Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-11-05 21:40:16
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,374
Melting Point
Melting Point
Gist
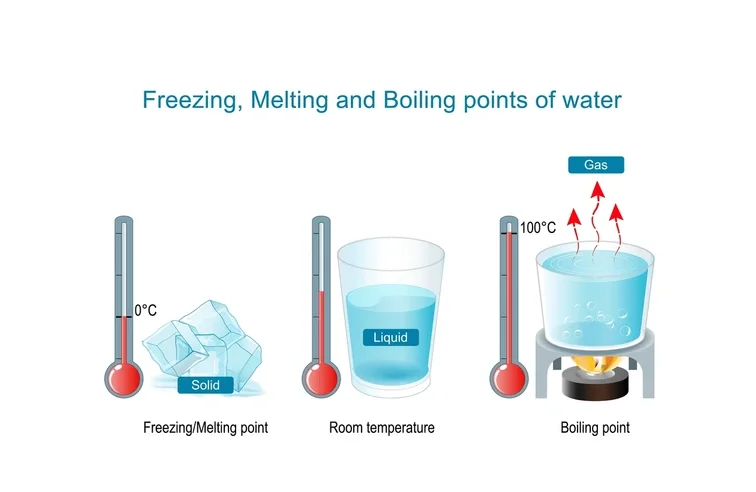
The melting point is the temperature at which a solid substance changes into a liquid under normal atmospheric pressure. At this temperature, the solid and liquid phases of the substance exist together in equilibrium. Melting occurs when added heat provides enough energy for the particles in the solid to break free from the rigid structure and move more freely as a liquid. For example, ice melts at 0 degrees Celsius (32 degrees Fahrenheit), which is also the freezing point for water.
Melting point is the temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. As heat is applied to a solid, its temperature will increase until the melting point is reached. More heat then will convert the solid into a liquid with no temperature change.
Summary
Melting point is the temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. As heat is applied to a solid, its temperature will increase until the melting point is reached. More heat then will convert the solid into a liquid with no temperature change. When all the solid has melted, additional heat will raise the temperature of the liquid. The melting temperature of crystalline solids is a characteristic figure and is used to identify pure compounds and elements. Most mixtures and amorphous solids melt over a range of temperatures.
The melting temperature of a solid is generally considered to be the same as the freezing point of the corresponding liquid; because a liquid may freeze in different crystal systems and because impurities lower the freezing point, however, the actual freezing point may not be the same as the melting point. Thus, for characterizing a substance, the melting point is preferred.
Details
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.
When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point."
Examples
For most substances, melting and freezing points are approximately equal. For example, the melting and freezing points of mercury is 234.32 kelvins (−38.83 °C; −37.89 °F). However, certain substances possess differing solid-liquid transition temperatures. For example, agar melts at 85 °C (185 °F; 358 K) and solidifies from 31 °C (88 °F; 304 K); such direction dependence is known as hysteresis. The melting point of ice at 1 atmosphere of pressure is very close to 0 °C (32 °F; 273 K); this is also known as the ice point. In the presence of nucleating substances, the freezing point of water is not always the same as the melting point. In the absence of nucleators water can exist as a supercooled liquid down to −48.3 °C (−54.9 °F; 224.8 K) before freezing.
The metal with the highest melting point is tungsten, at 3,414 °C (6,177 °F; 3,687 K); this property makes tungsten excellent for use as electrical filaments in incandescent lamps. The often-cited carbon does not melt at ambient pressure but sublimes at about 3,700 °C (6,700 °F; 4,000 K); a liquid phase only exists above pressures of 10 MPa (99 atm) and estimated 4,030–4,430 °C (7,290–8,010 °F; 4,300–4,700 K). Hafnium carbonitride (HfCN) is a refractory compound with the highest known melting point of any substance to date and the only one confirmed to have a melting point above 4,273 K (4,000 °C; 7,232 °F) at ambient pressure. Quantum mechanical computer simulations predicted that this alloy (HfN0.38C0.51) would have a melting point of about 4,400 K. This prediction was later confirmed by experiment, though a precise measurement of its exact melting point has yet to be confirmed. At the other end of the scale, helium does not freeze at all at normal pressure even at temperatures arbitrarily close to absolute zero; a pressure of more than twenty times normal atmospheric pressure is necessary.
Melting point measurements
Many laboratory techniques exist for the determination of melting points. A Kofler bench is a metal strip with a temperature gradient (range from room temperature to 300 °C). Any substance can be placed on a section of the strip, revealing its thermal behaviour at the temperature at that point. Differential scanning calorimetry gives information on melting point together with its enthalpy of fusion.
A basic melting point apparatus for the analysis of crystalline solids consists of an oil bath with a transparent window (most basic design: a Thiele tube) and a simple magnifier. Several grains of a solid are placed in a thin glass tube and partially immersed in the oil bath. The oil bath is heated (and stirred) and with the aid of the magnifier (and external light source) melting of the individual crystals at a certain temperature can be observed. A metal block might be used instead of an oil bath. Some modern instruments have automatic optical detection.
The measurement can also be made continuously with an operating process. For instance, oil refineries measure the freeze point of diesel fuel "online", meaning that the sample is taken from the process and measured automatically. This allows for more frequent measurements as the sample does not have to be manually collected and taken to a remote laboratory.[citation needed]
Techniques for refractory materials
For refractory materials (e.g. platinum, tungsten, tantalum, some carbides and nitrides, etc.) the extremely high melting point (typically considered to be above, say, 1,800 °C) may be determined by heating the material in a black body furnace and measuring the black-body temperature with an optical pyrometer. For the highest melting materials, this may require extrapolation by several hundred degrees. The spectral radiance from an incandescent body is known to be a function of its temperature. An optical pyrometer matches the radiance of a body under study to the radiance of a source that has been previously calibrated as a function of temperature. In this way, the measurement of the absolute magnitude of the intensity of radiation is unnecessary. However, known temperatures must be used to determine the calibration of the pyrometer. For temperatures above the calibration range of the source, an extrapolation technique must be employed. This extrapolation is accomplished by using Planck's law of radiation. The constants in this equation are not known with sufficient accuracy, causing errors in the extrapolation to become larger at higher temperatures. However, standard techniques have been developed to perform this extrapolation.
Consider the case of using gold as the source (mp = 1,063 °C). In this technique, the current through the filament of the pyrometer is adjusted until the light intensity of the filament matches that of a black-body at the melting point of gold. This establishes the primary calibration temperature and can be expressed in terms of current through the pyrometer lamp. With the same current setting, the pyrometer is sighted on another black-body at a higher temperature. An absorbing medium of known transmission is inserted between the pyrometer and this black-body. The temperature of the black-body is then adjusted until a match exists between its intensity and that of the pyrometer filament. The true higher temperature of the black-body is then determined from Planck's Law. The absorbing medium is then removed and the current through the filament is adjusted to match the filament intensity to that of the black-body. This establishes a second calibration point for the pyrometer. This step is repeated to carry the calibration to higher temperatures. Now, temperatures and their corresponding pyrometer filament currents are known and a curve of temperature versus current can be drawn. This curve can then be extrapolated to very high temperatures.
In determining melting points of a refractory substance by this method, it is necessary to either have black body conditions or to know the emissivity of the material being measured. The containment of the high melting material in the liquid state may introduce experimental difficulties. Melting temperatures of some refractory metals have thus been measured by observing the radiation from a black body cavity in solid metal specimens that were much longer than they were wide. To form such a cavity, a hole is drilled perpendicular to the long axis at the center of a rod of the material. These rods are then heated by passing a very large current through them, and the radiation emitted from the hole is observed with an optical pyrometer. The point of melting is indicated by the darkening of the hole when the liquid phase appears, destroying the black body conditions. Today, containerless laser heating techniques, combined with fast pyrometers and spectro-pyrometers, are employed to allow for precise control of the time for which the sample is kept at extreme temperatures. Such experiments of sub-second duration address several of the challenges associated with more traditional melting point measurements made at very high temperatures, such as sample vaporization and reaction with the container.
Additional Information
1. What Is Melting Point?
Melting point is a characteristic property of solid crystalline substances. It is the temperature at which the solid phase changes to the liquid phase. Melting point determination is the thermal analysis most frequently used to characterize solid crystalline materials. It is used in research and development as well as in quality control in various industry segments to identify solid crystalline substances and to check their purity.
Melting point is a characteristic property of solid crystalline substance. It is the temperature at which the solid phase changes to the liquid phase. This phenomenon occurs when the substance is heated. During the melting process, all of the energy added to the substance is consumed as heat of fusion, and the temperature remains constant (see diagram below). During the phase transition, the two physical phases of the material exist side-by-side.
Crystalline materials consist of fine particles that for a regular, 3-dimensional arrangement – a crystalline lattice. The particles within the lattice are held together by lattice forces. When the solid crystalline material is heated, the particles become more energetic and start to move more strongly, until finally the forces of attraction between them are no longer strong enough to hold them together. The crystalline structure is destroyed and the solid material melts.
The stronger the forces of attraction between the particles, the more energy is needed to overcome them. The more energy is needed, the higher the melting point. The melting temperature of a crystalline solid is thus an indicator for the stability of its lattice.
At the melting point not only the aggregate state changes; quite a lot of other physical characteristics also change significantly. Amongst these are the thermodynamic values, specific heat capacity, enthalpy, and rheological properties such as volume or viscosity. Last but not least, the optical properties birefringence reflection and light transmission change. Compared to other physical values the change in light transmission can easily be determined and can therefore be used for melting point detection.
2. Why Measure Melting Points?
Melting points are often used to characterize organic and inorganic crystalline compounds and to ascertain their purity. Pure substances melt at a sharp, highly-defined temperature (very small temperature range of 0.5 – 1 °C) whereas impure, contaminated substances generally exhibit a large melting interval. The temperature at which all material of a contaminated substance is molten is usually lower than that of a pure substance. This behavior is known as melting point depression and can be used to obtain qualitative information about the purity of a substance.
In general, melting point determination is used in the lab in research and development as well as in quality control in various industry segments to identify and check the purity of different substances.
3. Melting Point Determination Principle
At the melting point, there is a change in light transmission. Compared to other physical values the change in light transmission can easily be determined and can therefore be used for melting point detection. Powdered crystalline materials are opaque in the crystalline state and transparent in the liquid state. This distinct difference in optical properties can be measured in order to determine the melting point by recording the percentage of light intensity shining through the substance in the capillary, the transmittance, in relation to the measured furnace temperature.
There are different stages of the melting point process of a solid crystalline substance: At the collapse point, the substance is mostly solid and comprises only a small amount of molten material. At the meniscus point, most of the substance has melted but some solid material is still present. At the clear point, the substance has completely melted.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1