Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#76 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-11-14 14:17:33
Hi,
#9805.
#77 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-11-14 13:51:17
Hi,
6300.
.#78 Re: Exercises » Compute the solution: » 2025-11-14 13:46:36
Hi,
2651.
#79 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-11-13 17:38:17
2393) Julian Schwinger
Gist:
Work
Following the establishment of the theory of relativity and quantum mechanics, an initial relativistic theory was formulated for the interaction between charged particles and electromagnetic fields. However, partly because the electron’s magnetic moment proved to be somewhat larger than expected, the theory had to be reformulated. Julian Schwinger solved this problem in 1948 through “renormalization” and thereby contributed to a new quantum electrodynamics.
Summary
Julian Seymour Schwinger (born Feb. 12, 1918, New York, N.Y., U.S.—died July 16, 1994, Los Angeles, Calif.) was an American physicist and joint winner, with Richard P. Feynman and Tomonaga Shin’ichirō, of the Nobel Prize for Physics in 1965 for introducing new ideas and methods into quantum electrodynamics.
Schwinger was a child prodigy, publishing his first physics paper at age 16. He earned a bachelor’s degree (1937) and a doctorate (1939) from Columbia University in New York City, before engaging in postdoctoral studies at the University of California at Berkeley with physicist J. Robert Oppenheimer. Schwinger left Berkeley in the summer of 1941 to accept an instructorship at Purdue University, West Lafayette, Ind., and in 1943 he joined the Radiation Laboratory at the Massachusetts Institute of Technology, where many scientists had been assembled to help with wartime research on radar. In the fall of 1945 Schwinger accepted an appointment at Harvard University and in 1947 became one of the youngest full professors in the school’s history. From 1972 until his death, Schwinger was a professor in the physics department at the University of California at Los Angeles.
Schwinger was one of the participants at the meeting held in June 1947 on Shelter Island, Long Island, N.Y., at which reliable experimental data were presented that contradicted the predictions of the English theoretical physicist P.A.M. Dirac’s relativistic quantum theory of the electron. In particular, experimental data contradicted Dirac’s prediction that certain hydrogen electron stationary states were degenerate (i.e., had the same energy as certain other states) as well as Dirac’s prediction for the value of the magnetic moment of the electron. Schwinger made a quantum electrodynamical calculation that made use of the notions of mass and charge renormalization, which brought agreement between theory and experimental data. This was a crucial breakthrough that initiated a new era in quantum field theory. Richard Feynman and Tomonaga Shin’ichirō independently had carried out similar calculations, and in 1965 the three of them shared the Nobel Prize. Their work created a new and very successful quantum mechanical description of the interaction between electrically charged entities and the electromagnetic field that conformed with the principles of Albert Einstein’s special theory of relativity.
Schwinger’s work extended to almost every frontier of modern theoretical physics. He had a profound influence on physics both directly and through being the academic adviser for more than 70 doctoral students and more than 20 postdoctoral fellows, many of whom became the outstanding theorists of their generation.
Details
Julian Seymour Schwinger (February 12, 1918 – July 16, 1994) was a Nobel Prize-winning American theoretical physicist. He is best known for his work on quantum electrodynamics (QED), in particular for developing a relativistically invariant perturbation theory, and for renormalizing QED to one loop order. Schwinger was a physics professor at several universities.
Schwinger is recognized as an important physicist, responsible for much of modern quantum field theory, including a variational approach, and the equations of motion for quantum fields. He developed the first electroweak model, and the first example of confinement in 1+1 dimensions. He is responsible for the theory of multiple neutrinos, Schwinger terms, and the theory of the spin-3/2 field.
Biography:
Early life and career
Julian Seymour Schwinger was born in New York City, to Ashkenazi Jewish parents, Belle (née Rosenfeld) and Benjamin Schwinger, a garment manufacturer, who had emigrated from Poland to the United States. Both his father and his mother's parents were prosperous clothing manufacturers, although the family business declined after the Wall Street Crash of 1929. The family followed the Orthodox Jewish tradition. Julian's older brother Harold Schwinger was born in 1911, seven years before Julian who was born in 1918.
Schwinger was a precocious student. He attended the Townsend Harris High School from 1932 to 1934, a highly regarded high school for gifted students at the time. During high school, Julian had already started reading Physical Review papers by authors such as Paul Dirac in the library of the City College of New York, in whose campus Townsend Harris was then located.
In the fall of 1934, Schwinger entered the City College of New York as an undergraduate. CCNY automatically accepted all Townsend Harris graduates at the time, and both institutions offered free tuition. Due to his intense interest in physics and mathematics, Julian performed very well in those subjects despite often skipping classes and learning directly from books. On the other hand, his lack of interest for other topics such as English led to academic conflicts with teachers of those subjects.
After Julian had joined CCNY, his brother Harold, who had previously graduated from CCNY, asked his ex-classmate Lloyd Motz to "get to know [Julian]". Lloyd was a CCNY physics instructor and Ph.D. candidate at Columbia University at the time. Lloyd made the acquaintance, and soon recognized Julian's talent. Noticing Schwinger's academic problems, Lloyd decided to ask Isidor Isaac Rabi who he knew at Columbia for help. Rabi also immediately recognized Schwinger's capabilities on their first meeting, and then made arrangements to award Schwinger with a scholarship to study at Columbia. At first Julian's bad grades in some subjects at CCNY prevented the scholarship award. But Rabi persisted and showed an unpublished paper on quantum electrodynamics written by Schwinger to Hans Bethe, who happened to be passing by New York. Bethe's approval of the paper and his reputation in that domain were then enough to secure the scholarship for Julian, who then transferred to Columbia. His academic situation at Columbia was much better than at CCNY. He was accepted into the Phi Beta Kappa society and received his B.A. in 1936.
During Schwinger's graduate studies, Rabi felt that it would be good for Julian to visit other institutions around the country, and Julian was awarded a travelling fellowship for the year 37/38 which he spent at working with Gregory Breit and Eugene Wigner. During this time, Schwinger, who previously had already had the habit of working until late at night, went further and made the day/night switch more complete, working at night and sleeping during the day, a habit he would carry throughout his career. Schwinger later commented that this switch was in part a way to retain greater intellectual independence and avoid being "dominated" by Breit and Wigner by simply reducing the duration of contact with them by working different hours.
Schwinger obtained his PhD overseen by Rabi in 1939 at the age of 21.
During the fall of 1939 Schwinger started working at the University of California, Berkeley under J. Robert Oppenheimer, where he stayed for two years as an NRC fellow.
Career
After having worked with Oppenheimer, Schwinger's first regular academic appointment was at Purdue University in 1941. While on leave from Purdue, he worked at the MIT Radiation Laboratory instead of at the Los Alamos National Laboratory during World War II. He provided theoretical support for the development of radar. After the war, Schwinger left Purdue for Harvard University, where he taught from 1945 to 1974. In 1966 he became the Eugene Higgins professor of physics at Harvard.
Schwinger developed an affinity for Green's functions from his radar work, and he used these methods to formulate quantum field theory in terms of local Green's functions in a relativistically invariant way. This allowed him to calculate unambiguously the first corrections to the electron magnetic moment in quantum electrodynamics. Earlier non-covariant work had arrived at infinite answers, but the extra symmetry in his methods allowed Schwinger to isolate the correct finite corrections.
Schwinger developed renormalization, formulating quantum electrodynamics unambiguously to one-loop order.
In the same era, he introduced non-perturbative methods into quantum field theory, by calculating the rate at which electron–positron pairs are created by tunneling in an electric field, a process now known as the "Schwinger effect." This effect could not be seen in any finite order in perturbation theory.
Schwinger's foundational work on quantum field theory constructed the modern framework of field correlation functions and their equations of motion. His approach started with a quantum action and allowed bosons and fermions to be treated equally for the first time, using a differential form of Grassman integration. He gave elegant proofs for the spin-statistics theorem and the CPT theorem, and noted that the field algebra led to anomalous Schwinger terms in various classical identities, because of short distance singularities. These were foundational results in field theory, instrumental for the proper understanding of anomalies.
In other notable early work, Rarita and Schwinger formulated the abstract Pauli and Fierz theory of the spin-3/2 field in a concrete form, as a vector of Dirac spinors, Rarita–Schwinger equation. In order for the spin-3/2 field to interact consistently, some form of supersymmetry is required, and Schwinger later regretted that he had not followed up on this work far enough to discover supersymmetry.
Schwinger discovered that neutrinos come in multiple varieties, one for the electron and one for the muon. Nowadays there are known to be three light neutrinos; the third is the partner of the tau lepton.
In the 1960s, Schwinger formulated and analyzed what is now known as the Schwinger model, quantum electrodynamics in one space and one time dimension, the first example of a confining theory.
Having supervised 73 doctoral dissertations, Schwinger is known as one of the most prolific graduate advisors in physics. Four of his students won Nobel prizes: Roy Glauber, Benjamin Roy Mottelson, Sheldon Glashow and Walter Kohn (in chemistry).
Schwinger had a mixed relationship with his colleagues, because he always pursued independent research, different from mainstream fashion. In particular, Schwinger developed the source theory, a phenomenological theory for the physics of elementary particles, which is a predecessor of the modern effective field theory. It treats quantum fields as long-distance phenomena and uses auxiliary 'sources' that resemble currents in classical field theories. The source theory is a mathematically consistent field theory with clearly derived phenomenological results. The criticisms by his Harvard colleagues led Schwinger to leave the faculty in 1972 for UCLA. It is a story widely told that Steven Weinberg, who inherited Schwinger's paneled office in Lyman Laboratory, there found a pair of old shoes, with the implied message, "think you can fill these?". Based on Schwinger's source theory, Weinberg set the underpinnings of the effective field theory, that is more appreciated among physicists. In spite of the shoes incident, Weinberg gave the credit to Schwinger for the inspiration.
At UCLA, and for the rest of his career, Schwinger continued to develop the source theory and its various applications. After 1989 Schwinger took a keen interest in the non-mainstream research of cold fusion. He wrote eight theory papers about it. He resigned from the American Physical Society after their refusal to publish his papers. He felt that cold fusion research was being suppressed and academic freedom violated. He wrote, "The pressure for conformity is enormous. I have experienced it in editors' rejection of submitted papers, based on venomous criticism of anonymous referees. The replacement of impartial reviewing by censorship will be the death of science."
In his last publications, Schwinger proposed a theory of sonoluminescence as a long-distance quantum radiative phenomenon associated not with atoms, but with fast-moving surfaces in the collapsing bubble, where there are discontinuities in the dielectric constant. The mechanism of sonoluminescence now supported by experiments focuses on superheated gas inside the bubble as the source of the light.
Schwinger was jointly awarded the Nobel Prize in Physics in 1965 for his work on quantum electrodynamics (QED), along with Richard Feynman and Shin'ichirō Tomonaga. Schwinger's awards and honors were numerous even before his Nobel win. They include the first Albert Einstein Award (1951), the U.S. National Medal of Science (1964), honorary D.Sc. degrees from Purdue University (1961) and Harvard University (1962), and the Nature of Light Award of the U.S. National Academy of Sciences (1949). In 1987, Schwinger received the Golden Plate Award of the American Academy of Achievement.

#80 Re: This is Cool » Miscellany » 2025-11-13 17:07:14
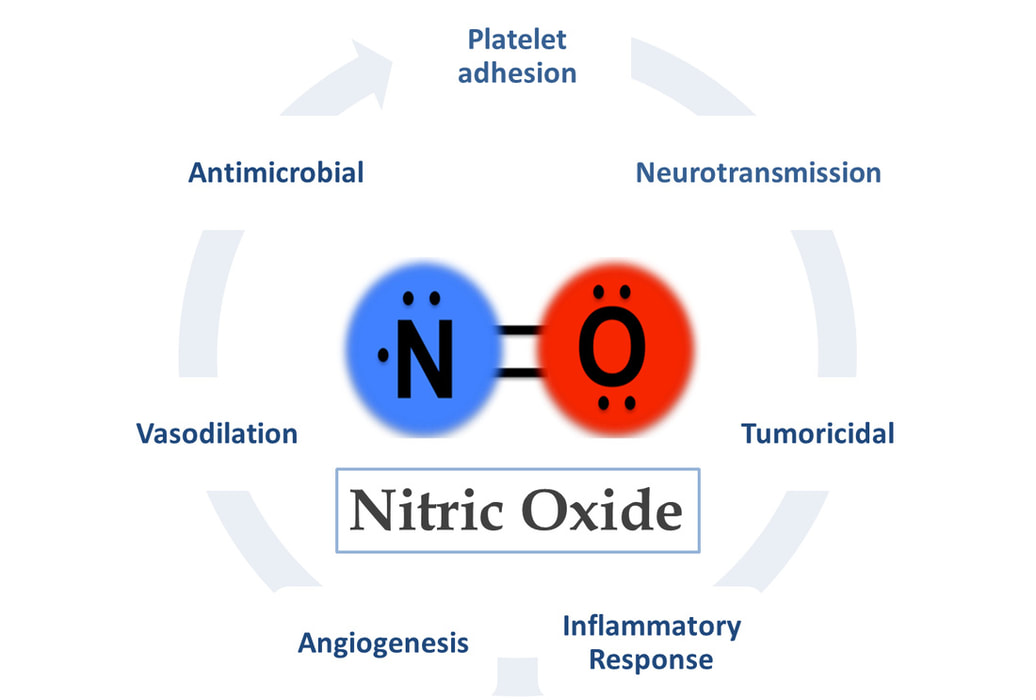
2445) Nitric Oxide
Gist
Nitric oxide (NO) is a colorless gas with the chemical formula NO, which acts as an important signaling molecule in the human body and is also an air pollutant. In the body, it relaxes blood vessels to improve blood flow, aids in neurotransmission, and plays a role in immune responses and other cellular processes. Industrially, it is a source of pollution and can be produced by high temperatures or chemical reactions.
Nitric oxide is used for medical purposes, such as treating respiratory failure in newborns and helping with erectile dysfunction, and is also used in industrial applications. In the body, it's crucial for vasodilation (widening blood vessels), which helps regulate blood flow, blood pressure, and other physiological processes.
Summary
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.
An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the "Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Its impact extends beyond biology, with applications in medicine, such as the development of sildenafil (Viagra), and in industry, including semiconductor manufacturing.
Nitric oxide should not be confused with nitrogen dioxide (NO2), a brown gas and major air pollutant, or with nitrous oxide (N2O), an anesthetic gas.
Details
Nitric oxide (NO) is a colorless, odorless gas molecule that plays a crucial role in various physiological processes within the human body. It was first discovered in the 18th century, but its significance in biological systems wasn’t fully understood until the late 20th century.
In the body, NO acts as a signaling molecule, participating in the regulation of numerous functions, including vasodilation (the widening of blood vessels), neurotransmission, immune response, and the regulation of inflammation. One of its most notable roles is in the regulation of blood pressure, where it helps to relax and widen blood vessels, thereby improving blood flow.
What is nitric oxide?
Nitric oxide (NO) is a molecule composed of one nitrogen atom and one oxygen atom. It is a colorless and odorless gas at room temperature. It is a crucial signaling molecule in the body involved in various physiological processes.
One of its primary roles is as a vasodilator, meaning it relaxes and widens blood vessels, leading to increased blood flow. This function is vital for regulating blood pressure and delivering oxygen and nutrients to tissues throughout the body.
Nitric oxide also acts as a neurotransmitter. Additionally, it plays a role in the immune system, helping to combat pathogens and regulate inflammation.
Synthesis
NO is synthesized in the body through a process involving the conversion of the amino acid arginine into nitric oxide and citrulline. This synthesis is catalyzed by a family of enzymes called nitric oxide synthases (NOS).
There are three isoforms of nitric oxide synthase:
* Endothelial NOS (eNOS): Found primarily in endothelial cells lining blood vessels, eNOS produces nitric oxide in response to various physiological stimuli, such as shear stress and certain hormones.
* Neuronal NOS (nNOS or NOS1): Present in neurons of the central and peripheral nervous systems, nNOS is involved in neurotransmission and neuromodulation, synthesizing nitric oxide in response to calcium influx during neuronal activation.
* Inducible NOS (iNOS or NOS2): Induced in response to inflammatory stimuli, such as cytokines and bacterial endotoxins, iNOS produces large amounts of NO for immune defense mechanisms and inflammation regulation.
The synthesis of nitric oxide by nitric oxide synthases involves several steps:
* L-arginine binding: The enzyme binds the substrate L-arginine, along with other cofactors such as tetrahydrobiopterin (BH4) and oxygen (O2).
* Conversion to L-citrulline and nitric oxide: Through a series of enzymatic reactions involving the cofactors and molecular oxygen, nitric oxide synthase catalyzes the conversion of L-arginine into nitric oxide and L-citrulline.
* Release of nitric oxide: Once synthesized, NO is released from the enzyme and diffuses freely across cell membranes to exert its physiological effects.
Functions
NO is a versatile molecule with numerous functions in the human body. Some of its key functions include:
* Vasodilation: NO acts as a potent vasodilator, meaning it relaxes and widens blood vessels. This helps to improve blood flow and regulate blood pressure.
* Neurotransmission: Serves as a signaling molecule in the nervous system, facilitating communication between nerve cells (neurons). It plays a role in neurotransmission, synaptic plasticity, and other neurological processes.
* Immune response: It is involved in the immune system’s defense against pathogens. It helps to regulate inflammation and can be produced by immune cells to kill invading bacteria, viruses, and parasites.
* Regulation of platelet function: Helps to regulate platelet aggregation, which is important for preventing excessive blood clotting and maintaining cardiovascular health.
* Smooth muscle relaxation: It relaxes smooth muscles found in various organs and tissues, including the gastrointestinal tract, airways, and urinary bladder. This relaxation helps to regulate processes such as digestion, breathing, and urination.
* Angiogenesis: It plays a role in angiogenesis, the formation of new blood vessels from existing ones. This process is important for tissue repair, wound healing, and the growth of tumors.
* Penile erection: It is a key mediator of penile erection. It stimulates the relaxation of smooth muscle cells in the erectile tissue of the male reproductive organ, leading to increased blood flow and the attainment of an erection.
* Regulation of mitochondrial function: It can modulate mitochondrial respiration and energy production within cells, influencing cellular metabolism and overall energy balance.
* Regulation of gene expression: It can also act as a signaling molecule within cells to regulate gene expression and various cellular processes, including cell proliferation, differentiation, and apoptosis (programmed cell death).
NO rich foods
There are several foods that are naturally rich in nitrates, which can be converted into NO in the body. These foods include:
* Leafy greens: Spinach, kale, arugula, and other leafy greens are high in nitrates.
* Celery: Celery is another vegetable that contains nitrates and can contribute to nitric oxide production.
* Garlic: Garlic contains compounds that can stimulate nitric oxide production and promote cardiovascular health.
* Citrus fruits: Oranges, lemons, and other citrus fruits are rich in vitamin C, which can help support nitric oxide production.
* Pomegranate: Pomegranate juice and seeds contain antioxidants and nitrates that may support NO levels and cardiovascular health.
* Watermelon: Watermelon contains an amino acid called citrulline, which can be converted into arginine, a precursor to nitric oxide.
* Nitrates in Vegetables: Nitrates are compounds found in certain vegetables that can be converted into NO in the body. Examples of nitrate-rich vegetables include spinach, arugula, kale, beetroot, and lettuce.
* Beets and Beetroot Juice: Beets and beetroot juice are well-known for their high nitrate content, which can be converted into nitric oxide.
* Dark Chocolate: Dark chocolate contains flavonoids, which have been shown to support nitric oxide production and cardiovascular health.
Interaction with other drugs
NO can interact with various drugs due to its role as a signaling molecule in many physiological processes. Some interactions include:
* Blood pressure medications: NO donors or drugs that increase NO levels, such as nitroglycerin or other nitrate medications, can enhance the effects of blood pressure-lowering medications like antihypertensives. This interaction can lead to excessive hypotension (low blood pressure).
* Erectile dysfunction drugs: Drugs used to treat erectile dysfunction, such as sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra), work by enhancing the effects of NO, leading to vasodilation and improved blood flow. Combining these drugs with other NO donors or medications that increase NO levels can potentiate their effects and may cause a dangerous drop in blood pressure.
* Anticoagulants and antiplatelet drugs: NO inhibits platelet aggregation and can increase bleeding risk. Combining NO donors or medications that increase NO levels with anticoagulants (e.g., warfarin, heparin) or antiplatelet drugs (e.g., aspirin, clopidogrel) may further enhance the risk of bleeding.
* Drugs affecting nitric oxide synthesis: Medications that affect the synthesis of NO, such as inhibitors of NO synthase (NOS), may interact with drugs that rely on NO signaling for their effects. These interactions can affect cardiovascular function, neurotransmission, and immune response.
* Alpha-blockers: Alpha-blockers, used to treat conditions like benign prostatic hyperplasia (BPH) and hypertension, can interact with NO donors or drugs that increase NO levels, leading to additive effects on blood pressure lowering.
* Drugs affecting cytochrome P450 enzymes: Some drugs can affect the activity of cytochrome P450 enzymes, which are involved in the metabolism of NO donors and other drugs. Interactions with these drugs can alter the metabolism and effectiveness of medications that affect NO levels.
Side effects
While NO is essential for various physiological functions in the body, including vasodilation and neurotransmission, excessive intake or production of nitric oxide can lead to potential complications. Here are some of the possible complications associated with taking excessive amounts of nitric oxide:
* Hypotension: Excessive nitric oxide can cause a significant drop in blood pressure (hypotension). This can lead to symptoms such as dizziness, lightheadedness, fainting, and in severe cases, shock.
* Headaches: Increased levels of nitric oxide can cause headaches, which may range from mild to severe and can be debilitating for some individuals.
* Increased bleeding risk: NO can inhibit platelet aggregation and contribute to increased bleeding risk. Excessive nitric oxide production may lead to prolonged bleeding and difficulty in clot formation.
* Worsening of respiratory conditions: In individuals with certain respiratory conditions such as asthma, excessive nitric oxide production can exacerbate symptoms by causing airway dilation and inflammation.
* Nitric oxide toxicity: In rare cases, excessive exposure to NO gas, particularly in industrial or occupational settings, can lead to toxicity, causing respiratory distress, lung damage, and even death.
* Formation of reactive nitrogen species: Excessive nitric oxide can react with other molecules in the body to form reactive nitrogen species, such as peroxynitrite, which can contribute to oxidative stress, tissue damage, and inflammation.
Additional Information
Nitric oxide (NO) is a colourless toxic gas that is formed by the oxidation of nitrogen. Nitric oxide performs important chemical signaling functions in humans and other animals and has various applications in medicine. It has few industrial applications. It is a serious air pollutant generated by automotive engines and thermal power plants.
Nitric oxide is formed from nitrogen and oxygen by the action of electric sparks or high temperatures or, more conveniently, by the action of dilute nitric acid upon copper or mercury. It was first prepared about 1620 by the Belgian scientist Jan Baptista van Helmont, and it was first studied in 1772 by the English chemist Joseph Priestley, who called it “nitrous air.”
Nitric oxide liquefies at −151.8 °C (−241.2 °F) and solidifies at −163.6 °C (−262.5 °F); both the liquid and the solid are blue in colour. The gas is almost insoluble in water, but it dissolves rapidly in a slightly alkaline solution of sodium sulfite, forming the compound sodium dinitrososulfite, Na2(NO)2SO3. It reacts rapidly with oxygen to form nitrogen dioxide, NO2. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). The molecule can gain or lose one electron to form the ions NO− or NO+.
In the chemical industry, nitric oxide is an intermediate compound formed during the oxidation of ammonia to nitric acid. An industrial procedure for the manufacture of hydroxylamine is based on the reaction of nitric oxide with hydrogen in the presence of a catalyst. The formation of nitric oxide from nitric acid and mercury is applied in a volumetric method of analysis for nitric acid or its salts.
Though it is a toxic gas at high concentrations, nitric oxide functions as an important signaling molecule in animals. It acts as a messenger molecule, transmitting signals to cells in the cardiovascular, nervous, and immune systems. The nitric oxide molecule’s possession of a free radical makes it much more reactive than other signaling molecules, and its small size enables it to diffuse through cell membranes and walls to perform a range of signaling functions in various bodily systems. The body synthesizes nitric oxide from the amino acid L-arginine by means of the enzyme nitric oxide synthase.
The main site of the molecule’s synthesis is the inner layer of blood vessels, the endothelium, though the molecule is also produced by other types of cells. From the endothelium, nitric oxide diffuses to underlying smooth muscle cells and causes them to relax. This relaxation causes the walls of blood vessels to dilate, or widen, which in turn increases blood flow through the vessels and decreases blood pressure. Nitric oxide’s role in dilating blood vessels makes it an important controller of blood pressure. Nitric oxide is also produced by neurons (nerve cells) and is used by the nervous system as a neurotransmitter to regulate functions ranging from digestion to blood flow to memory and vision. In the immune system, nitric oxide is produced by macrophages, which are a type of leukocyte (white blood cell) that engulfs bacteria and other foreign particles that have invaded the body. The nitric oxide released by macrophages kills bacteria, other parasites, and tumour cells by disrupting their metabolism.
Nitric oxide’s role in regulating blood flow and pressure is used by modern medicine in several ways. The drug nitroglycerin has been used since the late 19th century to relieve the condition known as angina pectoris, which is caused by an insufficient supply of blood to the heart muscle. Nitroglycerin was long known to achieve its therapeutic effect by dilating the coronary arteries (thereby increasing the flow of blood to the heart), but why it did so remained unknown until the late 1980s, when researchers realized that the drug serves to replenish the body’s supply of nitric oxide, more of which is then available to relax, and thereby widen, the coronary blood vessels.
Nitric oxide is an important component of the air pollution generated by automotive engines and thermal power-generating plants. When a mixture of air and hydrocarbon fuel is burned in an internal-combustion engine or a power plant, the ordinarily inert nitrogen in the air combines with oxygen at very high temperatures to form nitric oxide. The nitric oxide and hydrocarbon vapours emitted by automotive exhausts and power-plant smokestacks undergo complex photochemical reactions in the lower atmosphere to form various secondary pollutants called photochemical oxidants, which make up photochemical smog. Nitric oxide combines with water vapour in the atmosphere to form nitric acid, which is one of the components of acid rain. Heightened levels of atmospheric nitric oxide resulting from industrial activity were also one of the causes of gradual depletion of the ozone layer in the upper atmosphere. Sunlight causes nitric oxide to react chemically with ozone (O3), thereby converting the ozone to molecular oxygen (O2).
#81 Dark Discussions at Cafe Infinity » Coin Quotes - I » 2025-11-13 16:33:12
- Jai Ganesh
- Replies: 0
Coin Quotes - I
1. All the perplexities, confusion and distress in America arise, not from defects in their Constitution or Confederation, not from want of honor or virtue, so much as from the downright ignorance of the nature of coin, credit and circulation. - John Adams
2. Peace and justice are two sides of the same coin. - Dwight D. Eisenhower
3. Leadership is the other side of the coin of loneliness, and he who is a leader must always act alone. And acting alone, accept everything alone. - Ferdinand Marcos
4. Silver and gold are not the only coin; virtue too passes current all over the world. - Euripides
5. If you call yourself a leader, then you have to be decisive. If you're decisive, then you have the chance to be a leader. These are two sides to the same coin. - Narendra Modi
6. German and European unification are two sides of the same coin. - Helmut Kohl
7. When the machine had been fastened with a wire to the track, so that it could not start until released by the operator, and the motor had been run to make sure that it was in condition, we tossed a coin to decide who should have the first trial. Wilbur won. - Orville Wright
8. I said, yet again, for Germany, Europe is not only indispensable, it is part and parcel of our identity. We've always said German unity, European unity and integration, that's two parts of one and the same coin. But we want, obviously, to boost our competitiveness. - Angela Merkel.
#82 Jokes » Bread Jokes - VII » 2025-11-13 16:16:12
- Jai Ganesh
- Replies: 0
Q: What did the yeast confess to the bag of flour?
A: I loaf you dough much!
* * *
Q: Why did Mama Flour and Papa Yeast tell Baby Bread to get a job?
A: He was just loafing around!
* * *
Q: Why doesn't anyone want to work in a bakery?
A: It's a crumby place to work.
* * *
Q: What Kind of Biscuits Can Fly?
A: Plain Ones.
* * *
Q: When does sourdough bread rise?
A: When you yeast expect it.
* * *
#83 Science HQ » Isotope » 2025-11-13 16:02:01
- Jai Ganesh
- Replies: 0
Isotope
Gist
An isotope is a form of a chemical element with the same number of protons but a different number of neutrons than other atoms of the same element. This difference in neutron count gives isotopes the same atomic number but different mass numbers, meaning they have identical chemical properties but different physical properties. For example, carbon-12 and carbon-14 are both isotopes of carbon; they both have six protons, but carbon-12 has six neutrons, while carbon-14 has eight.
An isotope is a version of a chemical element with the same number of protons but a different number of neutrons. This difference in neutrons gives isotopes the same atomic number but different mass numbers, leading to nearly identical chemical properties but different physical properties. For example, carbon-12, carbon-13, and carbon-14 are all isotopes of carbon, each with six protons but a different number of neutrons.
Summary
Isotopes are distinct nuclear species (or nuclides) of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have virtually the same chemical properties, they have different atomic masses and physical properties.
The term isotope comes from the Greek roots isos ("equal") and topos ("place"), meaning "the same place": different isotopes of an element occupy the same place on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term.
The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.
For example, carbon-12, carbon-13, and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13, and 14, respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons so that the neutron numbers of these isotopes are 6, 7, and 8 respectively.
Details
An isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behaviour but with different atomic masses and physical properties. Every chemical element has one or more isotopes.
An atom is first identified and labeled according to the number of protons in its nucleus. This atomic number is ordinarily given the symbol Z. The great importance of the atomic number derives from the observation that all atoms with the same atomic number have nearly, if not precisely, identical chemical properties. A large collection of atoms with the same atomic number constitutes a sample of an element. A bar of pure uranium, for instance, would consist entirely of atoms with atomic number 92. The periodic table of the elements assigns one place to every atomic number, and each of these places is labeled with the common name of the element, as, for example, calcium, radon, or uranium.
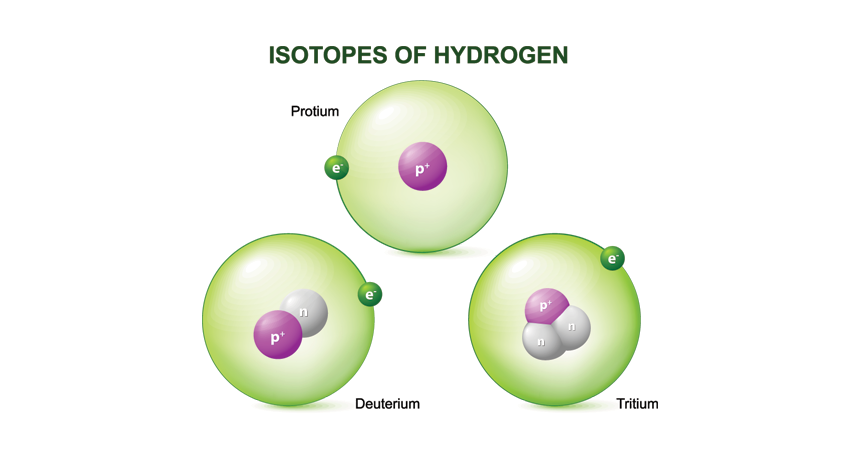
Not all the atoms of an element need have the same number of neutrons in their nuclei. In fact, it is precisely the variation in the number of neutrons in the nuclei of atoms that gives rise to isotopes. Hydrogen is a case in point. It has the atomic number 1. Three nuclei with one proton are known that contain 0, 1, and 2 neutrons, respectively. The three share the place in the periodic table assigned to atomic number 1 and hence are called isotopes (from the Greek isos, meaning “same,” and topos, signifying “place”) of hydrogen.
Many important properties of an isotope depend on its mass. The total number of neutrons and protons (symbol A), or mass number, of the nucleus gives approximately the mass measured on the so-called atomic-mass-unit (amu) scale. The numerical difference between the actual measured mass of an isotope and A is called either the mass excess or the mass defect.
The term nuclide is used to describe particular isotopes, notably in cases where the nuclear rather than the chemical properties of an atom are to be emphasized. The lexicon of isotopes includes three other frequently used terms: isotones for isotopes of different elements with the same number of neutrons, isobars for isotopes of different elements with the same mass number, and isomers for isotopes identical in all respects except for the total energy content of the nuclei.
The discovery of isotopes
Evidence for the existence of isotopes emerged from two independent lines of research, the first being the study of radioactivity. By 1910 it had become clear that certain processes associated with radioactivity, discovered some years before by French physicist Henri Becquerel, could transform one element into another. In particular, ores of the radioactive elements uranium and thorium had been found to contain small quantities of several radioactive substances never before observed. These substances were thought to be elements and accordingly received special names. Uranium ores, for example, yielded ionium, and thorium ores gave mesothorium. Painstaking work completed soon afterward revealed, however, that ionium, once mixed with ordinary thorium, could no longer be retrieved by chemical means alone. Similarly, mesothorium was shown to be chemically indistinguishable from radium. As chemists used the criterion of chemical indistinguishability as part of the definition of an element, they were forced to conclude that ionium and mesothorium were not new elements after all, but rather new forms of old ones. Generalizing from these and other data, English chemist Frederick Soddy in 1910 observed that “elements of different atomic weights [now called atomic masses] may possess identical (chemical) properties” and so belong in the same place in the periodic table. With considerable prescience, he extended the scope of his conclusion to include not only radioactive species but stable elements as well. A few years later, Soddy published a comparison of the atomic masses of the stable element lead as measured in ores rich in uranium and thorium, respectively. He expected a difference because uranium and thorium decay into different isotopes of lead. The lead from the uranium-rich ore had an average atomic mass of 206.08 compared to 207.69 for the lead from the thorium-rich ore, thus verifying Soddy’s conclusion.
The unambiguous confirmation of isotopes in stable elements not associated directly with either uranium or thorium followed a few years later with the development of the mass spectrograph (see mass spectrometry) by Francis William Aston. His work grew out of the study of positive rays (sometimes called canal rays), discovered in 1886 by Eugen Goldstein and soon thereafter recognized as beams of positive ions. As a student in the laboratory of J.J. Thomson, Aston had learned that the gaseous element neon produced two positive rays. The ions in the heavier ray had masses about two units, or 10 percent, greater than the ions in the lighter ray. To prove that the lighter neon had a mass very close to 20 and that the heavier ray was indeed neon and not a spurious signal of some kind, Aston had to construct an instrument that was considerably more precise than any other of the time. By 1919 he had done so and convincingly argued for the existence of neon-20 and neon-22. Information from his and other laboratories accumulated rapidly in the ensuing years, and by 1935 the principal isotopes and their relative proportions were known for all but a handful of elements.
Additional Information
A family of people often consists of related but not identical individuals. Elements have families as well, known as isotopes. Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons.
The number of protons in a nucleus determines the element’s atomic number on the Periodic Table. For example, carbon has six protons and is atomic number 6. Carbon occurs naturally in three isotopes: carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes.
The addition of even one neutron can dramatically change an isotope’s properties. Carbon-12 is stable, meaning it never undergoes radioactive decay. Carbon-14 is unstable and undergoes radioactive decay with a half-life of about 5,730 years (meaning that after 5,730 years half of the material will have decayed to the stable isotope nitrogen-14). This decay means the amount of carbon-14 in an object serves as a clock, showing the object’s age in a process called “carbon dating.”
Isotopes have unique properties, and these properties make them useful in diagnostics and treatment applications. They are important in nuclear medicine, oil and gas exploration, basic research, and national security.
#84 Re: Jai Ganesh's Puzzles » General Quiz » 2025-11-13 14:45:02
Hi,
#10665. What does the term in Geography City-state mean?
#10666. What does the term in Geography Cliff mean?
#85 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-11-13 14:33:03
Hi,
#5861. What does the noun blues mean?
#5862. What does the noun blueprint mean?
#86 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-11-13 14:17:48
Hi,
#2525. What does the medical term Azotemia mean?
#87 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-11-13 14:01:56
Hi,
#9804.
#88 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-11-13 13:47:15
Hi,
#6299.
#89 Re: Exercises » Compute the solution: » 2025-11-13 13:33:03
Hi,
2650.
#90 Re: Exercises » Compute the solution: » 2025-11-12 23:08:45
Hi,
Good!
2649.
#91 This is Cool » Ultraviolet » 2025-11-12 22:54:33
- Jai Ganesh
- Replies: 0
Ultraviolet
Gist
Ultraviolet (UV) is a type of electromagnetic radiation from the sun and artificial sources, with a shorter wavelength than visible light but longer than X-rays. It is invisible to the human eye and includes three main types: UVA, UVB, and UVC. While beneficial for vitamin D production, excessive UV exposure can cause skin damage, premature aging, and increase the risk of skin cancer.
Ultraviolet (UV) light has shorter wavelengths than visible light. Although UV waves are invisible to the human eye, some insects, such as bumblebees, can see them. This is similar to how a dog can hear the sound of a whistle just outside the hearing range of humans.
Summary
Ultraviolet radiation or UV is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights.
The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. These interactions can involve exciting orbital electrons to higher energy states in molecules potentially breaking chemical bonds. In contrast, the main effect of longer wavelength radiation is to excite vibrational or rotational states of these molecules, increasing their temperature. Short-wave ultraviolet light is ionizing radiation. Consequently, short-wave UV damages DNA and sterilizes surfaces with which it comes into contact.
For humans, suntan and sunburn are familiar effects of exposure of the skin to UV, along with an increased risk of skin cancer. The amount of UV radiation produced by the Sun means that the Earth would not be able to sustain life on dry land if most of that light were not filtered out by the atmosphere. More energetic, shorter-wavelength "extreme" UV below 121 nm ionizes air so strongly that it is absorbed before it reaches the ground. However, UV (specifically, UVB) is also responsible for the formation of vitamin D in most land vertebrates, including humans. The UV spectrum, thus, has effects both beneficial and detrimental to life.
The lower wavelength limit of the visible spectrum is conventionally taken as 400 nm. Although ultraviolet rays are not generally visible to humans, 400 nm is not a sharp cutoff, with shorter and shorter wavelengths becoming less and less visible in this range. Insects, birds, and some mammals can see near-UV (NUV), i.e., somewhat shorter wavelengths than what humans can see.
Details
Ultraviolet (UV) light has shorter wavelengths than visible light. Although UV waves are invisible to the human eye, some insects, such as bumblebees, can see them. This is similar to how a dog can hear the sound of a whistle just outside the hearing range of humans.
The Sun is a source of the full spectrum of ultraviolet radiation, which is commonly subdivided into UV-A, UV-B, and UV-C. These are the classifications most often used in Earth sciences. UV-C rays are the most harmful and are almost completely absorbed by our atmosphere. UV-B rays are the harmful rays that cause sunburn. Exposure to UV-B rays increases the risk of DNA and other cellular damage in living organisms. Fortunately, about 95 percent UV-B rays are absorbed by ozone in the Earth's atmosphere.
Scientists studying astronomical objects commonly refer to different subdivisions of ultraviolet radiation: near ultraviolet (NUV), middle ultraviolet (MUV), far ultraviolet (FUV), and extreme ultraviolet (EUV). NASA's SDO spacecraft captured the image below in multiple wavelengths of extreme ultraviolet (EUV) radiation. The false-color composite reveals different gas temperatures. Reds are relatively cool (about 60,000 Celsius) while blues and greens are hotter (greater than one million Celsius).
In 1801, Johann Ritter conducted an experiment to investigate the existence of energy beyond the violet end of the visible spectrum. Knowing that photographic paper would turn black more rapidly in blue light than in red light, he exposed the paper to light beyond violet. Sure enough, the paper turned black, proving the existence of ultraviolet light.
Since the Earth's atmosphere absorbs much of the high-energy ultraviolet radiation, scientists use data from satellites positioned above the atmosphere, in orbit around the Earth, to sense UV radiation coming from our Sun and other astronomical objects. Scientists can study the formation of stars in ultraviolet since young stars shine most of their light at these wavelengths. This image from NASA's Galaxy Evolution Explorer (GALEX) spacecraft reveals new young stars in the spiral arms of galaxy M81.
Chemical processes in the upper atmosphere can affect the amount of atmospheric ozone that shields life at the surface from most of the Sun's harmful UV radiation. Each year, a "hole" of thinning atmospheric ozone expands over Antarctica, sometimes extending over populated areas of South America and exposing them to increased levels of harmful UV rays. The Dutch Ozone Monitoring Instrument (OMI) onboard NASA's Aura satellite measures amounts of trace gases important to ozone chemistry and air quality. The image above shows the amount of atmospheric ozone in Dobson Units—the common unit for measuring ozone concentration. These data enable scientists to estimate the amount of UV radiation reaching the Earth's surface and forecast high-UV-index days for public health awareness.
Aurorae are caused by high-energy waves that travel along a planet's magnetic poles, where they excite atmospheric gases and cause them to glow. Photons in this high-energy radiation bump into atoms of gases in the atmosphere causing electrons in the atoms to excite, or move to the atom's upper shells. When the electrons move back down to a lower shell, the energy is released as light, and the atom returns to a relaxed state. The color of this light can reveal what type of atom was excited. Green light indicates oxygen at lower altitudes. Red light can be from oxygen molecules at a higher altitude or from nitrogen. On Earth, aurorae around the north pole are called the Northern Lights.
The Hubble Space Telescope captured this image of Jupiter's aurora in ultraviolet wrapping around Jupiter's north pole like a lasso.
Additional Information
Ultraviolet (UV) radiation covers the wavelength range of 100–400 nm, which is a higher frequency and lower wavelength than visible light. UV radiation comes naturally from the sun, but it can also be created by artificial sources used in industry, commerce and recreation.
The UV region covers the wavelength range 100-400 nm and is divided into three bands:
* UVA (315-400 nm)
* UVB (280-315 nm)
*UVC (100-280 nm).
As sunlight passes through the atmosphere, all UVC and approximately 90% of UVB radiation is absorbed by ozone, water vapour, oxygen and carbon dioxide. UVA radiation is less affected by the atmosphere. Therefore, the UV radiation reaching the Earth’s surface is largely composed of UVA with a small UVB component.
The amount of UV radiation from the sun that hits the Earth’s surface depends on several factors, including the sun’s height in the sky, latitude, cloud cover, altitude, the thickness of the ozone layer and ground reflection. Reductions in the ozone layer due to human-created pollution increase the amount of UVA and UVB that reaches the surface. This can impact human health, animals, marine organisms and plant life. In humans, increased UV exposure can cause skin cancers, cataracts and immune system damage.
Ultraviolet radiation is that portion of the electromagnetic spectrum extending from the violet, or short-wavelength, end of the visible light range to the X-ray region. Ultraviolet (UV) radiation is undetectable by the human eye, although, when it falls on certain materials, it may cause them to fluoresce—i.e., emit electromagnetic radiation of lower energy, such as visible light. Many insects, however, are able to see ultraviolet radiation.
Ultraviolet radiation lies between wavelengths of about 400 nanometres (1 nanometre [nm] is {10}{-9} metre) on the visible-light side and about 10 nm on the X-ray side, though some authorities extend the short-wavelength limit to 4 nm. In physics, ultraviolet radiation is traditionally divided into four regions: near (400–300 nm), middle (300–200 nm), far (200–100 nm), and extreme (below 100 nm). Based on the interaction of wavelengths of ultraviolet radiation with biological materials, three divisions have been designated: UVA (400–315 nm), also called black light; UVB (315–280 nm), responsible for the radiation’s best-known effects on organisms; and UVC (280–100 nm), which does not reach Earth’s surface.
Ultraviolet radiation is produced by high-temperature surfaces, such as the Sun, in a continuous spectrum and by atomic excitation in a gaseous discharge tube as a discrete spectrum of wavelengths. Most of the ultraviolet radiation in sunlight is absorbed by oxygen in Earth’s atmosphere, which forms the ozone layer of the lower stratosphere. Of the ultraviolet that does reach Earth’s surface, almost 99 percent is UVA radiation.
When the ozone layer becomes thin, however, more UVB radiation reaches Earth’s surface and may have hazardous effects on organisms. For example, studies have shown that UVB radiation penetrates the ocean’s surface and may be lethal to marine plankton to a depth of 30 metres (about 100 feet) in clear water. In addition, marine scientists have suggested that a rise in UVB levels in the Southern Ocean between 1970 and 2003 was strongly linked to a simultaneous decline in fish, krill, and other marine life.
Unlike X-rays, ultraviolet radiation has a low power of penetration; hence, its direct effects on the human body are limited to the surface skin. The direct effects include reddening of the skin (sunburn), pigmentation development (suntan), aging, and carcinogenic changes. Ultraviolet sunburns can be mild, causing only redness and tenderness, or they can be so severe as to produce blisters, swelling, seepage of fluid, and sloughing of the outer skin. The blood capillaries (minute vessels) in the skin dilate with aggregations of red and white blood cells to produce the red coloration. Tanning is a natural body defense relying on melanin to help protect the skin from further injury. Melanin is a chemical pigment in the skin that absorbs ultraviolet radiation and limits its penetration into tissues. A suntan occurs when melanin pigments in cells in the deeper tissue portion of the skin are activated by ultraviolet radiation, and the cells migrate to the surface of the skin. When these cells die, the pigmentation disappears. Persons of light complexion have less melanin pigment and so experience the harmful effects of ultraviolet radiation to a greater degree. The application of sunscreen to the skin can help to block absorption of ultraviolet radiation in such persons.
Constant exposure to the Sun’s ultraviolet radiation induces most of the skin changes commonly associated with aging, such as wrinkling, thickening, and changes in pigmentation. There is also a much higher frequency of skin cancer, particularly in persons with fair skin. The three basic skin cancers, basal- and squamous-cell carcinoma and melanoma, have been linked to long-term exposure to ultraviolet radiation and probably result from changes generated in the DNA of skin cells by ultraviolet rays.
Ultraviolet radiation also has positive effects on the human body, however. It stimulates the production of vitamin D in the skin and can be used as a therapeutic agent for such diseases as psoriasis. Because of its bactericidal capabilities at wavelengths of 260–280 nm, ultraviolet radiation is useful as both a research tool and a sterilizing technique. Fluorescent lamps exploit the ability of ultraviolet radiation to interact with materials known as phosphors that emit visible light; compared with incandescent lamps, fluorescent lamps are a more energy-efficient form of artificial lighting.

#92 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-11-12 17:50:02
2392) Shin'ichirō Tomonaga
Gist:
Work
Following the establishment of the theory of relativity and quantum mechanics, an initial relativistic theory was formulated for the interaction between charged particles and electromagnetic fields. The theory had to be reformulated, however, partly due to the observation of the Lamb shift in 1947, in which the supposed single energy level within a hydrogen atom was instead proven to be two similar levels. Sin-Itiro Tomonga solved this problem in 1948 through a “renormalization” and thereby contributed to a new quantum electrodynamics.
Summary
Tomonaga Shin’ichirō (born March 31, 1906, Kyōto, Japan—died July 8, 1979, Tokyo) was a Japanese physicist, joint winner, with Richard P. Feynman and Julian S. Schwinger of the United States, of the Nobel Prize for Physics in 1965 for developing basic principles of quantum electrodynamics.
Tomonaga became professor of physics at Bunrika University (later Tokyo University of Education) in 1941, the year he began his investigations of the problems of quantum electrodynamics. World War II isolated him from Western scientists, but in 1943 he completed and published his research. Tomonaga’s theoretical work made quantum electrodynamics (the theory of the interactions of charged subatomic particles with the electromagnetic field) consistent with the theory of special relativity. It was only after the war, in 1947, that his work came to the attention of the West, at about the same time that Feynman and Schwinger published the results of their research. It was found that all three had achieved essentially the same result from different approaches and had resolved the inconsistencies of the old theory without making any drastic changes.
Tomonaga was president of the Tokyo University of Education from 1956 to 1962, and the following year he was named chairman of the Japan Science Council. Throughout his life Tomonaga actively campaigned against the spread of nuclear weapons and urged that resources be spent on the peaceful use of nuclear energy. Most notable of his works available in English translation are Quantum Mechanics (1962) and his Nobel lecture Development of Quantum Electrodynamics: Personal Recollections (1966).
Details
Shinichiro Tomonaga (Tomonaga Shin'ichirō; March 31, 1906 – July 8, 1979), usually cited as Sin-Itiro Tomonaga in English, was a Japanese physicist, influential in the development of quantum electrodynamics, work for which he was jointly awarded the Nobel Prize in Physics in 1965 along with Richard Feynman and Julian Schwinger.
Biography
Tomonaga was born in Tokyo in 1906. He was the second child and eldest son of a Japanese philosopher, Tomonaga Sanjūrō. He entered the Kyoto Imperial University in 1926. Hideki Yukawa, also a Nobel laureate, was one of his classmates during undergraduate school. During graduate school at the same university, he worked as an assistant in the university for three years. In 1931, after graduate school, he joined Nishina's group in RIKEN. In 1937, while working at Leipzig University (Leipzig), he collaborated with the research group of Werner Heisenberg. Two years later, he returned to Japan due to the outbreak of the Second World War, but finished his doctoral degree (Dissertation PhD from University of Tokyo) on the study of nuclear materials with his thesis on work he had done while in Leipzig.
In Japan, he was appointed to a professorship in the Tokyo University of Education (a forerunner of Tsukuba University). During the war he studied the magnetron, meson theory, and his super-many-time theory. In 1948, he and his students re-examined a 1939 paper by Sidney Dancoff that attempted, but failed, to show that the infinite quantities that arise in quantum electrodynamics (QED) can be canceled with each other. Tomonaga applied his super-many-time theory and a relativistic method based on the non-relativistic method of Wolfgang Pauli and Fierz to greatly speed up and clarify the calculations. Then he and his students found that Dancoff had overlooked one term in the perturbation series. With this term, the theory gave finite results; thus Tomonaga discovered the renormalization method independently of Julian Schwinger and calculated physical quantities such as the Lamb shift at the same time.
In 1949, he was invited by Robert Oppenheimer to work at the Institute for Advanced Study in Princeton. He studied a many-body problem on the collective oscillations of a quantum-mechanical system. In the following year, he returned to Japan and proposed the Tomonaga–Luttinger liquid. In 1955, he took the leadership in establishing the Institute for Nuclear Study, University of Tokyo. In 1965, he was awarded the Nobel Prize in Physics, with Julian Schwinger and Richard P. Feynman, for the study of QED, specifically for the discovery of the renormalization method. He died of throat cancer in Tokyo in 1979.
Tomonaga was married in 1940 to Ryōko Sekiguchi. They had two sons and one daughter. He was awarded the Order of Culture in 1952, and the Grand Cordon of the Order of the Rising Sun in 1976.
In recognition of three Nobel laureates' contributions, the bronze statues of Shin'ichirō Tomonaga, Leo Esaki, and Makoto Kobayashi was set up in the Central Park of Azuma 2 in Tsukuba City in 2015.

#93 Re: This is Cool » Miscellany » 2025-11-12 17:13:49
2444) Glycerol
Gist
Glycerol is a colorless, odorless, viscous liquid that is sweet-tasting and can be used in a wide range of applications, including cosmetics, food, and pharmaceuticals, due to its moisturizing and solvent properties. It is also a component of explosives like nitroglycerin, and in the body, it is a part of fats known as glycerides. In excess, it can cause side effects like diarrhea, bloating, and nausea.
While glycerol is considered safe for most people, excessive intake can cause side effects such as headaches, dizziness, bloating, nausea, and diarrhoea. In some cases, high or fast consumption may lead to glycerol intoxication, characterised by symptoms such as hypoglycaemia and loss of consciousness.
Summary
Glycerol is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.
Modern use of the word glycerine (alternatively spelled glycerin) refers to commercial preparations of less than 100% purity, typically 95% glycerol.
Structure
Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a sn- prefix before the stem name of the molecule.
Details
Glycerol is a clear, colourless, viscous, sweet-tasting liquid belonging to the alcohol family of organic compounds; molecular formula HOCH2CHOHCH2OH. Until 1948 all glycerol was obtained as a by-product in making soaps from animal and vegetable fats and oils, but industrial syntheses based on propylene or sugar has accounted for an increasingly large percentage of production since that time. The term glycerin (or glycerine), introduced in 1811 by French chemist Michel-Eugène Chevreul, is ordinarily applied to commercial materials containing more than 95 percent glycerol. Though Chevreul gave glycerin its name, the substance was first isolated in 1783 by German Swedish chemist Carl Wilhelm Scheele, who described it as the “sweet principle of fat.”
Glycerol has numerous uses. It is a basic ingredient in the gums and resins used to make many modern protective coatings such as automotive enamels and exterior house paints. Glycerin reacted with nitric and sulfuric acid forms the explosive nitroglycerin (or nitroglycerine).
Glycerol is also a component of mono- and diglyceride emulsifiers, which are used as softening agents in baked goods, plasticizers in shortening, and stabilizers in ice cream. Its varied uses in the pharmaceutical and toilet goods fields include skin lotions, mouthwashes, cough medicines, drug solvents, serums, vaccines, and suppositories. Another significant use is as a protective medium for freezing red blood cells, sperm cells, eye corneas, and other living tissues. At one time, its largest single use was as automotive antifreeze; methanol and ethylene glycol have replaced it for this purpose.
Fats and oils are valued chiefly as sources of the carboxylic acids that are present, combined in the form of esters with glycerol. When the acids are set free from these compounds, glycerol remains as a solution in water and is purified by coagulating and settling extraneous matter, evaporating the water, and distilling.
Additional Information
Glycerol is the simplest alkane triol. It was historically called glycerine (or glycerin), but that name is misleading because the -ine suffix denotes an amine, not an alcohol.
Pioneering Swedish chemist Carl Wilhelm Scheele reported the discovery of glycerol in a 1783 article titled “Findings concerning a particular sweet substance in expressed oils and fatty substances”. In doing so, he established that glycerol is sweet-tasting and that it is the alcohol portion of natural mono-, di-, and triglyceride esters.
Glycerol/glycerine/glycerin appears in some 330,000 references listed in Chemical Abstracts Service’s SciFindern. The earliest citations are from 1878, in an article titled “A new test for glycerin” and in patents titled “Manufacture of pigments” and “Frees lime saccharate from salts and coloring matters”.
Glycerol can be synthesized from propylene through intermediates such as epichlorohydrin, acrolein, or propylene oxide. But for economic reasons, almost all commercial glycerol comes from hydrolyzing glycerides in natural fats, especially since the advent of using fatty acids to make biodiesel fuels. Some now-inexpensive glycerol is used as a raw material for producing epichlorohydrin and acrolein.
Glycerol has myriad uses in foods, pharmaceuticals, personal-care products, antifreezes, inks, lubricants, industrial lubricants, and many more. It is a key starting material for preparing nitroglycerol. As long ago as 1945, a book by Georgia Leffingwell and Milton A. Lesser titled Glycerin, Its Industrial and Commercial Applications listed 1583 specific uses of the compound.
Finally, glycerol is the base ingredient in commonly used face paints, as described in the facepaint.com blog. It is the medium in which the paint pigments are dispersed. If you paint your face for Halloween, Mardi Gras, or your favorite team’s game, give a shout-out for glycerol.

#94 Dark Discussions at Cafe Infinity » Coffee Quotes - III » 2025-11-12 16:37:56
- Jai Ganesh
- Replies: 0
Coffee Quotes - III
1. I start every morning at 7 or 7:30 in the same place - my little office where it's dark and cozy - with a cup of the same really strong black coffee. It's my little cocoon. There's no phone or fax or Internet. And no music. - John Grisham
2. I did work at Christie's for a couple of weeks, getting ready for 'The Devil Wears Prada,' getting people coffee and doing whatever they needed around the office. It was amazing. I got to see some wonderful art, and everybody was really nice. It was great. - Anne Hathaway
3. When I'm waiting to bat I try to watch the game and make sure I know what is going on so I am ready when I get out there but I spend a lot of time hydrating. It is very important for your performance and concentration. If it is cold I might have a coffee but mainly I am trying to stay really hydrated. - Joe Root
4. My whole approach to wardrobe is, throw it in a suitcase and make sure they don't press it, for Pete's sake, so I can try to display some rumpled charm. Actually, I'm just a pig. I've got coffee stains on my pants. I think they're coffee stains, anyway. - Mel Gibson.
#95 Re: Jai Ganesh's Puzzles » General Quiz » 2025-11-12 15:54:30
Hi,
#10663. What does the term in Geography City mean?
#10664. What does the term in Geography City centre mean?
#96 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-11-12 15:39:51
Hi,
#5859. What does the noun modicum mean?
#5860. What does the adjective modish mean?
#97 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-11-12 15:16:02
Hi,
#2524. What does the medical term Diabetes medication mean?
#98 Jokes » Bread Jokes - V » 2025-11-12 14:40:45
- Jai Ganesh
- Replies: 0
Q: What did the yeast say to the bag of flour?
A: Come on we Knead to be serious!
* * *
Q: Can you make a sandwich with corned meat, sauerkraut, and Swiss cheese?
A: Rye not?
* * *
Q: What is a bakers favorite Beatles song?
A: "Loaf is all you knead."
* * *
Q: What happens when you burn bread?
A: You loaf it to death.
* * *
Q: How does a loaf of bread validate it's anger against grapes?
A: Raisining!
* * *
#99 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-11-12 14:27:37
Hi,
#9803.
#100 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-11-12 13:58:00
Hi,
#6298.