International Member of the American Philosophical Society in 1954. In 1960, Granit was elected a Foreign Member of the Royal Society.
In 1967 he was awarded the Nobel Prize in Physiology or Medicine. Granit said that he was a "fifty-fifty" Finnish and Swedish Nobel laureate. He was elected an International Member of the United States National Academy of Sciences the following year. In 1971, he was elected an International Honorary Member of the American Academy of Arts and Sciences.
Death
Granit died on 12 March 1991 in Stockholm at the age of 90. Granit and his wife Marguerite, who died the same year, were buried in a church cemetery on the Finnish island of Korpo.

#178 Re: This is Cool » Miscellany » 2025-11-05 17:05:37
2437) Liquified Petroleum Gas
Gist
LPG stands for Liquefied Petroleum Gas. It is a flammable mixture of hydrocarbon gases, primarily propane and butane, which is stored and transported in liquid form under pressure. This process makes it easier to handle and store, and it is commonly used for cooking, heating, and as a fuel for vehicles.
Summary
Liquefied petroleum gas (LPG) is any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. It was used as early as 1860 for a portable fuel source, and its production and consumption for both domestic and industrial use have expanded ever since. A typical commercial mixture may also contain ethane and ethylene, as well as a volatile mercaptan, an odorant added as a safety precaution.
Liquefied petroleum gas (LPG) is recovered from “wet” natural gas (gas with condensable heavy petroleum compounds) by absorption. The recovered product has a low boiling point and must be distilled to remove the lighter fractions and then be treated to remove hydrogen sulfide, carbon dioxide, and water. The finished product is transported by pipeline and by specially built seagoing tankers. Transportation by truck, rail, and barge has also developed, particularly in the United States.
LPG reaches the domestic consumer in cylinders under relatively low pressures. The largest part of the LPG produced is used in central heating systems, and the next largest as raw material for chemical plants. LPG commonly is used as fuel for gas barbecue grills and gas cooktops and ovens, for gas fireplaces, and in portable heaters. In Europe, LPG water heaters are common. It is also used as an engine fuel and for backup generators. Unlike diesel, LPG can be stored nearly indefinitely without degradation.
Details
Liquefied petroleum gas, also referred to as liquid petroleum gas (LPG or LP gas), is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, n-butane and isobutane. It can also contain some propylene, butylene, and isobutylene/isobutene.
LPG is used as a fuel gas in heating appliances, cooking equipment, and vehicles, and is used as an aerosol propellant and a refrigerant, replacing chlorofluorocarbons in an effort to reduce the damage it causes to the ozone layer. When specifically used as a vehicle fuel, it is often referred to as autogas or just as gas.
Varieties of LPG that are bought and sold include mixes that are mostly propane (C3H8), mostly butane (C4H10), and, most commonly, mixes including both propane and butane. In the northern hemisphere winter, the mixes contain more propane, while in summer, they contain more butane. In the United States, mainly two grades of LPG are sold: commercial propane and HD-5. These specifications are published by the Gas Processors Association (GPA) and the American Society of Testing and Materials. Propane/butane blends are also listed in these specifications.
Propylene, butylenes and various other hydrocarbons are usually also present in small concentrations such as C2H6, CH4, and C3H8. HD-5 limits the amount of propylene that can be placed in LPG to 5% and is utilized as an autogas specification. A powerful odorant, ethanethiol, is added so that leaks can be detected easily. The internationally recognized European Standard is EN 589. In the United States, tetrahydrothiophene (thiophane) or amyl mercaptan are also approved odorants, although neither is currently being utilized.
LPG is prepared by refining petroleum or "wet" natural gas, and is almost entirely derived from fossil fuel sources, being manufactured during the refining of petroleum (crude oil), or extracted from petroleum or natural gas streams as they emerge from the ground. It was first produced in 1910 by Walter O. Snelling, and the first commercial products appeared in 1912. It currently provides about 3% of all energy consumed, and burns relatively cleanly with no soot and very little sulfur emission. As it is a gas, it does not pose ground or water pollution hazards, but it can cause air pollution. LPG has a typical specific calorific value of 46.1 MJ/kg compared with 42.5 MJ/kg for fuel oil and 43.5 MJ/kg for premium grade petrol (gasoline). However, its energy density per volume unit of 26 MJ/L is lower than either that of petrol or fuel oil, as its relative density is lower (about 0.5–0.58 kg/L, compared to 0.71–0.77 kg/L for gasoline). As the density and vapor pressure of LPG (or its components) change significantly with temperature, this fact must be considered every time when the application is connected with safety or custody transfer operations, e.g. typical cuttoff level option for LPG reservoir is 85%.
Besides its use as an energy carrier, LPG is also a promising feedstock in the chemical industry for the synthesis of olefins such as ethylene and propylene.
As its boiling point is below room temperature, LPG will evaporate quickly at normal temperatures and pressures and is usually supplied in pressurized steel vessels. They are typically filled to 80–85% of their capacity to allow for thermal expansion of the contained liquid. The ratio of the densities of the liquid and vapor varies depending on composition, pressure, and temperature, but is typically around 250:1. The pressure at which LPG becomes liquid, called its vapour pressure, likewise varies depending on composition and temperature; for example, it is approximately 220 kilopascals (32 psi) for pure butane at 20 °C (68 °F), and approximately 2,200 kilopascals (320 psi) for pure propane at 55 °C (131 °F). LPG in its gaseous phase is still heavier than air, unlike natural gas, and thus will flow along floors and tend to settle in low spots, such as basements. There are two main dangers to this. The first is a possible explosion if the mixture of LPG and air is within the explosive limits and there is an ignition source. The second is suffocation due to LPG displacing air, causing a decrease in oxygen concentration.
A full LPG gas cylinder contains 86% liquid; the ullage volume will contain vapour at a pressure that varies with temperature.
Additional Information
Liquefied Petroleum Gas (LPG) can pose a serious health hazard in the workplace. The chemical properties of LPG, combined with its potential for combustion and the associated risks of leaks and exposure to combustion byproducts, make it essential to handle, store, and use LPG safely to mitigate health hazards and prevent accidents.
What Is Liquified Petroleum Gas?
LPG is a colourless, odourless, flammable gas. It is a mixture of propane and butane with smaller amounts of isobutane, butylene, and other hydrocarbons. When sold and shipped, an odorant (such as Methyl Mercaptan) is added.
What are the Common Uses of Liquified Petroleum Gas?
LPG is widely used as a fuel for domestic or camping heating and cooking appliances. It is also used as a lighter fuel, refrigerant, propellant in aerosols, substitute for gasoline and in the production of other chemicals and plastics.
Why Is Liquified Petroleum Gas Harmful?
LPG is an asphyxiant gas that can cause unconsciousness and/or death if oxygen levels are sufficiently reduced. May displace oxygen and cause rapid suffocation. LPG is also an extremely flammable gas and is a gas under pressure; which may explode if heated.
Side Effects of Inhaling Liquified Petroleum Gas
Exposure to LPG is mainly by inhalation or by eye and skin contact. Inhaling LPG vapor at high concentration even for a short time can cause asphyxiation, seizures, comas, heart problems and death.
Inhalation of LPG may cause drowsiness or dizziness and respiratory irritation (cough, sneezing, headache, nose and throat pain). Long-term exposure may lead to central nervous system damage, nosebleeds, rhinitis, oral/nasal ulcerations, conjunctivitis, weight loss and fatigue.
Eye and skin irritation may occur due to contact with LPG. LPG released under pressure can cause frostbite burn due to rapid temperature decrease. Symptoms of frostbite include permanent eye damage or blindness, change in skin color to white or grayish-yellow.
Who Needs to Be Concerned About LPG Hazards in the Workplace
Both employers and employees need to be aware of LPG hazards so that safety precautions are taken.
Exposure to LPG may be due to accidental emissions of vapours or gases from pressurized equipment, a leak from containment or release during breaking containment in the following workplaces:
• Natural gas processing facilities.
• Crude oil treatment processes in petroleum refineries, distillation, cracking or reforming.
• Manufacture of refrigerants and aerosols.
• Manufacture of chemicals using LPG as chemical feedstock.
• Loading, unloading, transportation and storage.
• Filling/transfer stations.

#179 Dark Discussions at Cafe Infinity » Code Quotes - III » 2025-11-05 16:29:03
- Jai Ganesh
- Replies: 0
Code Quotes - III
1. The best book I read this year was 'The Da Vinci Code.' - Goran Ivanisevic
2. Theosophy has no code of morals, being itself the embodiment of the highest morality; it presents to its students the highest moral teachings of all religions, gathering the most fragrant blossoms from the gardens of the world-faiths. - Annie Besant
3. There has to be some kind of order and some moral code. I don't know how people can function without a belief in a deity. - Mel Gibson
4. The thing about boxers is that there's respect there. You beat me, and I may not like it, but you know what, deep down inside, I respect you. And that's the code of honor. - Sugar Ray Leonard
5. The Russian people have their own cultural code, their own tradition. - Vladimir Putin
6. A lot of people assume that creating software is purely a solitary activity where you sit in an office with the door closed all day and write lots of code. - Bill Gates
7. Proprietary software keeps users divided and helpless. Divided because each user is forbidden to redistribute it to others, and helpless because the users can't change it since they don't have the source code. They can't study what it really does. So the proprietary program is a system of unjust power. - Richard Stallman
8. We have a rule that if you check in code, you have to maintain it. So I mostly code on the side. I don't check in code anymore. - Mark Zuckerberg.
#180 Re: Jai Ganesh's Puzzles » General Quiz » 2025-11-05 16:01:22
Hi,
#10649. What does the term in Biology Egg mean?
#10650. What does the term in Biology Electrochemical gradient mean?
#181 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-11-05 15:42:12
Hi,
#5845. What does the verb (used with object) downplay mean?
#5846. What does the noun downpour mean?
#182 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-11-05 15:24:20
Hi,
#2517. What does the medical term HELLP syndrome mean?
#183 Jokes » Blackberry Jokes » 2025-11-05 14:29:03
- Jai Ganesh
- Replies: 0
Q: What do you call blackberries playing the guitar?
A: A jam session.
* * *
Q: What did one blackberry say to the other blackberry?
A: If you weren't so sweet, we wouldn't be in this jam!
* * *
Patient: Doctor, there is a berry growing out of my head.
Doctor: Oh, that's easy. Just put some cream on it!
* * *
Q: What do you call a blackberry that uses foul language?
A: Berry Rude.
* * *
Q: How many grams of protein are in a blackberry pi?
A: 3.14159265...
* * *
#184 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-11-05 14:12:16
Hi,
#9796.
#185 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-11-05 13:56:01
Hi,
#6291.
#186 Re: Exercises » Compute the solution: » 2025-11-05 13:44:32
Hi,
2639.
#187 Science HQ » Boiling Point » 2025-11-04 22:17:49
- Jai Ganesh
- Replies: 0
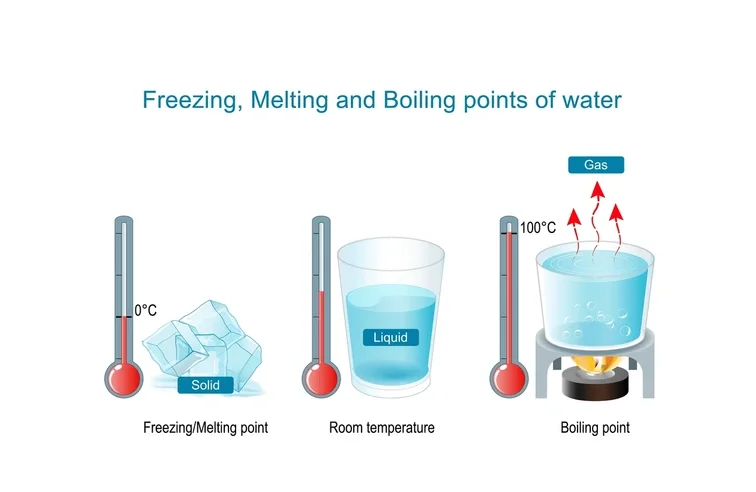
Boiling Point
Gist
The boiling point is the temperature at which a liquid turns into a vapor or gas. This occurs when the liquid's vapor pressure equals the surrounding environmental pressure, and the substance's particles gain enough energy to break free from their intermolecular forces. For example, water boils at 100 degrees Centigrade (212 degrees Fahrenheit) under standard atmospheric pressure.
The boiling point is the specific temperature at which a liquid transitions to gas, occurring when its vapor pressure matches the external atmospheric pressure.
Summary
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: 99.97 °C (211.95 °F)) under standard pressure at sea level, but at 93.4 °C (200.1 °F) at 1,905 metres (6,250 ft) altitude. For a given pressure, different liquids will boil at different temperatures.
The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pressure and allow bubbles of vapor to form inside the bulk of the liquid. The standard boiling point has been defined by IUPAC since 1982 as the temperature at which boiling occurs under a pressure of one bar.
The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) of a substance from a liquid into a gas at a given pressure (often atmospheric pressure).
Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation. Evaporation is a surface phenomenon in which molecules located near the liquid's edge, not contained by enough liquid pressure on that side, escape into the surroundings as vapor. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid.
Details
At the boiling point, the transition from the liquid to the gaseous phase occurs in a pure substance. Therefore, the boiling point is the temperature at which the vapor pressure of the liquid is equal to the applied pressure on the liquid. The boiling point at a pressure of 1 atmosphere is called the normal boiling point
For a pure substance at a particular pressure P, the stable phase is the vapor phase at temperatures immediately above the boiling point and is the liquid phase at temperatures immediately below the boiling point. The liquid-vapor equilibrium line on the phase diagram of a pure substance gives the boiling point as a function of pressure. Alternatively, this line gives the vapor pressure of the liquid as a function of temperature. The vapor pressure of water is 1 atm (101.325 kilopascals) at 100°C, the normal boiling point of water. The vapor pressure of water is 3.2 kPa (0.031 atm) at 25°C, so the boiling point of water at 3.2 kPa is 25°C. The liquid-vapor equilibrium line on the phase diagram of a pure substance begins at the triple point (where solid, liquid, and vapor coexist in equilibrium) and ends at the critical point, where the densities of the liquid and vapor phases have become equal. For pressures below the triple-point pressure or above the critical-point pressure, the boiling point is meaningless. Carbon dioxide has a triple-point pressure of 5.11 atm (518 kPa), so carbon dioxide has no normal boiling point.
The normal boiling point is high for liquids with strong intermolecular attractions and low for liquids with weak intermolecular attractions. Helium has the lowest normal boiling point, 4.2 kelvin (−268.9°C). Some other normal boiling points are 111.1 K (−162°C) for methane (CH4), 450°C for triacontane (n-C30H62), 1465°C for sodium chloride (NaCl), and 5555°C for tungsten (W).
When a pure liquid is boiled at fixed pressure, the temperature remains constant until all the liquid has vaporized. When a solution is boiled at fixed pressure, the composition of the vapor usually differs from that of the liquid, and the change in liquid composition during boiling changes the boiling point. Thus the boiling process occurs over a range of temperatures for a solution. An exception is an azeotrope, which is a solution that boils entirely at a constant temperature because the vapor in equilibrium with the solution has the same composition as the solution. In fractional distillation, the variation of boiling point with composition is used to separate liquid mixtures into their components.
Additional Information
Boiling point is the temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; under this condition, addition of heat results in the transformation of the liquid into its vapour without raising the temperature.
At any temperature a liquid partly vaporizes into the space above it until the pressure exerted by the vapour reaches a characteristic value called the vapour pressure of the liquid at that temperature. As the temperature is increased, the vapour pressure increases; at the boiling point, bubbles of vapour form within the liquid and rise to the surface. The boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). At sea level, water boils at 100° C (212° F). At higher altitudes the temperature of the boiling point is lower.
#188 This is Cool » Alum » 2025-11-04 20:26:06
- Jai Ganesh
- Replies: 0
Alum
Gist
Alum is a natural crystalline compound with antiseptic, astringent, and deodorizing properties, commonly known as a double sulfate salt of aluminum. It is used for water purification, in skincare for acne and tightening, and in traditional medicine for issues like bleeding gums and as a deodorant. It is also used in the food and textile industries.
Alum is a crystalline double sulfate salt of a monovalent cation (like potassium) and aluminium, which has properties like antiseptic, astringent, and deodorizing effects. It is used in various applications including water purification, food, and medicine.
Summary
An alum is a type of chemical compound, usually a hydrated double sulfate salt of aluminium with the general formula XAl(SO4)2·12H2O, such that X is a monovalent cation such as potassium or ammonium. By itself, alum often refers to potassium alum, with the formula KAl(SO4)2·12H2O. Other alums are named after the monovalent ion, such as sodium alum and ammonium alum.
The name alum is also used, more generally, for salts with the same formula and structure, except that aluminium is replaced by another trivalent metal ion like chromiumIII, or sulfur is replaced by another chalcogen like selenium. The most common of these analogs is chrome alum KCr(SO4)2·12H2O.
In most industries, the name alum (or papermaker's alum) is used to refer to aluminium sulfate, Al2(SO4)3·n2O, which is used for most industrial flocculation (the variable n is an integer whose size depends on the amount of water absorbed into the alum). For medicine, the word alum may also refer to aluminium hydroxide gel used as a vaccine adjuvant.
Production
Some alums occur as minerals, the most important being alunite.
The most important alums – potassium, sodium, and ammonium – are produced industrially. Typical recipes involve combining aluminium sulfate and the sulfate monovalent cation. The aluminium sulfate is usually obtained by treating minerals like alum schist, bauxite and cryolite with sulfuric acid.
Details
Alum is a type of chemical compound that is commonly used in everyday and industrial applications. You'll find alum in everything from baking powder and toothpaste to cosmetics and some fire extinguishers, but there are also various types of alum with different use cases.
Usually, when you hear about alum it is in reference to potassium alum, which is the hydrated form of potassium aluminum sulfate and has the chemical formula KAl(SO4)2·12H2O.
However, any of the compounds with the empirical formula AB(SO4)2·12H2O are also considered to be alum. Sometimes alum is seen in its crystalline form, although it is most often sold as a powder. Potassium alum is a fine white powder that you can find sold with kitchen spices or pickling ingredients. It is also sold as a large crystal as a "deodorant rock" for underarm use.
Key Takeaways: Alum
* Alum refers to a collection of chemical compounds that are hydrated sulfate salts of aluminum and usually one other metal.
* Common forms of alum include hydrated potassium aluminum sulfate, ammonium aluminum sulfate, and sodium aluminum sulfate.
* The different compounds have different functions. Alum finds use in vaccines, baking powder, tanning agents, deodorants, and antiseptics.
What Are the Types of Alum?
* Potassium Alum: Potassium alum is also known as potash alum or tawas. It is aluminum potassium sulfate. This is the type of alum that you find in the grocery store for pickling. It is also used in leather tanning, as a flocculant in water purification, as an ingredient in aftershave, and as a treatment for fireproof textiles. Its chemical formula is KAl(SO4)2.
* Soda Alum: Soda alum has the formula NaAl(S O4)2·12H2O. It is used in baking powder and as an acidulant in food.
* Ammonium Alum: Ammonium alum has the formula NH4Al(SO4)2·12H2O. Ammonium alum is used for many of the same purposes as potassium alum and soda alum. Ammonium alum finds applications in tanning, dyeing textiles, purifying water, and making textiles flame retardant. It's also used in the manufacture of porcelain cement, vegetable glues, and some deodorants.
* Chrome Alum: Chrome alum or chromium alum has the formula KCr(S O4)2·12H2O. This deep violet compound is used in tanning and can be added to other alums to grow lavender or purple crystals.
* Selenate Alums: Selenate alums occur when selenium takes the place of sulfur so that instead of a sulfate you get a selenate, (SeO42-). The selenium-containing alums are strong oxidizing agents, so they can be used as antiseptics, among other uses.
* Aluminum Sulfate: This compound is also known as papermaker's alum. However, it is not technically an alum.
What Is Alum Used for?
Alum has several household and industrial uses. Potassium alum is used most often, although ammonium alum, ferric alum, and soda alum may be used for many of the same purposes.
* purification of drinking water as a chemical flocculant
* in styptic pencil to stop bleeding from minor cuts
* the adjuvant in vaccines ( a chemical that enhances the immune response)
* deodorant "rock"
* pickling agent to help keep pickles crisp
* flame retardant
* the acidic component of some types of baking powder
* an ingredient in some homemade and commercial modeling clay
* an ingredient in some depilatory (hair removal) waxes
* skin whitener
* ingredient in some brands of toothpaste.
Alum in Science Projects
Several interesting science projects use alum. In particular, it is used to grow stunning non-toxic crystals. Clear crystals result from potassium alum, while purple crystals grow from chrome alum.
How Is Alum Produced?
Several minerals are used as the source material to produce alum, including alum schist, alunite, bauxite, and cryolite.
The specific process used to obtain the alum depends on the original mineral. When alum is obtained from alunite, the alunite is calcined. The resulting material is kept moist and exposed to air until it turns to a powder, which is lixiviated, or extracted, with sulfuric acid and hot water. The liquid is decanted, and the alum crystallizes out of the solution.
Additional Information
Alum is any of a group of hydrated double salts, usually consisting of aluminum sulfate, water of hydration, and the sulfate of another element. A whole series of hydrated double salts results from the hydration of the sulfate of a singly charged cation (e.g., K+) and the sulfate of any one of a number of triply charged cations (e.g., Al3+). Aluminum sulfate can thus form alums with sulfates of the singly charged cations of potassium, sodium, ammonium, cesium, and other elements and compounds. In similar fashion, sulfates of the triply charged cations of iron, chromium, manganese, cobalt, and other metals may take the place of aluminum sulfate. The most important alums are potassium aluminum sulfate, ammonium aluminum sulfate, and sodium aluminum sulfate. Potassium aluminum sulfate, also known as potassium alum or potash alum, has a molecular formula of K2(SO4)·Al2(SO4)3·24H2O or KAl(SO4)2·12H2O.
Alums can easily be produced by precipitation from an aqueous solution. In producing potassium alum, for example, aluminum sulfate and potassium sulfate are dissolved in water, and then upon evaporation the alum crystallizes out of the solution. A more common production method is to treat bauxite ore with sulfuric acid and then with potassium sulfate. Ammonium alum is produced by the evaporation of a water solution containing ammonium sulfate and aluminum sulfate. It can also be obtained by treating a mixture of aluminum sulfate and sulfuric acid with ammonia. Alums occur naturally in various minerals. Potassium alum, for example, is found in the minerals kalinite, alunite, and leucite, which can be treated with sulfuric acid to obtain crystals of the alum.
Most alums have an astringent and acid taste. They are colourless, odourless, and exist as a white crystalline powder. Alums are generally soluble in hot water, and they can be readily precipitated from aqueous solutions to form large octahedral crystals.
Alums have many uses, but they have been partly supplanted by aluminum sulfate itself, which is easily obtainable by treating bauxite ore with sulfuric acid. The commercial uses of alums mainly stem from the hydrolysis of the aluminum ions, which results in the precipitation of aluminum hydroxide. This chemical has various industrial uses. Paper is sized, for example, by depositing aluminum hydroxide in the interstices of the cellulose fibres. Aluminum hydroxide adsorbs suspended particles from water and is thus a useful flocculating agent in water-purification plants. When used as a mordant (binder) in dyeing, it fixes dye to cotton and other fabrics, rendering the dye insoluble. Alums are also used in pickling, in baking powder, in fire extinguishers, and as astringents in medicine.

#189 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-11-04 16:52:57
2384) George Porter
Gist:
Work
During chemical reactions, atoms and molecules regroup and form new constellations. Chemical reactions are affected by heat and light, among other things. The sequence of events can proceed very quickly. At the end of the 1940s, George Porter and Ronald Norrish built an extremely powerful lamp that emitted very short bursts of light. The light’s energy triggered reactions among the molecules or split them into parts that were inclined to react. By registering the light spectrums that are characteristic for different substances, the progress of the reaction could be monitored.
Summary
Sir George Porter, Baron Porter of Luddenham (born December 6, 1920, Stainforth, Yorkshire, England—died August 31, 2002, Canterbury) was an English chemist, corecipient with fellow Englishman Ronald George Wreyford Norrish and Manfred Eigen of West Germany of the 1967 Nobel Prize for Chemistry. All three were honoured for their studies in flash photolysis, a technique for observing the intermediate stages of very fast chemical reactions.
After undergraduate work at the University of Leeds, Porter earned a doctorate at the University of Cambridge under Norrish in 1949. He continued on there, developing the technique of flash photolysis with Norrish. In this technique, a gas or liquid in equilibrium is illuminated with an ultrashort burst of light that causes photochemical reactions in the substance. The extremely short-lived intermediate products of these reactions are illuminated by a second burst of light that enables an absorption spectrum to be taken of the reaction products before the gas has returned to a state of equilibrium. Porter specifically studied the equilibrium of chlorine atoms and molecules. In 1955 he joined the faculty of chemistry at the University of Sheffield, where he taught until 1966, becoming in that year director of the Royal Institution of Great Britain and Fullerian professor of chemistry. Porter was knighted in 1972 and created a life peer in 1990.
Details
George Porter, Baron Porter of Luddenham, (6 December 1920 – 31 August 2002) was a British chemist. He was awarded the Nobel Prize in Chemistry in 1967.
Education and early life
Porter was born in Stainforth, near Thorne, in the then West Riding of Yorkshire. He was educated at Thorne Grammar School, then won a scholarship to the University of Leeds and gained his first degree in chemistry. During his degree, Porter was taught by Meredith Gwynne Evans, who he said was the most brilliant chemist he had ever met. He was awarded a PhD from the University of Cambridge in 1949 for research investigating free radicals produced by photochemical means. He later became a Fellow of Emmanuel College, Cambridge.
Career and research
Porter served in the Royal Naval Volunteer Reserve during the Second World War. Porter then went on to do research at the University of Cambridge supervised by Ronald George Wreyford Norrish where he began the work that ultimately led to them becoming Nobel Laureates.
His original research in developing the technique of flash photolysis to obtain information on short-lived molecular species provided the first evidence of free radicals. His later research utilised the technique to study the detailed aspects of the light-dependent reactions of photosynthesis, with particular regard to possible applications to a hydrogen economy, of which he was a strong advocate.
He was Assistant Director of the British Rayon Research Association from 1953 to 1954, where he studied the phototendering of dyed cellulose fabrics in sunlight.
Porter served as professor in the Chemistry department at the University of Sheffield in 1954–65. It was here he started his work on flash photolysis with equipment designed and made in the departmental workshop. During this tenure he also took part in a television programme describing his work. This was in the "Eye on Research" series. Porter became Fullerian Professor of Chemistry and Director of the Royal Institution in 1966. During his directorship of the Royal Institution, Porter was instrumental in the setting up of Applied Photophysics, a company created to supply instrumentation based on his group's work. He was awarded the Nobel Prize in Chemistry in 1967 along with Manfred Eigen and Ronald George Wreyford Norrish. In the same year he became a visiting professor in University College London.
Porter was a major contributor to the Public Understanding of science. He became president of the British Association in 1985 and was the founding Chair of the Committee on the Public Understanding of Science (COPUS). He gave the Romanes Lecture, entitled "Science and the human purpose", at the University of Oxford in 1978; and in 1988 he gave the Dimbleby Lecture, "Knowledge itself is power." From 1990 to 1993 he gave the Gresham lectures in astronomy.
Awards and honours
Porter was elected a Fellow of the Royal Society (FRS) in 1960, a member of the American Academy of Arts and Sciences in 1979, a member of the American Philosophical Society in 1986, and served as President of the Royal Society from 1985 to 1990. He was also awarded the Davy Medal in 1971, the Rumford Medal in 1978, the Ellison-Cliffe Medal in 1991 and the Copley Medal in 1992.
Porter also received an Honorary Doctorate from Heriot-Watt University in 1971.
He was knighted in 1972, appointed to the Order of Merit in 1989, and was made a life peer as Baron Porter of Luddenham, of Luddenham in the County of Kent, in 1990. In 1995, he was awarded an Honorary Degree (Doctor of Laws) from the University of Bath.
In 1976 he gave the Royal Institution Christmas Lecture on The Natural History of a Sunbeam.
Porter served as Chancellor of the University of Leicester between 1984 and 1995. In 2001, the university's chemistry building was named the George Porter Building in his honour.
Family
In 1949 Porter married Stella Jean Brooke.

#190 Re: This is Cool » Miscellany » 2025-11-04 16:26:45
2436) Citric Acid
Gist
Citric acid is used in various applications, including acting as a flavoring agent and preservative in food and drinks, a descaling and cleaning agent in household products, an ingredient in cosmetics and personal care items, and a chelating agent in industrial processes. It is also used in some pharmaceuticals and supplements to aid mineral absorption, buffer solutions, or prevent kidney stone formation.
Summary
Citric acid is a colourless crystalline organic compound belonging to the family of carboxylic acids, present in practically all plants and in many animal tissues and fluids. It is one of a series of compounds involved in the physiological oxidation of fats, proteins, and carbohydrates to carbon dioxide and water.
Citric acid was first isolated from lemon juice by Swedish chemist Carl Wilhelm Scheele in 1784 and is manufactured by fermentation of cane sugar or molasses in the presence of a fungus, Aspergillus niger. It is used in confections and soft drinks (as a flavouring agent), in metal-cleaning compositions, and in improving the stability of foods and other organic substances (by suppressing the deleterious action of dissolved metal salts).
Details
Citric acid is an important natural compound that has been known since the late 18th century. The pioneering Swedish–German chemist Carl Wilhelm Scheele isolated it from lemon juice in 1784. It has since been found in other citrus fruits, pineapples, and even animal tissues.
Citric acid is a major industrial chemical, produced at >2 million t/year worldwide. Its main source is not from fruit, but from the fermentation of crude sugars (e.g., molasses and corn starch) by the mold Aspergillus niger. It has a myriad of uses, mostly in foods and pharmaceuticals; these uses include acidifying agent/pH adjustment, antioxidant, flavoring agent, and as metal salts in dietary supplements. In industry and domestic applications, citric acid is a chelating and buffering agent in many cleaning products and a starting material for synthesizing citrate esters, itaconic acid, acetonedicarboxylic acid, and other compounds.
Biochemists are familiar with the citric acid cycle, which is a major life process in all respiring organisms. Also called the Krebs cycle or the tricarboxylic acid cycle, the process begins with sugar-derived pyruvate, which enzymatically generates acetyl-coenzyme A (CoA) to start the cycle. Acetate released from acetyl-CoA reacts with oxaloacetic acid produced at the end of the previous cycle to form citric acid; this is followed by several steps, during which an oxidation reaction releases energy to the body in the form of adenosine triphosphate.
Many scientists contributed to the discovery and establishment of the citric acid cycle. The two key researchers were Albert Szent-Györgyi at the University of Szeged (Hungary) and Hans Adolf Krebs at the University of Sheffield (UK); they were awarded the Nobel Prize in Physiology or Medicine in 1937 and 1953, respectively.
Additional Information
Citric acid is an organic compound with the formula C6H8O7. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
More than two million tons of citric acid are manufactured every year. It is used widely as acidifier, flavoring, preservative, and chelating agent.
A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solutions and salts of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.
Natural occurrence and industrial production
Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in the juices).[a] The concentrations of citric acid in citrus fruits range from 0.005 mol/L for oranges and grapefruits to 0.30 mol/L in lemons and limes; these values vary within species depending upon the cultivar and the circumstances under which the fruit was grown.
Citric acid was first isolated in 1784 by the chemist Carl Wilhelm Scheele, who crystallized it from lemon juice.
Industrial-scale citric acid production first began in 1890 based on the Italian citrus fruit industry, where the juice was treated with hydrated lime (calcium hydroxide) to precipitate calcium citrate, which was isolated and converted back to the acid using diluted sulfuric acid. In 1893, C. Wehmer discovered Penicillium mold could produce citric acid from sugar. However, microbial production of citric acid did not become industrially important until World War I disrupted Italian Citrus exports.
In 1917, American food chemist James Currie discovered that certain strains of the mold Aspergillus niger could be efficient citric acid producers, and the pharmaceutical company Pfizer began industrial-level production using this technique two years later, followed by Citrique Belge in 1929. In this production technique, which is still the major industrial route to citric acid used today, cultures of Aspergillus niger are fed on a sucrose or glucose-containing medium to produce citric acid. The source of sugar is corn steep liquor, molasses, hydrolyzed corn starch, or other inexpensive, carbohydrate solution. After the mold is filtered out of the resulting suspension, citric acid is isolated by precipitating it with calcium hydroxide to yield calcium citrate salt, from which citric acid is regenerated by treatment with sulfuric acid, as in the direct extraction from citrus fruit juice.
In 1977, a patent was granted to Lever Brothers for the chemical synthesis of citric acid starting either from aconitic or isocitrate (also called alloisocitrate) calcium salts under high pressure conditions; this produced citric acid in near quantitative conversion under what appeared to be a reverse, non-enzymatic Krebs cycle reaction.
Although industrial-scale production of citric acid by chemical synthesis or extraction from citrus fruits are both feasible, fermentation by molds (and sometimes yeasts) is almost exclusively the only method actually practiced.
Global production was in excess of 2,000,000 tons in 2018. More than 50% of this volume was produced in China. More than 50% was used as an acidity regulator in beverages, some 20% in other food applications, 20% for detergent applications, and 10% for applications other than food, such as cosmetics, pharmaceuticals, and in the chemical industry.

#191 Dark Discussions at Cafe Infinity » Code Quotes - II » 2025-11-04 15:47:43
- Jai Ganesh
- Replies: 0
Code Quotes - II
1. The manual for WordStar, the most popular word-processing program, is 400 pages thick. To write a novel, you have to read a novel - one that reads like a mystery to most people. They're not going to learn slash q-z any more than they're going to learn Morse code. That is what Macintosh is all about. - Steve Jobs
2. Hackathons are these things where just all of the Facebook engineers get together and stay up all night building things. And, I mean, usually at these hackathons, I code too, just alongside everyone. - Mark Zuckerberg
3. I start 'The Code Book' with the story of Mary Queen of Scots and the Babington Plot, which was foiled when Mary's enciphered messages fell into the hands of Elizabeth I's codebreakers. - Simon Singh
4. Trying to read our DNA is like trying to understand software code - with only 90% of the code riddled with errors. It's very difficult in that case to understand and predict what that software code is going to do. - Elon Musk
5. Proprietary software tends to have malicious features. The point is with a proprietary program, when the users don't have the source code, we can never tell. So you must consider every proprietary program as potential malware. - Richard Stallman
6. It now seems very likely that many of the 64 triplets, possibly most of them, may code one amino acid or another, and that in general several distinct triplets may code one amino acid. - Francis Crick
7. In fact, the best thing we could do on taxes for all Americans is to simplify the individual tax code. This will be a tough job, but members of both parties have expressed an interest in doing this, and I am prepared to join them. - Barack Obama
8. I want to reform the tax code so that it's simple, fair, and asks the wealthiest households to pay higher taxes on incomes over $250,000 - the same rate we had when Bill Clinton was president; the same rate we had when our economy created nearly 23 million new jobs, the biggest surplus in history, and a lot of millionaires to boot. - Barack Obama.
#192 Re: Jai Ganesh's Puzzles » General Quiz » 2025-11-04 15:25:41
Hi,
#10647. What does the term in Biology Ectotherm mean?
#10648. What does the term in Biology Effector mean?
#193 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-11-04 15:08:59
Hi,
#5843. What does the noun echelon mean?
#5844. What does the noun echinoderm mean?
#194 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-11-04 14:58:06
Hi,
#2516. What does the medical term Leptomeningeal cancer mean?
#195 Jokes » Beverage Jokes - III » 2025-11-04 14:16:14
- Jai Ganesh
- Replies: 0
Q: What has eight arms and an IQ of 60?
A: Four guys drinking Bud Light and watching a football game!
* * *
Q: When do women drink alcohol?
A: Wine O'Clock.
* * *
Q: When does it rain money?
A: When there is "change" in the weather.
* * *
Q: What do you say when you're gonna drunk dial someone?
A: Al-cohol you.
* * *
Q: If H20 is water what is H204?
A: Drinking, bathing, washing, swimming. . .
* * *
#196 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-11-04 14:07:43
Hi,
#9795.
#197 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-11-04 13:53:43
Hi,
#6290.
#198 Re: Exercises » Compute the solution: » 2025-11-04 13:41:19
Hi,
2638.
#199 This is Cool » Carbonic Acid » 2025-11-03 21:45:49
- Jai Ganesh
- Replies: 0
Carbonic Acid
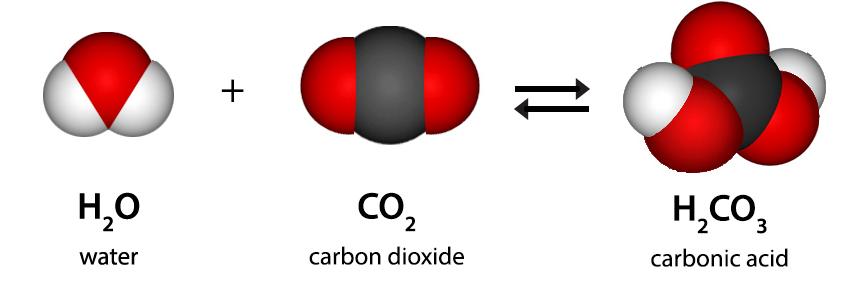
Gist
Carbonic acid (H2CO3) is a weak acid formed when carbon dioxide (CO2)) dissolves in water. It is an unstable compound that can dissociate into a hydrogen ion (H+) and a bicarbonate ion (HCO3-), and it plays a critical role in regulating blood pH in the human body. Carbonic acid is also found in carbonated beverages and is responsible for weathering limestone.
Carbonic acid is used in the food and beverage industry for carbonating soft drinks, wine, and sparkling water; in medicine for its role as a blood buffer and for certain topical treatments; and in industrial applications for pH control in water treatment, as a cleaning agent, and in the production of other chemicals.
Summary
Carbonic acid is a chemical compound with the chemical formula H2CO3. The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters.
In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis.
Terminology in biochemical literature
In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula H2CO3. Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid.
In physiology, carbon dioxide excreted by the lungs may be called volatile acid or respiratory acid.
Details
Carbonic acid, (H2CO3) is a compound of the elements hydrogen, carbon, and oxygen. It is formed in small amounts when its anhydride, carbon dioxide (CO2), dissolves in water.
The predominant species are simply loosely hydrated CO2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be formed—namely, hydrogen carbonates.
However, the acid-base behaviour of carbonic acid depends on the different rates of some of the reactions involved, as well as their dependence on the pH of the system.
Between pH values of 8 and 10, all the above equilibrium reactions are significant.
Carbonic acid plays a role in the assembly of caves and cave formations like stalactites and stalagmites. The largest and most common caves are those formed by dissolution of limestone or dolomite by the action of water rich in carbonic acid derived from recent rainfall. The calcite in stalactites and stalagmites is derived from the overlying limestone near the bedrock/soil interface. Rainwater infiltrating through the soil absorbs carbon dioxide from the carbon dioxide-rich soil and forms a dilute solution of carbonic acid. When this acid water reaches the base of the soil, it reacts with the calcite in the limestone bedrock and takes some of it into solution. The water continues its downward course through narrow joints and fractures in the unsaturated zone with little further chemical reaction. When the water emerges from the cave roof, carbon dioxide is lost into the cave atmosphere, and some of the calcium carbonate is precipitated. The infiltrating water acts as a calcite pump, removing it from the top of the bedrock and redepositing it in the cave below.
Carbonic acid is important in the transport of carbon dioxide in the blood. Carbon dioxide enters blood in the tissues because its local partial pressure is greater than its partial pressure in blood flowing through the tissues. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). Blood acidity is minimally affected by the released hydrogen ions because blood proteins, especially hemoglobin, are effective buffering agents. (A buffer solution resists change in acidity by combining with added hydrogen ions and, essentially, inactivating them.) The natural conversion of carbon dioxide to carbonic acid is a relatively slow process; however, carbonic anhydrase, a protein enzyme present inside the red blood cell, catalyzes this reaction with sufficient rapidity that it is accomplished in only a fraction of a second. Because the enzyme is present only inside the red blood cell, bicarbonate accumulates to a much greater extent within the red cell than in the plasma. The capacity of blood to carry carbon dioxide as bicarbonate is enhanced by an ion transport system inside the red blood cell membrane that simultaneously moves a bicarbonate ion out of the cell and into the plasma in exchange for a chloride ion. The simultaneous exchange of these two ions, known as the chloride shift, permits the plasma to be used as a storage site for bicarbonate without changing the electrical charge of either the plasma or the red blood cell. Only 26 percent of the total carbon dioxide content of blood exists as bicarbonate inside the red blood cell, while 62 percent exists as bicarbonate in plasma; however, the bulk of bicarbonate ions is first produced inside the cell, then transported to the plasma. A reverse sequence of reactions occurs when blood reaches the lung, where the partial pressure of carbon dioxide is lower than in the blood.
Additional Information
Carbonic Acid is an inorganic, weak, and unstable acid. The molecule of Carbonic Acid consists of one carbon atom, two hydrogen atoms, and three oxygen atoms. Carbonic Acid is also referred to as a respiratory acid as it is the only acid that is exhaled in the gaseous state by human lungs.
Carbonic acid is studied majorly in various fields of science because of its vast applications and uses. It helps improve marine life due to its natural process of ocean acidification. It is essential for the human body as well.
What is Carbonic Acid?
Carbonic acid is a chemical compound made of carbon dioxide, oxygen, and hydrogen as its elements. It is a weak acid with the chemical formula H2CO3. It is formed when carbon dioxide is dissolved in water. Carbonic acid is a diprotic acid, which means it can form two types of salts: carbonate and bicarbonate. Carbonic Acid is also known as dihydrogen carbonate (because it is made of two hydrogen atoms and a carbonate ion), aerial acid (or acid of air), Oxidocarboxylic acid, Hydroxyformic acid, etc.
It was discovered by the French chemist Antoine Lavoisier in the late 18th century. He found that when carbon dioxide reacts with water an acidic solution is formed which he named as carbonic acid.
Formula of Carbonic Acid
The compound of carbonic acid consists of one carbon atom, two hydrogen atoms, and three oxygen atoms. It can also be represented as OC(OH)2 due to the presence of a carbon-oxygen double bond.
Structure of Carbonic Acid
Carbonic acid consists of a carboxyl functional group along with a hydroxyl group. The structure of carbonic acids consists of one carbon-oxygen double bond and further, this carbon is single bonded with two hydroxyl groups (OH).
Preparation of Carbonic Acid
When strong acid reacts with calcium carbonate, carbon dioxide gas is evolved which when dissolved in water, Carbonic acid is formed.
Properties of Carbonic Acid
Carbonic acid has a variety of properties which are classified into physical and chemical properties. The physical and chemical properties of carbonic acid are described below:
Physical Properties of Carbonic Acid
* State: Carbonic Acid generally occurs as a solution, but according to recent research it was found that Carbonic acid is also being prepared in a solid state by NASA scientists.
* Odor: Carbonic Acid are generally odorless.
* pKa value: The pKa value of Carbonic acid is 6.35. The pKa value is inversely proportional to the acidity of the compound. Hence, carbonic acid is termed under weak acid.
* Boiling Point: The boiling point of carbonic acid is around 127° Celsius.
* Melting Point: The melting point of carbonic acid is around -53° Celsius.
* Density: Carbonic acid has a density of around 1.668 grams per cubic centimeter.
* Molecular weight: The molecular weight of Carbonic acid is 62.024 g/mol.
* Conjugate base: The conjugate bases of Carbonic acid are bicarbonate and carbonate.
Chemical Properties of Carbonic Acid
* Stability: Carbonic acids are unstable under normal circumstances. However, they can be stabilized under high pressure and high temperatures.
* pH: Being a weak acidic, carbonic acid has a pH value of around 4.68
* Diprotic acid: It is a diprotic acid, i.e. it can form two types of salts namely carbonate and bicarbonate.
* Dissociation reaction: In the presence of water, Carbonic acid can partially dissociate into bicarbonate ions (HCO3-) and hydrogen ions (H+).
* Reaction with base: Carbonic acids form bicarbonate salt when reacted with a moderate amount of base and it form carbonate salts if reacted with excess amount of base.
* Decomposition reaction: Carbonic acid can easily decompose into carbon dioxide and water. However, this reaction is reversible under suitable conditions.
Uses of Carbonic Acid
* Carbonated Drinks: Carbonic acid provides the fizzy and bubbly taste in carbonated drinks like sparkling water, sodas, soft drinks, beer, champagne and other carbonated beverages
* Buffering System: Carbonic acid acts as a buffer system in human bodies which helps in maintaining the pH balance in the blood.
* pH regulation: Carbonic acid is also useful in regulating the pH level of water in swimming pools and in regulating pH balance in various other industrial processes.
* Medicinal uses: It is used in medications to balance acid-base levels in the body and in ointments to treat various fungal infections like ringworm. It is also consumed to induce vomiting (when required).
* pH indicator: Carbonic acid is used as a pH indicator to check whether the solution is acidic or basic.
* Precipitation: Carbonic acid is used as a solvent in the precipitation of various ammonium salts like ammonium persulfate.
Cleansing agent: Carbonic acid is also used as a cleaning agent to clean contact lenses.
#200 Science HQ » Chromosome » 2025-11-03 16:46:26
- Jai Ganesh
- Replies: 0
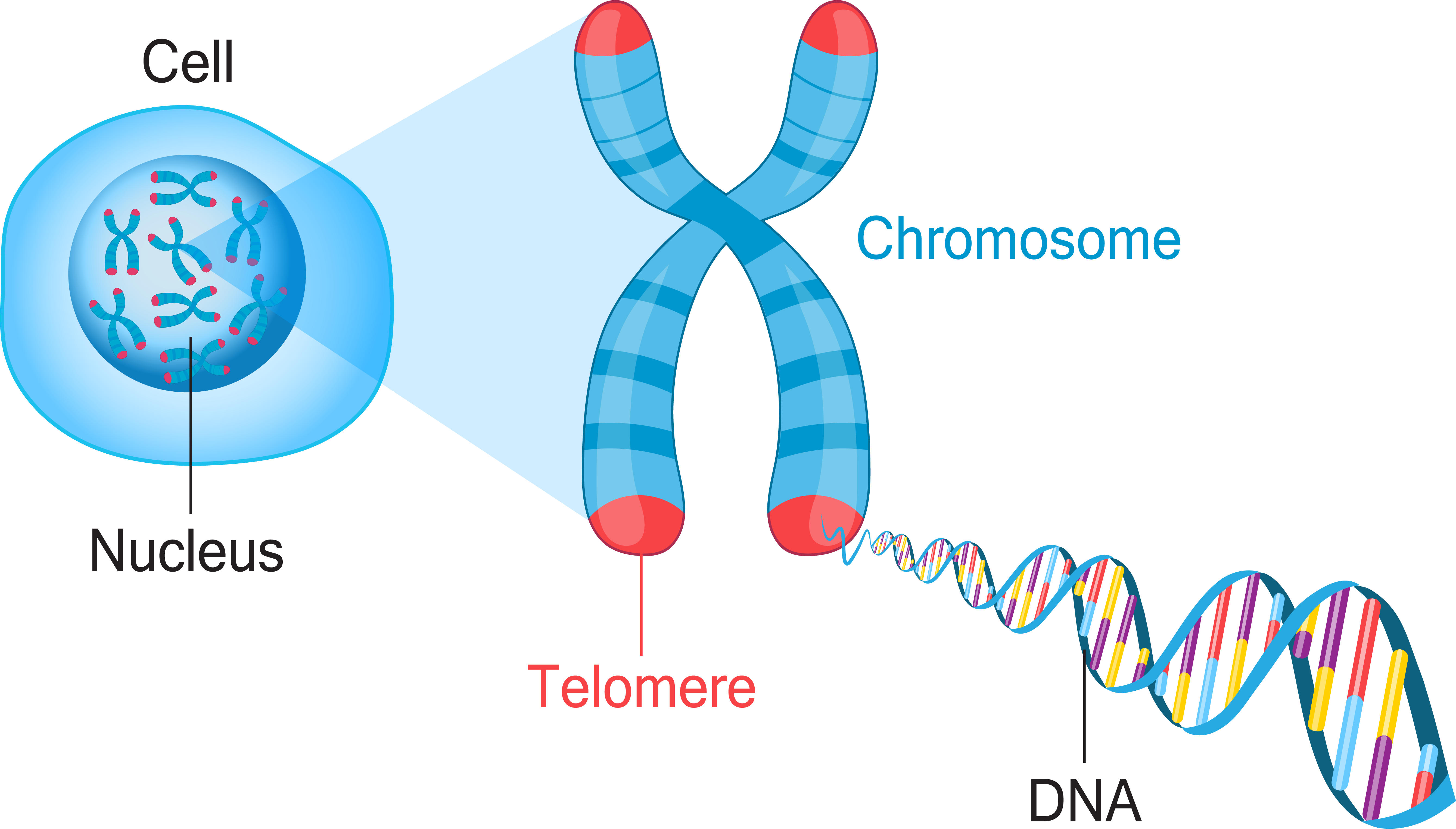
Chromosome
Gist
A chromosome is a thread-like structure made of proteins and a single molecule of DNA that carries the genetic information of an organism. Located in the nucleus of cells, chromosomes are a highly organized way to package the DNA, which is a very long molecule. Humans typically have 46 chromosomes in 23 pairs, with 22 pairs of autosomes and one pair of sex chromosomes (X and Y).
Chromosomes are long strings of DNA wrapped around proteins to make them compact. They're a way for cells to organize and store your DNA. Humans typically have 23 pairs of chromosomes for a total of 46 chromosomes. The first 22 are called autosomes and the last pair are sex chromosomes.
Summary
Chromosomes are threadlike structures made of protein and a single molecule of DNA that serve to carry the genomic information from cell to cell. In plants and animals (including humans), chromosomes reside in the nucleus of cells. Humans have 22 pairs of numbered chromosomes (autosomes) and one pair of sex chromosomes (XX or XY), for a total of 46. Each pair contains two chromosomes, one coming from each parent, which means that children inherit half of their chromosomes from their mother and half from their father. Chromosomes can be seen through a microscope when the nucleus dissolves during cell division.
Chromosomes vary in number and shape among living organisms. Most bacteria have one or two circular chromosomes. Humans, along with other animals and plants, have linear chromosomes . In fact, each species of plants and animals has a set number of chromosomes. A fruit fly, for example, has four pairs of chromosomes, while a rice plant has 12 and a dog, 39. In humans, the twenty-third pair is the sex chromosomes, while the first 22 pairs are called autosomes. Typically, biologically female individuals have two X chromosomes (XX) while those who are biologically male have one X and one Y chromosome (XY). However, there are exceptions to these rules. Chromosomes are also different sizes. The human X chromosome is about three times larger than the human Y chromosome, containing about 900 genes, while the Y chromosome has about 55 genes. The unique structure of chromosomes keeps DNA tightly wound around spool-like proteins, called histones. Without such packaging, DNA molecules would be too long to fit inside cells! For example, if all of the DNA molecules in a single human cell were unwound from their histones and placed end-to-end, they would stretch 6 feet.
Details
A chromosome is a package of DNA containing part or all of the genetic material of an organism. In most chromosomes, the very long thin DNA fibers are coated with nucleosome-forming packaging proteins; in eukaryotic cells, the most important of these proteins are the histones. Aided by chaperone proteins, the histones bind to and condense the DNA molecule to maintain its integrity. These eukaryotic chromosomes display a complex three-dimensional structure that has a significant role in transcriptional regulation.
Normally, chromosomes are visible under a light microscope only during the metaphase of cell division, where all chromosomes are aligned in the center of the cell in their condensed form. Before this stage occurs, each chromosome is duplicated (S phase), and the two copies are joined by a centromere—resulting in either an X-shaped structure if the centromere is located equatorially, or a two-armed structure if the centromere is located distally; the joined copies are called 'sister chromatids'. During metaphase, the duplicated structure (called a 'metaphase chromosome') is highly condensed and thus easiest to distinguish and study. In animal cells, chromosomes reach their highest compaction level in anaphase during chromosome segregation.
Chromosomal recombination during meiosis and subsequent sexual reproduction plays a crucial role in genetic diversity. If these structures are manipulated incorrectly, through processes known as chromosomal instability and translocation, the cell may undergo mitotic catastrophe. This will usually cause the cell to initiate apoptosis, leading to its own death, but the process is occasionally hampered by cell mutations that result in the progression of cancer.
The term 'chromosome' is sometimes used in a wider sense to refer to the individualized portions of chromatin in cells, which may or may not be visible under light microscopy. In a narrower sense, 'chromosome' can be used to refer to the individualized portions of chromatin during cell division, which are visible under light microscopy due to high condensation.
Additional Information
A chromosome is a microscopic threadlike part of the cell that carries hereditary information in the form of genes. A defining feature of any chromosome is its compactness. For instance, the 46 chromosomes found in human cells have a combined length of 200 nm (1 nm = {10}^{-9} metre); if the chromosomes were to be unraveled, the genetic material they contain would measure roughly 2 metres (about 6.5 feet) in length. The compactness of chromosomes plays an important role in helping to organize genetic material during cell division and enabling it to fit inside structures such as the nucleus of a cell, the average diameter of which is about 5 to 10 μm (1 μm = 0.00l mm, or 0.000039 inch), or the polygonal head of a virus particle, which may be in the range of just 20 to 30 nm in diameter.
The structure and location of chromosomes are among the chief differences between viruses, prokaryotes, and eukaryotes. The nonliving viruses have chromosomes consisting of either DNA (deoxyribonucleic acid) or RNA (ribonucleic acid); this material is very tightly packed into the viral head. Among organisms with prokaryotic cells (i.e., bacteria and blue-green algae), chromosomes consist entirely of DNA. The single chromosome of a prokaryotic cell is not enclosed within a nuclear membrane. Among eukaryotes, the chromosomes are contained in a membrane-bound cell nucleus. The chromosomes of a eukaryotic cell consist primarily of DNA attached to a protein core. They also contain RNA. The remainder of this article pertains to eukaryotic chromosomes.
Every eukaryotic species has a characteristic number of chromosomes (chromosome number). In species that reproduce asexually, the chromosome number is the same in all the cells of the organism. Among sexually reproducing organisms, the number of chromosomes in the body (somatic) cells is diploid (2n; a pair of each chromosome), twice the haploid (1n) number found in the sex cells, or gametes. The haploid number is produced during meiosis. During fertilization, two gametes combine to produce a zygote, a single cell with a diploid set of chromosomes.
Somatic cells reproduce by dividing, a process called mitosis. Between cell divisions the chromosomes exist in an uncoiled state, producing a diffuse mass of genetic material known as chromatin. The uncoiling of chromosomes enables DNA synthesis to begin. During this phase, DNA duplicates itself in preparation for cell division.
Following replication, the DNA condenses into chromosomes. At this point, each chromosome actually consists of a set of duplicate chromatids that are held together by the centromere. The centromere is the point of attachment of the kinetochore, a protein structure that is connected to the spindle fibres (part of a structure that pulls the chromatids to opposite ends of the cell). During the middle stage in cell division, the centromere duplicates, and the chromatid pair separates; each chromatid becomes a separate chromosome at this point. The cell divides, and both of the daughter cells have a complete (diploid) set of chromosomes. The chromosomes uncoil in the new cells, again forming the diffuse network of chromatin.
Among many organisms that have separate sexes, there are two basic types of chromosomes: sex chromosomes and autosomes. Autosomes control the inheritance of all the characteristics except the sex-linked ones, which are controlled by the sex chromosomes. Humans have 22 pairs of autosomes and one pair of sex chromosomes. All act in the same way during cell division.
Chromosome breakage is the physical breakage of subunits of a chromosome. It is usually followed by reunion (frequently at a foreign site, resulting in a chromosome unlike the original). Breakage and reunion of homologous chromosomes during meiosis are the basis for the classical model of crossing over, which results in unexpected types of offspring of a mating.