Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#26 Science HQ » Platinum » 2025-09-10 18:44:16
- Jai Ganesh
- Replies: 0
Platinum
Gist
Platinum is known for its superior durability, resisting scratches and tarnish better than gold. It has a higher density and doesn't wear down as quickly as gold, making it ideal for rings and bracelets. Gold, particularly in higher purity forms like 24-carat, is softer and more prone to bending or scratching.
The auto industry uses platinum for catalytic converters, which can help reduce the toxicity of gases and pollutants in the exhaust that an internal combustion engine creates. Platinum and other platinum-grade metals in catalytic converters have led to a secondary market for scrap converters, which scrap businesses will buy in order to extract the metal for resale. The metal is also used in thermometers, laboratory equipment, electrodes, and dentistry equipment.
Summary
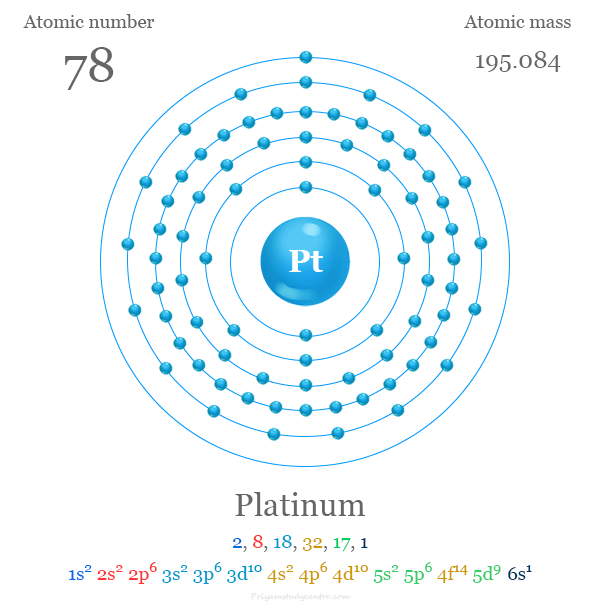
Platinum is a chemical element; it has symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish platina, a diminutive of plata "silver".
Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg. It occurs in some nickel and copper ores along with some native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable as well as a major precious metal commodity.
Platinum has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. Consequently, platinum is often found chemically uncombined as native platinum. Because it occurs naturally in the alluvial sands of various rivers, it was first used by pre-Columbian South American natives to produce artifacts. It was referenced in European writings as early as the 16th century, but it was not until Antonio de Ulloa published a report on a new metal of Colombian origin in 1748 that it began to be investigated by scientists.
Platinum is used in catalytic converters, laboratory equipment, electrical contacts and electrodes, platinum resistance thermometers, dentistry equipment, and jewelry. Platinum is used in the glass industry to manipulate molten glass, which does not "wet" platinum. Elemental platinum has not been linked to adverse health effects. Compounds containing platinum, such as cisplatin, oxaliplatin and carboplatin, are applied in chemotherapy against certain types of cancer.
Details
Platinum (Pt) is a chemical element, the best known and most widely used of the six platinum metals of Groups 8–10, Periods 5 and 6, of the periodic table. A very heavy, precious, silver-white metal, platinum is soft and ductile and has a high melting point and good resistance to corrosion and chemical attack. For example, its surface remains bright after being brought to white heat in air, and, though it readily dissolves in aqua regia, it is scarcely attacked by simple acids. (It does dissolve slowly in hydrochloric acid in the presence of air.) Small amounts of iridium are commonly added to give a harder, stronger alloy that retains the advantages of pure platinum.
Platinum, one of the most abundant platinum metals, and its alloys are indispensable in the chemical laboratory for electrodes and for crucibles and dishes in which materials can be heated to high temperatures. Platinum is used for electrical contacts and sparking points because it resists both the high temperatures and chemical attack of electric arcs. Jewelry and dental alloys account for much of its use; platinum-iridium is used for surgical pins. The prototype international standard kilogram of mass was made from an alloy of 90 percent platinum and 10 percent iridium. The electrical resistivity of platinum is relatively high and depends markedly upon the temperature; the International Temperature Scale from −259.35 to 961.78 °C (−434.83 to 1,763.2 °F)is defined in terms of a resistance thermometer made with platinum wire. As a catalyst, platinum has many applications, notably in automotive catalytic converters and in petroleum refining.
The Italian-French physician Julius Caesar Scaliger alluded (1557) to a refractory metal, probably platinum, found between Darién and Mexico. The first certain discovery was in the alluvial deposits of the Río Pinto, Colombia. The Spaniards called the new metal platina del Pinto for its resemblance to silver. The world’s most important deposits occur in the Transvaal of South Africa. Other deposits are found in Russia, Finland, Ireland, Borneo, New South Wales, New Zealand, Brazil, Peru, and Madagascar. In North America native platinum is found in Alaska, California, and Oregon, in British Columbia, and in Alberta. Placer deposits are the most productive sources of the native element. The ordinary variety of native platinum is called polyxene; it is 80 percent to 90 percent platinum, with 3 percent to 11 percent iron, plus the other platinum metals, and gold, copper, and nickel. For mineralogical properties, see native element (table). Platinum is also found in the very rare native alloy platiniridium. Platinum occurs combined with math as sperrylite (PtAs2) in the copper–nickel mining district near Sudbury, Ontario, and with sulfur as cooperite (PtS) in the Transvaal. (For information about the mining, recovery, and production of platinum, see platinum processing.)
Platinum is rapidly attacked by fused alkali oxides and peroxides and also by fluorine and chlorine at about 500 °C. It is capable of absorbing large volumes of hydrogen, and, with palladium, it is one of the most reactive platinum metals.
Platinum forms an important series of compounds with the oxidation states of +2 and +4. Many of these compounds contain coordination complexes in which chloride ion (Cl−), ammonia (NH3), or other groups are bonded to a central platinum atom. Among the transition metals, platinum has one of the greatest tendencies to form bonds directly with carbon. Platinum also combines with a number of nonmetallic elements on heating, such as phosphorus, math, antimony, silicon, sulfur, and selenium.
Natural platinum is a mixture of six isotopes: platinum-190 (0.012 percent), platinum-192 (0.782 percent), platinum-194 (32.86 percent), platinum-195 (33.78 percent), platinum-196 (25.21 percent), and platinum-198 (7.36 percent). All are stable except platinum-190, which has been reported as a long-lived alpha emitter.
Element Properties
atomic number : 78
atomic weight : 195.09
melting point : 1,769 °C (3,216 °F)
boiling point : 3,827 °C (6,920 °F)
specific gravity : 21.45 (20 °C)
oxidation states : +2, +4.
Additional Information
Appearance:
A shiny, silvery-white metal as resistant to corrosion as gold.
Uses
Platinum is used extensively for jewellery. Its main use, however, is in catalytic converters for cars, trucks and buses. This accounts for about 50% of demand each year. Platinum is very effective at converting emissions from the vehicle’s engine into less harmful waste products.
Platinum is used in the chemicals industry as a catalyst for the production of nitric acid, silicone and benzene. It is also used as a catalyst to improve the efficiency of fuel cells.
The electronics industry uses platinum for computer hard disks and thermocouples.
Platinum is also used to make optical fibres and LCDs, turbine blades, spark plugs, pacemakers and dental fillings.
Platinum compounds are important chemotherapy drugs used to treat cancers.
Biological role
Platinum has no known biological role. It is non-toxic.
Natural abundance
Platinum is found uncombined in alluvial deposits. Most commercially produced platinum comes from South Africa, from the mineral cooperite (platinum sulfide). Some platinum is prepared as a by-product of copper and nickel refining.
#27 Jokes » Librarian Jokes - IV » 2025-09-10 14:28:30
- Jai Ganesh
- Replies: 0
Q: Why is a math book always unhappy?
A: Because it always has lots of problems.
* * *
Q: What do dogs and story tellers have in common?
A: They both have tails!
* * *
Q: What is it called when someone gets suffocated by a book?
A: Literally murder!
* * *
Q: Have you seen the Bruce Willis movie where an entire library gets destroyed?
It's called "Die Hardcover".
* * *
Q: Why can't lawyers trick a librarian?
A: Because librarians can read the fine print.
* * *
Q: What did the surfer say to the librarian?
A: Is my book over dude?
* * *
#28 Re: Exercises » Compute the solution: » 2025-09-10 14:09:20
Hi,
You are correct!
2579.
#29 Jokes » Librarian Jokes - III » 2025-09-09 15:42:18
- Jai Ganesh
- Replies: 0
Q: Why should you be careful about what you say around a librarian?
A: Because they can read lips.
* * *
Q: What has a spine but, no bones?
A: A book.
* * *
Q: Who's the biggest liar in school?
A: The Lie-brarian.
* * *
Q: What did the librarian say to John Cusack?
A: Shhhhh! Don't Say Anything.
* * *
Q: What did the librarian say to the astronaut?
A: Find space for a book.
* * *
#30 Re: Introductions » Hello Math Is Fun! » 2025-09-09 14:45:03
Hi GretaJKelly,
Welcome to the forum!
See the links:
and
Systems of Linear and Quadratic Equations.
Links In the MathsIsFun website!
#31 Re: This is Cool » Miscellany » 2025-09-08 20:23:09
2384) Seahorse
Gist
The seahorse is a marine fish of the genus Hippocampus and family Syngnathidae, named for its horse-like head and upright posture, resembling a miniature horse in the sea. These bony fish have distinctive features such as a prehensile tail, independently moving eyes, and segmented bony armour, and they are found in shallow coastal waters worldwide.
Summary
A seahorse, (genus Hippocampus), any of about 50 species of marine fishes allied to pipefishes in the family Syngnathidae (order Gasterosteiformes). Seahorses are found in shallow coastal waters in latitudes from about 52° N to 45° S. Their habitats include coral reefs, mangroves, sea grass beds, and estuaries. They are unique in appearance, with their horselike head, prehensile tail, independently moving eyes, and brood pouch. They have long, tubular snouts and small, toothless mouths. Their bodies are covered with consecutive rings of bony plates. The name of the genus that contains seahorses is taken from the Greek words hippos (meaning “horse”) and kampos (meaning “sea monster”).
Seahorses vary in size, ranging in length from about 2 to 35 cm (about 0.8 to 14 inches). Adults of some of the smallest species—such as Denise’s pygmy seahorse (Hippocampus denise), found in the tropical western Pacific from Indonesia to Vanuatu, and Satomi’s pygmy seahorse (H. satomiae), found in the tropical Indian and Pacific Oceans from the Bay of Bengal to the Coral Sea—are less than 2 cm long. The largest species, the big-bellied seahorse (H. abdominalis), which inhabits the waters off South Australia and New Zealand, can grow up to 35 cm (13.8 inches) in length.
Seahorses are rather immobile, swimming more slowly than other fishes. When swimming they maintain a vertical position and propel themselves forward using a soft-rayed dorsal fin. They use pectoral fins located on the side of the head to maneuver. Some scientists contend that this upright swimming posture evolved shortly after the expansion of sea grasses in the western Pacific roughly 25 million years ago. These plants provided seahorses with useful hiding places to avoid enemies and to capture unsuspecting prey, and ancestors of the seahorse evolved to maximize the opportunities offered by this new habitat.
Seahorses are usually found clinging to plants or corals with their tails. Their sedentary habits coupled with excellent camouflage abilities render them successful ambush predators. When small organisms swim nearby, a seahorse may capture them by rapidly sucking them into the mouth. Seahorses also rely upon camouflage to avoid predators such as crabs and other fishes.
The reproductive behaviour of seahorses is notable in that the male carries the fertilized eggs. After an elaborate courtship, the female uses an ovipositor (egg duct) to place her eggs into a brood pouch located at the base of the male’s tail where the eggs are later fertilized. Depending on the species, the eggs remain in the pouch between 10 days and six weeks. During this time the male nurtures the developing young by regulating the chemistry of the fluid inside the pouch, slowly transforming it from that of his internal body fluids to that of salt water as pregnancy progresses. To nourish the growing young, the male also produces inorganic compounds and releases the hormone prolactin, which helps break down the proteins contributed by the female. Once the eggs hatch, the male convulses his body and expels the young through a single opening in the pouch. The young are miniature versions of their parents that receive no further care. The male can receive another brood of eggs almost immediately after giving birth. In some species a male and female will maintain a monogamous pair bond throughout the breeding season and produce many broods.
Commercially, seahorses are traded live as aquarium animals and dead for use in traditional medicines and as curios. Threatened by direct overfishing, accidental capture (bycatch) in other fisheries, and the destruction of their coastal habitats, some species—such as the Pacific seahorse (H. ingens)—face extinction.
Details:
Scientific classification
Kingdom: Animalia
Phylum: Chordata
Class: Actinopterygii
Order: Syngnathiformes
Family: Syngnathidae
Subfamily: Hippocampinae
Genus: Hippocampus
A seahorse (also written sea-horse and sea horse) is any of 46 species of small marine bony fish in the genus Hippocampus. The genus name comes from the Ancient Greek hippókampos (ἱππόκαμπος), itself from híppos (ἵππος) meaning "horse" and kámpos meaning "sea monster" or "sea animal". Having a head and neck suggestive of a horse, seahorses also feature segmented bony armour, an upright posture and a curled prehensile tail. Along with the pipefishes and seadragons (Phycodurus and Phyllopteryx) they form the family Syngnathidae.
Evolution and fossil record
Anatomical evidence, supported by molecular, physical, and genetic evidence, demonstrates that seahorses are highly modified pipefish. The fossil record of seahorses, however, is very sparse. The best known and best studied fossils are specimens of Hippocampus guttulatus (though literature more commonly refers to them under the synonym of H. ramulosus), from the Marecchia River formation of Rimini Province, Italy, dating back to the Lower Pliocene, about 3 million years ago. The earliest known seahorse fossils are of two pipefish-like species, H. sarmaticus and H. slovenicus, from the coprolitic horizon of Tunjice Hills, a middle Miocene lagerstätte in Slovenia dating back about 13 million years.
Molecular dating implies that pipefish and seahorses diverged during the Late Oligocene. This has led to speculation that seahorses evolved in response to large areas of shallow water, newly created as the result of tectonic events. The shallow water would have allowed the expansion of seagrass habitats that served as camouflage for the seahorses' upright posture. These tectonic changes occurred in the western Pacific Ocean, pointing to an origin there, with molecular data suggesting two later, separate invasions of the Atlantic Ocean. In 2016, a study published in Nature found the seahorse genome to be the most rapidly evolving fish genome studied so far.
The evolution of seahorses from pipefish may have been an adaptation related to the biomechanics of prey capture. The unique posture of the seahorse allows them to capture small shrimps at larger distances than the pipefish is capable of.
Description
Seahorses range in size from 1.5 to 35 cm (0.6 to 13.8 in). They are named for their equine appearance, with bent necks and long snouted heads and a distinctive trunk and tail. Although they are bony fish, they do not have scales, but rather thin skin stretched over a series of bony plates, which are arranged in rings throughout their bodies. Each species has a distinct number of rings. The armor of bony plates also protects them against predators, and because of this outer skeleton, they no longer have ribs. Seahorses swim upright, propelling themselves using the dorsal fin, another characteristic not shared by their close pipefish relatives, which swim horizontally. Razorfish are the only other fish that swim vertically. The pectoral fins, located on either side of the head behind their eyes, are used for steering. They lack the caudal fin typical of fishes. Their prehensile tail is composed of square-like rings. They are adept at camouflage, and can grow and reabsorb spiny appendages depending on their habitat.
Unusual among fish, a seahorse has a flexible, well-defined neck. It also sports a crown-like spine or horn on its head, termed a "coronet", which is distinct for each species.
Seahorses swim extremely poorly, rapidly fluttering a dorsal fin and using pectoral fins to steer. The slowest-moving fish in the world is H. zosterae (the dwarf seahorse), with a top speed of about 1.5 m (5 ft) per hour. Since they are poor swimmers, they are most likely to be found resting with their prehensile tail wound around a stationary object. They have long snouts, which they use to drag up food, and their eyes can move independently of each other like those of a chameleon.
Habitat
Seahorses are mainly found in shallow tropical and temperate salt water throughout the world, from about 45°S to 45°N. They live in sheltered areas such as seagrass beds, estuaries, coral reefs, and mangroves. Four species are found in Pacific waters from North America to South America. In the Atlantic, Hippocampus erectus ranges from Nova Scotia to Uruguay. H. zosterae, known as the dwarf seahorse, is found in the Bahamas.
Colonies have been found in European waters such as the Thames Estuary.
Two species live in the Mediterranean Sea: H. guttulatus (the long-snouted seahorse), and H. hippocampus (the short-snouted seahorse). These species form territories; males stay within 1 sq m (10 sq ft) of habitat, while females range over about one hundred times that.
Feeding habits
Seahorses rely on stealth to ambush small prey such as copepods. They use pivot feeding to catch the copepod, which involves rotating their snout at high speed and then sucking in the copepod.
Seahorses use their long snouts to eat their food with ease. However, they are slow to consume their food and have extremely simple digestive systems that lack a stomach, so they must eat constantly to stay alive.[28] Seahorses are not very good swimmers, and for this reason they need to anchor themselves to seaweed, coral or anything else that will keep the seahorse in place. They do this by using their prehensile tails to grasp their object of choice. Seahorses feed on small crustaceans floating in the water or crawling on the bottom. With excellent camouflage, seahorses ambush prey that floats within striking range, sitting and waiting until an optimal moment. Mysid shrimp and other small crustaceans are favorites, but some seahorses have been observed eating other kinds of invertebrates and even larval fish. In a study of seahorses, the distinctive head morphology was found to give them a hydrodynamic advantage that creates minimal interference while approaching an evasive prey. Thus the seahorse can get very close to the copepods on which it preys. After successfully closing in on the prey without alerting it, the seahorse gives an upward thrust and rapidly rotates the head aided by large tendons that store and release elastic energy, to bring its long snout close to the prey. This step is crucial for prey capture, as oral suction only works at a close range. This two-phase prey capture mechanism is termed pivot-feeding. Seahorses have three distinctive feeding phases: preparatory, expansive, and recovery. During the preparatory phase, the seahorse slowly approaches the prey while in an upright position, after which it slowly flexes its head ventrally. In the expansive phase, the seahorse captures its prey by simultaneously elevating its head, expanding the buccal cavity, and sucking in the prey item. During the recovery phase, the jaws, head, and hyoid apparatus of the seahorse return to their original positions.
The amount of available cover influences the seahorse's feeding behaviour. For example, in wild areas with small amounts of vegetation, seahorses will sit and wait, but an environment with extensive vegetation will prompt the seahorse to inspect its environment, feeding while swimming rather than sitting and waiting. Conversely, in an aquarium setting with little vegetation, the seahorse will fully inspect its environment and makes no attempt to sit and wait.
Additional Information
Seahorses are members of the pipefish family. In addition to their iconic appearance, seahorses possess many interesting attributes. Among them are specialized structures in their skin cells, called chromatophores, which allow the mostly sessile seahorses to change color to mimic their surroundings. Well camouflaged as they cling to stalks of seagrass in their shallow habitats, seahorses can be hard to see.
Their truly remarkable biological claim to fame, however, is that male seahorses and sea dragons get pregnant and bear young—a unique adaptation in the animal kingdom.
After completing an elaborate courtship dance that may go on for hours or days, the female seahorse transfers her mature eggs into the male’s brood pouch, where they are fertilized. At the end of a gestation period usually lasting from two to four weeks, the pregnant male’s abdominal area begins to undulate rhythmically, and strong muscular contractions eject from a few dozen to as many as 1,000 fully formed baby seahorses into the surrounding water. After that, the offspring must fend for themselves. Large litters are necessary because only about 0.5 percent will survive to adulthood.
Many, if not all, of the 47 known seahorse species—14 of which were identified only in the 21st century—are in decline worldwide.
Because seahorses generally live in shallow, near-coastal waters, human activities including development, pollution, fisheries, and traditional medicine have reduced their numbers. At the same time, their universal appeal has worked against them; until recently, wild seahorses were often captured for the aquarium trade. The delicate creatures tend to fare poorly in aquaria, however. In recent years, captive-bred seahorses have shown promise as hardier tank-dwellers than their wild relatives.

#32 Science HQ » Iridium » 2025-09-08 17:42:22
- Jai Ganesh
- Replies: 0
Iridium
Gist
Iridium (Ir) is a rare, hard, brittle, silvery-white transition metal in the platinum group, known for its exceptional corrosion resistance and high density. It is primarily used in alloys for electrical contacts, scientific equipment, and spark plugs. The element is also essential in catalysis, medical brachytherapy for cancer treatment (using Iridium-192), and as the basis for the global Iridium satellite constellation.
Iridium is used in high-tech applications because of its extreme resistance to heat and corrosion, including in spark plugs, crucibles for high-temperature crystal growth, and catalysts for industrial chemical processes like acetic acid production. Iridium alloys are also vital for specialized tools, deep-sea pipes, and components in aerospace and electronic devices, with its radioactive isotope (Iridium-192) used in cancer treatment (brachytherapy).
Summary
Iridium is a chemical element; it has symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density of 22.56 g/{cm}^{3} (0.815 lb/cu in) as defined by experimental X-ray crystallography. 191Ir and 193Ir are the only two naturally occurring isotopes of iridium, as well as the only stable isotopes; the latter is the more abundant. It is one of the most corrosion-resistant metals, even at temperatures as high as 2,000 °C (3,630 °F).
Iridium was discovered in 1803 in the acid-insoluble residues of platinum ores by the English chemist Smithson Tennant. The name iridium, derived from the Greek word iris (rainbow), refers to the various colors of its compounds. Iridium is one of the rarest elements in Earth's crust, with an estimated annual production of only 6,800 kilograms (15,000 lb) in 2023.
The dominant uses of iridium are the metal itself and its alloys, as in high-performance spark plugs, crucibles for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process. Important compounds of iridium are chlorides and iodides in industrial catalysis. Iridium is a component of some OLEDs.
Iridium is found in meteorites in much higher abundance than in the Earth's crust. For this reason, the unusually high abundance of iridium in the clay layer at the Cretaceous–Paleogene boundary gave rise to the Alvarez hypothesis that the impact of a massive extraterrestrial object caused the extinction of non-avian dinosaurs and many other species 66 million years ago, now known to be produced by the impact that formed the Chicxulub crater. Similarly, an iridium anomaly in core samples from the Pacific Ocean suggested the Eltanin impact of about 2.5 million years ago.
Details
Iridium (Ir) is a chemical element, one of the platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table. It is very dense and rare and is used in platinum alloys. A precious, silver-white metal, iridium is hard and brittle, but it becomes ductile and can be worked at a white heat, from 1,200° to 1,500° C (2,200° to 2,700° F). It is one of the densest terrestrial substances. In the massive state the metal is practically insoluble in acids and is not attacked even by aqua regia. It can be dissolved in concentrated hydrochloric acid in the presence of sodium perchlorate at 125° to 150° C (257° to 302° F).
Because of difficulties in preparation and fabrication, the pure metal has few applications. Iridium is chiefly used in the form of platinum alloys. Platinum-iridium alloys (5 to 10 percent iridium) are readily workable metals that are much harder and stiffer and more resistant to chemical attack than the soft pure platinum. Such alloys are used for jewelry, pen points, surgical pins and pivots, and electrical contacts and sparking points. The international prototype standard kilogram of mass is made from an alloy containing 90 percent platinum and 10 percent iridium.
Pure iridium probably does not occur in nature; its abundance in the Earth’s crust is very low, about 0.001 parts per million. Though rare, iridium does occur in natural alloys with other noble metals: in iridosmine up to 77 percent iridium, in platiniridium up to 77 percent, in aurosmiridium 52 percent, and in native platinum up to 7.5 percent. Iridium generally is produced commercially along with the other platinum metals as a by-product of nickel or copper production.
Iridium-containing ores are found in South Africa and Alaska, U.S., as well as in Myanmar (Burma), Brazil, Russia, and Australia. In the late 20th century South Africa was the world’s major producer of iridium.
The element was discovered in 1803 in the acid-insoluble residues of platinum ores by the English chemist Smithson Tennant; the French chemists H.-V. Collet-Descotils, A.-F. Fourcroy, and N.-L. Vauquelin identified it at about the same time. The name iridium, derived from the Greek word iris (“rainbow”), refers to the various colours of its compounds. Natural iridium consists of a mixture of two stable isotopes, iridium-191 (37.3 percent) and iridium-193 (62.7 percent). The chemistry of iridium centres on oxidation states of +1, +3, and +4, though compounds of all states from 0 to +6 are known with perhaps the exception of +2. Complexes in oxidation state +1 chiefly contain carbon monoxide, olefins, and phosphines as ligands. The anions hexachloroiridate, [IrCl6]2−, and hexabromoiridate, [IrBr6]2−, are the only notable chemical species containing iridium in the +4 oxidation state. Iridium is somewhat more reactive than ruthenium and osmium.
Element Properties
atomic number : 77
atomic weight : 192.2
melting point : 2,410° C (4,370° F)
boiling point : 4,527° C (8,181° F)
specific gravity : 22.4 (20° C)
oxidation states : +1, +3, +4.
Additional Information:
Appearance
Iridium is a hard, silvery metal. It is almost as unreactive as gold. It has a very high density and melting point.
Uses
Iridium is the most corrosion-resistant material known. It is used in special alloys and forms an alloy with osmium, which is used for pen tips and compass bearings. It was used in making the standard metre bar, which is an alloy of 90% platinum and 10% iridium. It is also used for the contacts in spark plugs because of its high melting point and low reactivity.
Biological role
Iridium has no known biological role, and has low toxicity.
Natural abundance
Iridium is one of the rarest elements on Earth. It is found uncombined in nature in sediments that were deposited by rivers. It is commercially recovered as a by-product of nickel refining.
A very thin layer of iridium exists in the Earth’s crust. It is thought that this was caused by a large meteor or asteroid hitting the Earth. Meteors and asteroids contain higher levels of iridium than the Earth’s crust. The impact would have caused a huge dust cloud depositing the iridium all over the world. Some scientists think that this could be the same meteor or asteroid impact that wiped out the dinosaurs.

#33 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-08 15:47:35
Hi,
#5743. What does the noun optimization mean?
#5744. What does the adjective optimum mean?
#34 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-08 15:15:56
Hi,
#2463. Where is the Mesangial cell found in the human body?
#35 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-08 14:57:37
Hi,
#9730.
#36 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-08 14:31:49
Hi,
#6236.
#37 Jokes » Librarian Jokes - II » 2025-09-08 14:10:34
- Jai Ganesh
- Replies: 0
Q: What's the longest word in the dictionary?
A: Smiles. Because there is a mile between each s.
* * *
Q: What section of the library can you get bitten by a snake?
A: Hissssssstory.
* * *
Q: Why did the book join the police?
A: He wanted to go undercover!
* * *
Q: What kind of berry wants a coloring book?
A: A crayon-berry.
* * *
Q: Why should you be careful about what you say around a librarian?
A: Because they can read lips.
* * *
#38 Re: Exercises » Compute the solution: » 2025-09-08 13:54:23
Hi,
2578.
#39 Jokes » Librarian Jokes - I » 2025-09-07 21:52:16
- Jai Ganesh
- Replies: 0
Q: What is the tallest building in the world?
A: The library, because it has the most stories.
* * *
Q: What do you call a nervous javelin thrower?
A: Shakespeare.
* * *
Q: What do you call a South American librarian who is always in a hurry?
A: Urgent Tina.
* * *
Q: What do you call a book that's about the brain?
A: A mind reader.
* * *
Q: Why did the librarian win a Lifetime Achievement Award?
A: She had a storied career.
* * *
#40 Re: This is Cool » Miscellany » 2025-09-07 21:28:45
2383) Ostrich
Gist
Ostriches have special features including being the world's largest and fastest flightless bird, with powerful two-toed legs, largest eyes of any land animal, and unique two-toed feet. Their specialized feathers help with temperature regulation, their large wings are used for balance, and their powerful kicks serve as a potent defense mechanism.
Summary
Details
Additional Information
The flightless ostrich is the world's largest bird. They roam African savanna and desert lands and get most of their water from the plants they eat.
Speed and Movement
Though they cannot fly, ostriches are fleet, strong runners. They can sprint up to 43 miles an hour and run over distance at 31 miles an hour. They may use their wings as "rudders" to help them change direction while running. An ostrich's powerful, long legs can cover 10 to 16 feet in a single stride. These legs can also be formidable weapons. Ostrich kicks can kill a human or a potential predator like a lion. Each two-toed foot has a long, sharp claw.
Herds and Reproduction
Ostriches live in small herds that typically contain less than a dozen birds. Alpha males maintain these herds, and mate with the group's dominant hen. The male sometimes mates with others in the group, and wandering males may also mate with lesser hens. All of the group's hens place their eggs in the dominant hen's nest—though her own are given the prominent center place. The dominant hen and male take turns incubating the giant eggs, each one of which weighs as much as two dozen chicken eggs.
Behavior and Diet
Contrary to popular belief, ostriches do not bury their heads in the sand. The old saw probably originates with one of the bird's defensive behaviors. At the approach of trouble, ostriches will lie low and press their long necks to the ground in an attempt to become less visible. Their plumage blends well with sandy soil and, from a distance, gives the appearance that they have buried their heads in the sand.
Ostriches typically eat plants, roots, and seeds but will also eat insects, lizards, or other creatures available in their sometimes harsh habitat.
Details:
Scientific classification
Kingdom : Animalia
Phylum : Chordata
Class : Aves
Infraclass : Palaeognathae
Order : Struthioniformes
Family : Struthionidae
Genus : Struthio
Ostriches are large flightless birds. Two living species are recognised; the common ostrich, native to large parts of Sub-Saharan Africa, and the Somali ostrich, native to the Horn of Africa.
They are the heaviest and largest living birds, with adult common ostriches weighing anywhere between 63.5 and 145 kilograms and laying the largest eggs of any living land animal. With the ability to run at 70 km/h (43.5 mph), they are the fastest birds on land. They are farmed worldwide, with significant industries in the Philippines and in Namibia. South Africa produces about 70% of global ostrich products, with the industry largely centered around the town of Oudtshoorn. Ostrich leather is a lucrative commodity, and the large feathers are used as plumes for the decoration of ceremonial headgear. Ostrich eggs and meat have been used by humans for millennia. Ostrich oil is another product that is made using ostrich fat.
Ostriches are of the genus Struthio in the order Struthioniformes, part of the infra-class Palaeognathae, a diverse group of flightless birds also known as ratites that includes the emus, rheas, cassowaries, kiwi and the extinct elephant birds and moa.
The common ostrich was historically native to the Arabian Peninsula, and ostriches were present across Asia as far east as China and Mongolia during the Late Pleistocene and possibly into the Holocene.
Taxonomic history
The genus Struthio was first described by Carl Linnaeus in 1758. The genus was used by Linnaeus and other early taxonomists to include the emu, rhea, and cassowary, until they each were placed in their own genera. The Somali ostrich (Struthio molybdophanes) has recently become recognized as a separate species by most authorities, while others are still reviewing the evidence.
Evolution
Struthionidae is a member of the Struthioniformes, a group of paleognath birds which first appeared during the Early Eocene, and includes a variety of flightless forms which were present across the Northern Hemisphere (Europe, Asia and North America) during the Eocene epoch. The closest relatives of Struthionidae within the Struthioniformes are the Ergilornithidae, known from the late Eocene to early Pliocene of Asia. It is therefore most likely that Struthionidae originated in Asia.
The earliest fossils of the genus Struthio are from the early Miocene ~21 million years ago of Namibia in Africa, so it is proposed that genus is of African origin. By the middle to late Miocene (5–13 mya) they had spread to and become widespread across Eurasia. While the relationship of the African fossil species is comparatively straightforward, many Asian species of ostrich have been described from fragmentary remains, and their interrelationships and how they relate to the African ostriches are confusing. In India, Mongolia and China, ostriches are known to have become extinct only around, or even after, the end of the last ice age; images of ostriches have been found prehistoric Chinese pottery and petroglyphs.
Distribution and habitat
Today, ostriches are only found natively in the wild in Africa, where they occur in a range of open arid and semi-arid habitats such as savannas and the Sahel, both north and south of the equatorial forest zone. The Somali ostrich occurs in the Horn of Africa, having evolved isolated from the common ostrich by the geographic barrier of the East African Rift. In some areas, the common ostrich's Masai subspecies occurs alongside the Somali ostrich, but they are kept from interbreeding by behavioral and ecological differences. The Arabian ostriches in Asia Minor and Arabia were hunted to extinction by the middle of the 20th century, and in Israel attempts to introduce North African ostriches to fill their ecological role have failed. Escaped common ostriches in Australia have established feral populations.
Species
In 2008, S. linxiaensis was transferred to the genus Orientornis. Three additional species, S. pannonicus, S. dmanisensis, and S. transcaucasicus, were transferred to the genus Pachystruthio in 2019. Several additional fossil forms are ichnotaxa (that is, classified according to the organism's trace fossils such as footprints rather than its body) and their association with those described from distinctive bones is contentious and in need of revision pending more good material.
Additional Information
Ostrich, (Struthio camelus), is a large flightless bird found only in open country in Africa. The largest living bird, an adult male may be 2.75 metres (about 9 feet) tall—almost half of its height is neck—and weigh more than 150 kg (330 pounds); the female is somewhat smaller. The ostrich’s egg, averaging about 150 mm (6 inches) in length by 125 mm (5 inches) in diameter and about 1.35 kg (3 pounds), is also the world’s largest. The male is mostly black but has white plumes in the wings and tail; females are mostly brown. The head and most of the neck, reddish to bluish in colour, is lightly downed; the legs, including the powerful thighs, are bare. The head is small, the bill short and rather wide; the big brown eyes have thick black lashes.
Ostriches are seen individually, in pairs, in small flocks, or in large aggregations, depending on the season. The ostrich relies on its strong legs—uniquely two-toed, with the main toe developed almost as a hoof—to escape its enemies, chiefly humans and the larger carnivores. A frightened ostrich can achieve a speed of 72.5 km (45 miles) per hour. If cornered, it can deliver dangerous kicks.
Ostriches live mainly on vegetation but also take some animal food, mainly insects; they can go without water for long periods. Breeding males emit lionlike roars and hisses as they fight for a harem of three to five hens. A communal nest scraped in the ground contains more than a dozen shiny, whitish eggs. The major hen of the harem may get rid of some of the eggs to make incubation more manageable. The male sits on the eggs by night; the females take turns during the day. The chicks hatch in about 40 days and when a month old can keep up with running adults. To escape detection, chicks as well as adults may lie on the ground with neck outstretched, a habit that may have given rise to the mistaken belief that the ostrich buries its head in the sand when danger threatens. Ostrich plumes adorned the helmets of medieval European knights, and in the 19th century such plumes were sold for women’s finery. This demand led to the establishment of ostrich farms in South Africa, the southern United States, Australia, and elsewhere, but the trade collapsed after World War I. Ostriches are now raised for their meat and hide, which provides a soft, fine-grained leather. The birds have been trained for saddle and sulky racing, but they tire easily and are not well suited for training. They do well in captivity and may live 50 years.
The ostrich is typical of a group of flightless birds called ratites. Ostrich populations differing slightly in skin colour, size, and egg features formerly were considered separate species, but now they are considered to be merely races of Struthio camelus. Most familiar is the North African ostrich, S. camelus camelus, ranging, in much-reduced numbers, from Morocco to Sudan. Ostriches also live in eastern and southern Africa. The Syrian ostrich (S. camelus syriacus) of Syria and Arabia became extinct in 1941. The ostrich is the only living species in the genus Struthio. Ostriches are the only members of the family Struthionidae in the order Struthioniformes—a group that also contains kiwis, emus, cassowaries, and rheas. The oldest fossil relatives of ostriches belong to the species Calciavis grandei, which were excavated from the Green River Formation in Wyoming and date to the Eocene Epoch, some 56 million to 34 million years ago.
#41 Dark Discussions at Cafe Infinity » Close Quotes - I » 2025-09-07 20:33:42
- Jai Ganesh
- Replies: 0
Close Quotes - I
1. Many of life's failures are people who did not realize how close they were to success when they gave up. - Thomas A. Edison
2. Keep close to Nature's heart... and break clear away, once in awhile, and climb a mountain or spend a week in the woods. Wash your spirit clean. - John Muir
3. Don't let your ego get too close to your position, so that if your position gets shot down, your ego doesn't go with it. - Colin Powell
4. In dwelling, live close to the ground. In thinking, keep to the simple. In conflict, be fair and generous. In governing, don't try to control. In work, do what you enjoy. In family life, be completely present. - Lao Tzu
5. I never attempt to make money on the stock market. I buy on the assumption that they could close the market the next day and not reopen it for five years. - Warren Buffett
6. Perhaps when we find ourselves wanting everything, it is because we are dangerously close to wanting nothing. - Sylvia Plath
7. When even one American - who has done nothing wrong - is forced by fear to shut his mind and close his mouth - then all Americans are in peril. - Harry S Truman
8. Nonsense and beauty have close connections. - E. M. Forster.
#42 Jokes » Lawyer Jokes - XIV » 2025-09-07 20:13:11
- Jai Ganesh
- Replies: 0
Q: What's the difference between a lawyer and a boxing referee?
A: A boxing referee doesn't get paid extra for a longer fight.
* * *
Q: What's the difference between a lawyer and a leech?
A: When you die, a leech will stop sucking your blood and drop off.
* * *
Q: What's the difference between a lawyer and an angry rhinoceros?
A: The lawyer charges more.
* * *
Q: What clothing do you wear to court?
A: A lawsuit.
* * *
Q: What did the lawyer name his daughter?
A: Sue.
* * *
#43 Science HQ » Osmium » 2025-09-07 19:35:13
- Jai Ganesh
- Replies: 0
Osmium
Gist
Osmium is a chemical element; it has symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium has the highest density of any stable element (22.59 g/{cm}^{3}). It is also one of the rarest elements in the Earth's crust, with an estimated abundance of 50 parts per trillion (ppt). Manufacturers use alloys of osmium with platinum, iridium, and other platinum-group metals for fountain pen nib tipping, electrical contacts, and other applications that require extreme durability and hardness.
Summary
Osmium (Os, atomic number 76) is a rare, hard, brittle, bluish-white transition metal in the platinum group, known for being the densest stable element. It's named for its "smell" in oxide form, is expensive, and has uses in specialized applications like electrical contacts, catalysts, and scientific instruments. However, osmium tetroxide is a highly toxic and flammable substance that is a strong oxidant and can cause serious health problems.
Osmium's primary uses are in producing extremely hard alloys for items like fountain pen tips, electrical contacts, and surgical implants such as pacemakers, and as a catalyst in the chemical industry. Additionally, osmium tetroxide, a volatile compound, is used for staining fatty tissues in microscopy and for detecting fingerprints.
Details
Osmium (Os), chemical element, is one of the platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table and the densest naturally occurring element. A gray-white metal, osmium is very hard, brittle, and difficult to work, even at high temperatures. Of the platinum metals, it has the highest melting point, so fusing and casting are difficult. Osmium wires were used for filaments of early incandescent lamps before the introduction of tungsten. It has been used chiefly as a hardener in alloys of the platinum metals, though ruthenium has generally replaced it. A hard alloy of osmium and iridium was used for tips of fountain pens and phonograph needles, and osmium tetroxide is used in certain organic syntheses.
Pure osmium metal does not occur in nature. Osmium has a low crustal abundance of about 0.001 part per million. Though rare, osmium is found in native alloys with other platinum metals: in siserskite (up to 80 percent), in iridosmine, in aurosmiridium (25 percent), and in slight amounts in native platinum. Processes for isolating it are an integral part of the metallurgical art that applies to all platinum metals.
The English chemist Smithson Tennant discovered the element together with iridium in the residues of platinum ores not soluble in aqua regia. He announced its isolation (1804) and named it for the unpleasant odour of some of its compounds (Greek osme, odour).
Of the platinum metals, osmium is the most rapidly attacked by air. The powdered metal, even at room temperature, exudes the characteristic odour of the poisonous volatile tetroxide, OsO4. Because solutions of OsO4 are reduced to the black dioxide, OsO2, by some biological materials, it is sometimes used to stain tissues for microscopic examinations.
Osmium is, with ruthenium, the most noble of the platinum metals, and cold and hot acids are without effect on them. It can be dissolved by fused alkalies, especially if an oxidizing agent such as sodium chlorate is present. Osmium will react at 200 °C with air or oxygen to form OsO4.
Osmium exhibits oxidation states from 0 to +8 in its compounds, with the exception of +1; well-characterized and stable compounds contain the element in +2, +3, +4, +6, and +8 states. There are also carbonyl and organometallic compounds in the low oxidation states −2, 0, and +1. Ruthenium is the only other element known to have an oxidation state of 8. (The chemistries of ruthenium and osmium are generally similar.) All compounds of osmium are easily reduced or decomposed by heating to form the free element as a powder or sponge. There is an extensive chemistry of the tetroxides, oxohalides, and oxo anions. There is little, if any, evidence that simple aquo ions exist, and virtually all their aqueous solutions, whatever the anions present, may be considered to contain complexes.
Natural osmium consists of a mixture of seven stable isotopes: osmium-184 (0.02 percent), osmium-186 (1.59 percent), osmium-187 (1.96 percent), osmium-188 (13.24 percent), osmium-189 (16.15 percent), osmium-190 (26.26 percent), and osmium-192 (40.78 percent).
Element Properties
atomic number : 76
atomic weight : 190.23
melting point : 3,000 °C (5,432 °F)
boiling point : about 5,000 °C (9,032 °F)
specific gravity : 22.59 (20 °C)
oxidation states : +2, +3, +4, +6, +8.
Additional Information:
Appearance
A shiny, silver metal that resists corrosion. It is the densest of all the elements and is twice as dense as lead.
Uses
Osmium has only a few uses. It is used to produce very hard alloys for fountain pen tips, instrument pivots, needles and electrical contacts. It is also used in the chemical industry as a catalyst.
Biological role
Osmium has no known biological role. The metal is not toxic, but its oxide is volatile and very toxic, causing lung, skin and eye damage.
Natural abundance
Osmium occurs uncombined in nature and also in the mineral osmiridium (an alloy with iridium). Most osmium is obtained commercially from the wastes of nickel refining.

#44 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-07 16:18:56
Hi,
#5741. What does the noun marathon mean?
#5742. What does the noun marauder mean?
#45 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-07 15:40:32
Hi,
#2462. What does the medical term Prenatal testing signify?
#46 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-07 15:10:43
Hi,
#9729.
#47 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-07 14:57:59
Hi,
#6235.
#48 Re: Exercises » Compute the solution: » 2025-09-07 14:05:17
Hi,
Correct!
2577.
#49 Re: This is Cool » Miscellany » 2025-09-06 22:44:37
2382) Congo River
Gist
The deepest river in the world is the Congo River in Africa, with recorded depths reaching at least 220 meters (720 feet). Recognized by Guinness World Records, its maximum depth surpasses that of any other river and was confirmed by scientists in 2008.
Summary
The basin of the deepest river in the world provides food, water, medicines and transportation to about 75 million people, according to Yale University’s Global Forest Atlas.
1 . Where does the Congo river flow through?
The Congo is the deepest river in the world. Its headwaters are in the north-east of Zambia, between Lake Tanganyika and Lake Nyasa (Malawi), 1760 metres above sea level; it flows into the Atlantic Ocean.
The Congo river flows through the Republic of the Congo, the Democratic Republic of the Congo, the Central African Republic, western Zambia, northern Angola, Cameroon and Tanzania. The lower course of the Congo river has large gorges and falls, which makes it one of the most dangerous rivers.
2 . How deep and how long is the Congo river?
The Congo river, formerly known as the Zaire, is the deepest river in the world: at some points the water can be up to 220 metres deep; the basin has a surface area of 3,457,000 square kilometres.
The Congo river is 4,700 kilometres long, which makes it the second longest river in Africa, the tenth longest river in the world and the second largest river by discharge volume in the world, only after the Amazon.
3 . What are the environmental threats in the Congo river basin?
One of the main threats in the Congo river basin is deforestation to accommodate modern agricultural practices; moreover, industrial logging accelerates the rate of deforestation.
The other major threat is food demand due to rapid population growth in the region, which leads to excessive hunting of wild animals such as bats, monkeys, rats and snakes.
Summary
he Congo River, formerly also known as the Zaire River, is the second-longest river in Africa, shorter only than the Nile, as well as the third-largest river in the world by discharge volume, following the Amazon and Ganges–Brahmaputra rivers. It is the world's deepest recorded river, with measured depths of around 220 m (720 ft). The Congo–Lualaba–Luvua–Luapula–Chambeshi River system has an overall length of 4,700 km (2,900 mi), which makes it the world's ninth-longest river. The Chambeshi is a tributary of the Lualaba River, and Lualaba is the name of the Congo River upstream of Boyoma Falls, extending for 1,800 km (1,100 mi).
Measured along with the Lualaba, the main tributary, the Congo River has a total length of 4,370 km (2,720 mi). It is the only major river to cross the equator twice. The Congo Basin has a total area of about 4,000,000 sq km (1,500,000 sq mi), or 13% of the entire African landmass.
Name
The name Congo/Kongo originates from the Kingdom of Kongo once located on the southern bank of the river. The kingdom in turn was named after the indigenous Bantu Kongo people, known in the 17th century as "Esikongo". South of the Kingdom of Kongo proper lay the similarly named Kakongo kingdom, mentioned in 1535. Abraham Ortelius labelled "Manicongo" as the city at the mouth of the river in his world map of 1564. The tribal names in Kongo possibly derive from a word for a public gathering or tribal assembly. The modern name of the Kongo people or Bakongo was introduced in the early 20th century.
The name Zaire is from a Portuguese adaptation of a Kikongo word, nzere ("river"), a truncation of nzadi o nzere ("river swallowing rivers"). The river was known as Zaire during the 16th and 17th centuries; Congo seems to have replaced Zaire gradually in English usage during the 18th century, and Congo is the preferred English name in 19th-century literature, although references to Zahir or Zaire as the name used by the inhabitants remained common. The Democratic Republic of the Congo and the Republic of the Congo are named after it, as was the previous Republic of the Congo which had gained independence in 1960 from the Belgian Congo. The Republic of Zaire during 1971–1997 was also named after the river's name in French and Portuguese.
Details
Congo River, river in west-central Africa. With a length of 2,900 miles (4,700 km), it is the continent’s second longest river, after the Nile. It rises in the highlands of northeastern Zambia between Lakes Tanganyika and Nyasa (Malawi) as the Chambeshi River at an elevation of 5,760 feet (1,760 metres) above sea level and at a distance of about 430 miles (700 km) from the Indian Ocean. Its course then takes the form of a giant counterclockwise arc, flowing to the northwest, west, and southwest before draining into the Atlantic Ocean at Banana (Banane) in the Democratic Republic of the Congo. Its drainage basin, covering an area of 1,335,000 square miles (3,457,000 square km), takes in almost the entire territory of that country, as well as most of the Republic of the Congo, the Central African Republic, eastern Zambia, and northern Angola and parts of Cameroon and Tanzania.
With its many tributaries, the Congo forms the continent’s largest network of navigable waterways. Navigability, however, is limited by an insurmountable obstacle: a series of 32 cataracts over the river’s lower course, including the famous Inga Falls. These cataracts render the Congo unnavigable between the seaport of Matadi, at the head of the Congo estuary, and Malebo Pool, a lakelike expansion of the river. It was on opposite banks of Malebo Pool—which represents the point of departure of inland navigation—that the capitals of the former states of the French Congo and the Belgian Congo were founded: on the left bank Kinshasa (formerly Léopoldville), now the capital of the Democratic Republic of the Congo, and on the right bank Brazzaville, now the capital of the Republic of the Congo.
The Amazon and the Congo are the two great rivers of the world that flow out of equatorial zones where heavy rainfall occurs throughout all or almost all of the year. Upstream from Malebo Pool, the Congo basin receives an average of about 60 inches (1,500 mm) of rain a year, of which more than one-fourth is discharged into the Atlantic. The drainage basin of the Congo is, however, only about half the size of that of the Amazon, and the Congo’s rate of flow—1,450,000 cubic feet (41,000 cubic metres) per second at its mouth—is considerably less than the Amazon’s flow of more than 6,180,000 cubic feet (175,000 cubic metres) per second.
While the Chambeshi River, as the remotest source, may form the Congo’s original main stream in terms of the river’s length, it is another tributary—the Lualaba, which rises near Musofi in southeastern Democratic Republic of the Congo—that carries the greatest quantity of water and thus may be considered as forming the Congo’s original main stream in terms of water volume.
When the river first became known to Europeans at the end of the 15th century, they called it the Zaire, a corruption of a word that is variously given as nzari, nzali, njali, nzaddi, and niadi and that simply means “river” in local African languages. It was only in the early years of the 18th century that the river was first called the “Rio Congo,” a name taken from the kingdom of Kongo that had been situated along the lower course of the river. During the period (1971–97) when the Democratic Republic of the Congo was called Zaire, the government also renamed the river the Zaire. Even during that time, however, the river continued to be known throughout the world as the Congo. To the literary-minded the river is evocative of the famous 1902 short story “Heart of Darkness” by Joseph Conrad. His book conjured up an atmosphere of foreboding, treachery, greed, and exploitation. Today, however, the Congo appears as the key to the economic development of the central African interior.
Physical features:
Physiography
The expression “Congo basin,” strictly speaking, refers to the hydrographic basin. This is not only vast but is also covered with a dense and ramified network of tributaries, subtributaries, and small rivers—with the exception of the sandy plateaus in the southwest.
The Congo basin is the most clearly distinguished of the various geographic depressions situated between the Sahara to the north, the Atlantic Ocean to the south and west, and the region of the East African lakes to the east. In this basin, a fan-shaped web of tributaries flows downward along concentric slopes that range from 900 to 1,500 feet (275 to 460 metres) in elevation and that enclose a central depression. The basin itself stretches for more than 1,200 miles (1,900 km) from north to south (from the Congo–Lake Chad watershed to the interior plateaus of Angola) and also measures about 1,200 miles from the Atlantic in the west to the Nile-Congo watershed in the east.
The central part of the Congo basin—often called the cuvette (literally “saucer” or “shallow bowl”)—is an immense depression containing Quaternary alluvial deposits that rest on thick sediments of continental origin, consisting principally of sands and sandstones. These underlying sediments form outcrops in valley floors at the eastern edge of the cuvette. The filling of the cuvette, however, began much earlier. Boreholes have revealed that since late Precambrian times (i.e., since at least 570 million years ago) considerable sediment has accumulated, derived from the erosion of formations situated around the periphery of the cuvette. The arrangement of surface relief, thick depositional strata, and substratum in amphitheatre-like fashion around the main Congo channel, which has been uniform across time, is evidence of a persistent tendency to subsidence in this part of the continent. This subsidence is accompanied by uplifting on the edges of the cuvette, principally on its eastern side—which has also been influenced by the formation of the Western Rift Valley.
From its sources to its mouth, the Congo River system has three contrasting sections—the upper Congo, middle Congo, and lower Congo. The upper reaches are characterized by three features—confluences, lakes, and waterfalls or rapids. To begin with, several streams of approximately equal size unite to form the river. In a little more than 60 miles (100 km), the upper Lualaba joins the Luvua and then the Lukuga. Each stream for part of its course undergoes at least a lacustrine type of expansion, even when it does not form a lake. Thus, Lake Upemba occurs on the upper Lualaba; Lakes Bangweulu and Mweru occur on the Chambeshi–Luapula–Luvua system; and Lake Tanganyika, which is fed by the Ruzizi (flowing from Lake Kivu) and by the Malagarasi, itself flows into the Lukuga. Rapids occur not only along the headstreams but also in several places along the course of the main stream. Navigation thus is possible only along sections of the upper Congo by vessels of low tonnage. Even so, these stretches are in danger of being overgrown by aquatic vegetation, particularly water hyacinths.
Kisangani (formerly Stanleyville)—located just downstream of the Boyoma Falls, a series of seven cataracts—marks the real beginning, upriver, of the navigable Congo. This central part of the river flows steadily for more than 1,000 miles (1,600 km) to within 22 miles (35 km) of Kinshasa. Its course at first is narrow but soon grows wider, after which many islands occur in midstream. This change in the character of the river corresponds to its entry into its alluvial plain. From that point onward, with the exception of a few rare narrow sections, the Congo divides into several arms, separated by strings of islands. It increases from a width of more than 3.5 miles (5.5 km) downstream from Isangi (where the Lomami enters the Congo) to a width of 5 to 7 miles (8 to 11 km) and on occasion—for example, at the mouth of the Mongala—to 8 miles (13 km). Beyond the natural levees (formed by silt deposits) occurring on either bank, some areas are subjected to extensive flooding that increases the river’s bounds still further. It is not always easy to distinguish such areas from the “rain swamps” in regions lying between rivers. The middle course of the Congo ends in a narrow section called the Chenal (“Channel”), or Couloir (“Corridor”). Between banks no more than half a mile to a mile wide, the riverbed deepens and the current becomes rapid, flowing through a valley that cuts down several hundreds of yards deep into the soft sandstone bedrock of the Batéké Plateau. Along this central reach the Congo receives its principal tributaries, primarily the Ubangi and the Sangha on the right bank and the Kwa on the left bank. An enormous increase in the average rate of flow results, rising from less than 250,000 cubic feet (7,000 cubic metres) a second at Kisangani to nearly its maximum flow at Kinshasa.
Upon leaving the Chenal, the Congo divides into two branches, forming Malebo Pool, a vast lacustrine area about 15 by 17 miles (24 by 27 km), which marks the end of the middle Congo. Immediately downstream occur the first waterfalls of the final section of the river’s course. Cataracts and rapids are grouped into two series, separated by a fairly calm central reach, in which the elevation drops from a little less than 900 feet (275 metres) to a few yards above sea level. The Congo’s estuary begins at Matadi, downstream from the rapids that close off the interior Congo; 83 miles (134 km) in length, it forms the border between Angola and the Democratic Republic of the Congo. At first the estuary is narrow—less than half a mile to about a mile and a half in width—with a central channel 65 to 80 feet (20 to 24 metres) deep, but it widens downstream of Boma. There the river, obstructed by islands, divides into several arms, and in some places the depth does not exceed 20 to 25 feet (6 to 7.5 metres), which makes dredging necessary to allow oceangoing vessels to reach Matadi. Beyond the estuary’s mouth, the course of the Congo continues offshore as a deep underwater canyon that extends for a distance of about 125 miles (200 km).
Hydrology of the Congo River
The Congo has a regular flow, which is fed by rains throughout the year. At Kinshasa the flow has for many years remained between the high level of 2,310,000 cubic feet (65,000 cubic metres) per second, recorded during the flood of 1908, and the low level of 756,000 cubic feet (21,000 cubic metres) per second, recorded in 1905. During the unusual flood of 1962, however, by far the highest for a century, the flow probably exceeded 2,600,000 cubic feet (73,000 cubic metres) per second.
At Brazzaville and Kinshasa, the river’s regime is characterized by a main maximum at the end of the year and a secondary maximum in May, as well as by a major low level during July and a secondary low level during March and April. In reality, the downstream regime of the Congo represents climatic influence extending over 20° of latitude on both sides of the Equator a distance of some 1,400 miles (2,250 km). Each tributary in its course modifies the level of the main stream. Thus, for example, the low level in July at Malebo Pool results from two factors: a drought that occurs for several months in the southern part of the basin at that time, as well as a delay before the floods of the Ubangi tributary flowing down from the north arrive, which does not happen before August. The Congo basin is so vast that no single meteorologic circumstance is capable of disturbing the slow movement of the waters’ rise and fall. The annual fluctuations may alter drastically, however, when floodwaters from different tributaries that normally coincide with each other arrive at different times.
Lake Tanganyika, apart from brief seiches caused by wind drift and sudden changes in atmospheric pressure, may experience considerable variations in its water level from year to year. In 1960, for example, its waters flooded parts of Kalemi, Democratic Republic of the Congo, and Bujumbura, Burundi. A series of particularly rainy years followed by a blocking of the outlet by floating vegetation may explain this phenomenon.
Climate
Typical climate in regions through which the Congo flows is that of Yangambi, a town situated on the river’s right bank slightly north of the Equator and a little downstream of Kisangani. Humidity is high throughout the year, and annual rainfall amounts to 67 inches (1,700 mm) and occurs fairly regularly; even in the driest month the rainfall totals more than 3 inches (76 mm). Temperatures are also uniformly high throughout the year, and there is little diurnal variability. The average temperature at Yangambi is in the mid-70s F (mid-20s C).
From the pluviometric equator (an imaginary east-west line indicating the region of heaviest rainfall), which is situated slightly to the north of the geographic equator, the amount of rainfall decreases regularly in proportion to latitude. The northernmost points of the basin, situated in the Central African Republic, receive only from 8 to 16 inches (200 to 400 mm) less during the course of a year than points near the Equator. The dry season, however, lasts for four or five months, and there is only one annual rainfall maximum, which occurs in summer. In the far southern part of the basin, at a latitude of 12° S, the climate becomes definitely Sudanic in character, with marked dry and wet seasons of approximately equal length and with rainfall of about 49 inches (1,250 mm) a year.
Plant life
The Congo basin is home to the second largest rainforest in the world. The equatorial climate that prevails over a significant part of the Congo basin is coextensive with a dense evergreen forest. The Congolese forest spreads out over the central depression, extending continuously from about 4° N to about 5° S; it is interrupted only by clearings, many of which have a natural origin. The forest region is bordered on either side by belts of savanna (grassy parkland). The forest and savanna often meet imperceptibly, blending together in a mosaic pattern; more rarely, strips of forest invade the grassland. Farther away from the Equator, and to the extent that the Sudanic features of the climate become evident, the wooded savanna region, with its thin deciduous forest, is progressively reached.
As it courses through the solid mass of the Congolese forest, the Congo and its tributaries are bordered by discontinuous grassy strips. Meadows of Echinochloa (a type of grass), papyrus, and Cyperaceae (sedge) occupy abandoned river channels, fringe the banks, or, behind a curtain of forest, blanket the depressions in the centre of the islands, They also spring up on sandbanks, as well as on the downstream ends of islands that are fertilized by the floods. A shrub, Alchornea, frequently marks the transition to the high forest that grows on the levees behind the banks.
Animal life
The animal life of the Congo basin is identified to a certain extent with that of the equatorial forest, which is sharply distinct from the wildlife of the savannas. Within this equatorial domain, the Congo and its principal tributaries form a separate ecological milieu. The animal population of the great waterways often has fewer affinities with the neighbouring marshes or the forests on dry land than it has with other river systems, whether of the coastal region or the savannas.
Numerous species of fish live in the waters of the Congo; more than 230 have been identified in Malebo Pool and the waters that flow into it alone. The riverine swamps, which often dry up at low water, are inhabited by lungfish, which survive the dry periods buried and encysted in cocoons of mucus. In the wooded marshlands, where the water is the colour of black tea, the black catfish there assume the colour of their environment. The wildlife of the marshes and that of the little parallel streams do not mix with the wildlife of the river itself.
The waters of the Congo contain various kinds of reptiles, of which crocodiles are the most striking species. Semiaquatic tortoises are also found, as are several species of water snakes.
The forest birdlife constitutes, together with the birdlife of the East African mountains, the most specifically indigenous birdlife found on the African continent. In the Congo region more than 265 species typical of the equatorial forest have been recorded. Occasionally or seasonally, however, nontypical birds may be observed. Seabirds, such as the sea swallow, fly upstream from the ocean. Migratory birds from Europe, including the blongios heron and the Ixobrychus minutus (little bittern), pass through the region. Species with a wide distribution within Africa, such as the Egyptian duck, also have been sighted. Ducks, herons, storks, and pelicans are abundantly represented.
Aquatic mammals are rare, consisting of the hippopotamus, two species of otters, and the manatee. The manatee (sea cow), which lives entirely in the water, has been officially identified only on the Sangha tributary but appears to have given rise to some curious legends on the lower Congo, including its association with a creature called Mami Wata (a kind of siren), stories of which were carried by African slaves to the Americas.
The people and the economy:
Life of the river peoples
Three types of environments are found, either juxtaposed or in succession, along the river and its tributaries: the narrower sections, bordered by firm ground; the wider stretches, dotted with islands and accompanied by backwaters; and the zones where flooding occurs or where there are extensive marshes.
Almost all the river peoples engage in fishing. Along the narrow sections, where rapids often occur, fishing is only of interest to a small number of villages. The Enya (Wagenia) of Boyoma Falls and the Manyanga living downstream from Malebo Pool attach fish traps to stakes or to dams built in the rapids themselves. Fishing of a very different nature, notably by poison, is conducted in the marshy areas, where the population is more extensive than might be imagined. Among these peoples are the Ngombe—“water people”—who inhabit the Itimbiri-Ngiri and the triangle formed by the Congo and the Ubangi. Other fisherfolk of the marshes dwell in the lagoons and the flooded forests of the region where the confluence of the Congo and the Alima, Likouala, and Sangha occurs.
Despite unfavourable conditions, all these peoples are also cultivators. They raise dikes, often of monumental size, to plant cassava (manioc) on the land thus sheltered from flooding. Other minor crops, such as sweet potatoes, bananas, and yams, are also found. The Congo basin has the continent’s most important timber resources, but the timber industry is developing slowly, mainly because the interior is so inaccessible and because the cost of transporting timber to the coast is so high.
Few modes of existence have undergone such profound changes as a result of contact with the modern world as has that of the river’s fisherfolk. The growth of the towns on the banks of Malebo Pool as well as the taste of urban dwellers for river fish have served to stimulate fishing by tying it to a cash economy. It is not just a question of villagers smoking fish that they sell to passing traders. Increasingly numerous fishing crews sail up the Congo, the Ubangi, and the Kasai, well above their confluences, to fish in the shallows.
Transportation
The Congo is an important navigational system in Africa. Within the territorial limits of the Democratic Republic of the Congo alone, there are some 8,700 miles (14,000 km) of navigable waterway. Of this total, 650 miles (1,050 km) are accessible at all seasons to barges with capacities between 800 and 1,100 tons, depending upon the height of the water. The amount of goods transported by water—consisting mainly of agricultural produce, wood, minerals, and fuel—is very modest in comparison with the traffic on European rivers (for example, the commercial traffic from the port of Kinshasa does not reach a million tons), but river transport remains essential for communications with regions that are inaccessible by road, especially in the cuvette. The three principal routes, all of which converge on the downstream terminus at Kinshasa on the Malebo Pool, run from Kisangani, from Ilebo (formerly Port-Francqui) on the Kasai, and from Bangui on the Ubangi. River transport, however, falls short of the role it could play in development. It has actually declined since the states of the Congo basin became independent in 1960, because of serious problems with aging equipment, a lack of maintenance of the infrastructure, and the poor functioning of the public waterway agencies. In the Democratic Republic of the Congo only the section from Ilebo to Kinshasa is still important, because it constitutes the river link (the other link being a railway between Kinshasa and Matadi) used to transport the copper production of Katanga to the coast.
This network has fostered economic development in inland areas, far from the coast. Varied activities include the production of palm oil on the banks of the Kwilu, centred on the port of Kikwit, and the establishment of plantations of robusta coffee in the Kisangani area.
Before such developments could be undertaken, however, it was necessary to overcome the barrier to the sea formed by the Congo’s lower course. That feat was accomplished in 1898 with the opening of the railway between Matadi and Léopoldville (now Kinshasa) and in 1934 by the completion of the Congo-Ocean rail line on the right bank between Brazzaville and Pointe-Noire.
While the river system facilitates navigation, it also hinders land transportation. Only a small number of bridges cross the Congo and its tributaries. The Kongolo rail-and-road bridge over the Lualaba was reconstructed in 1968, and a bridge over the Congo at Matadi was opened in 1983. Numerous projects to improve the situation nevertheless exist, notably a link between Kinshasa and Brazzaville. This project has long been under discussion, although to financial obstacles are added difficulties caused by political dissension. Several times since the two countries gained independence in 1960, dissension has interrupted the ferry traffic between the two capitals.
Power
It has been estimated that the hydroelectric potential of the Congo basin amounts to about one-sixth of the known world resources, but only a fraction of this potential has been harnessed. The single site of Inga, just upriver from Matadi, has a power potential estimated at more than 30,000 megawatts. Two hydroelectric projects, called Inga I and Inga II, have been completed there since the independence of the Democratic Republic of the Congo. Further development of the region’s hydropower potential, as outlined in the ambitious “Grand Inga” scheme, would create one of the world’s largest hydroelectric power systems.
Study and exploration
The question of the source of the Congo confronted European explorers from the time that the Portuguese navigator Diogo Cão encountered the river’s mouth in 1482, which he believed to be a strait providing access to the realm of the mythical Prester John, a Christian priest-king. It is virtually certain that, well before the Welsh explorer Henry Morton Stanley arrived in 1877, some 17th-century Capuchin missionaries reached the shores of Malebo Pool. This exploit, however, was not followed up, even by the amply supplied expedition led by James Kingston Tuckey, which was sent out by the British Admiralty in 1816 but was decimated and had to retrace its footsteps even before it had surmounted the cataracts. Preposterous hypotheses about the river continued to be entertained, connecting, for example, the upper Niger to the Congo or maintaining that the Congo and the Nile both flowed from a single great lake in the heart of Africa.
Even after the European discovery of Lake Tanganyika by the British explorers Richard Burton and John Speke (1858), then of the Lualaba (1867) and of Lake Bangweulu (1868) by the Scottish explorer David Livingstone, uncertainty remained—uncertainty that Stanley was to dissipate in the course of his famous expedition in 1876 and 1877 that took him by water from the Lualaba to the Congo’s mouth over a period of nine months. In the interior of the Congo basin and above all on the right bank, the final blank spaces on the map could not be filled in until about 1890, when the exploration of the upper course of the Ubangi was completed.
Additional Information
The Congo River (also known as Zaire River) is the largest river in Africa. Its overall length of 4,700 km (2,922 miles) makes it the second longest in Africa (after the Nile). The river and its tributaries flow through the second largest rain forest area in the world, second only to the Amazon Rainforest in South America.
The river also has the second-largest flow in the world, behind the Amazon, and the second-largest watershed of any river, again trailing the Amazon. Its watershed is a little larger than that of the Mississippi River. Because large parts of the river basin sit north and south of the equator, its flow is steady, as there is always at least one river having a rainy season. The Congo gets its name from the old Kingdom of Kongo which was at the mouth of the river. The Democratic Republic of the Congo and the Republic of the Congo, both countries sitting along the river's banks, are named after it. From 1971 to 1997, the Democratic Republic of the Congo was called Zaire and its government called the river the Zaire River.
The sources of the Congo are in the Highlands and mountains of the East African Rift, as well as Lake Tanganyika and Lake Mweru, which feed the Lualaba River. This then becomes the Congo below Boyoma Falls. The Chambeshi River in Zambia is usually taken as the source of the Congo because of the accepted practice worldwide of using the longest tributary, as with the Nile River.
The Congo flows mostly west from Kisangani just below the falls, then slowly bends southwest, passing by Mbandaka, joining with the Ubangi River, and running into the Pool Malebo (Stanley Pool). Kinshasa (formerly Léopoldville) and Brazzaville are on opposite sides of the river at the Pool, where the river narrows and falls through a few cataracts in deep canyons (collectively known as the Livingstone Falls), running by Matadi and Boma, and into the sea at the small town of Muanda.
History of exploration
The mouth of the Congo was visited by Europeans in 1482, by the Portuguese Diogo Cão, and in 1817, by a British exploration under James Kingston Tuckey that went up the river as far as Isangila. Henry Morton Stanley was the first European to travel along the whole river.
Economic importance
Although the Livingstone Falls stop ships coming in from the sea, almost all of the Congo is navigable in parts, especially between Kinshasa and Kisangani. Railways cross the three major falls that interrupt navigation, and much of the trade of central Africa passes along the river. Goods include copper, palm oil, sugar, coffee, and cotton. The river can also be valuable for hydroelectric power, and Inga Dams below Pool Malebo have been built.
In February of 2005, South Africa's state owned power company, Eskom, said that they had a proposal to increase the amount of electric power that the Inga can make through improvements and the building of a new hydroelectric dam. The project would bring the highest output of the dam to 40 GW, twice that of China's Three Gorges Dam.
Geological history
In the Mesozoic period before the continental drift opened the South Atlantic Ocean, the Congo was the upper part of a river about 12,000 km (7,500 miles) long that flowed west across the parts of Gondwanaland, now called Africa and South America.

#50 This is Cool » Sodium Bicarbonate » 2025-09-06 21:32:34
- Jai Ganesh
- Replies: 0
Sodium Bicarbonate
Gist
Sodium bicarbonate , also known as baking soda, is used to relieve heartburn, sour stomach, or acid indigestion by neutralizing excess stomach acid. When used for this purpose, it is said to belong to the group of medicines called antacids. It may be used to treat the symptoms of stomach or duodenal ulcers.
People commonly use sodium bicarbonate for indigestion. It is also used for stomach ulcers, athletic performance, kidney damage, dental plaque, tooth discoloration, and many other conditions, but there is no good scientific evidence to support many of these uses.
Summary
Sodium bicarbonate (NaHCO3) is awhite crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking powders, in effervescent salts and beverages, and as a constituent of dry-chemical fire extinguishers. Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis. It is also employed in certain industrial processes, as in tanning and the preparation of wool.
Many bakery products are leavened by carbon dioxide from added baking soda or sodium bicarbonate in baking powder. When added without the offsetting amounts of dry acids or acid salts present in baking powder, sodium bicarbonate tends to make dough or batter alkaline, causing flavour deterioration and discoloration and slowing carbon dioxide release. Addition of an acid-reacting substance promotes vigorous gas evolution and maintains acidity within a favourable range. The rate of gas release affects the size of the bubbles produced in the dough or batter, consequently influencing the grain, volume, and texture of the finished product.
Details
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda (or simply "bicarb" especially in the UK) is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite, although it is more commonly found as a component of the mineral trona.
As it has long been known and widely used, the salt has many different names such as baking soda, bread soda, cooking soda, brewing soda and bicarbonate of soda and can often be found near baking powder in stores. The term baking soda is more common in the United States, while bicarbonate of soda is more common in Australia, the United Kingdom, and New Zealand. Abbreviated colloquial forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common.
The prefix bi- in "bicarbonate" comes from an outdated naming system predating molecular knowledge. It is based on the observation that there is twice as much carbonate per sodium in sodium bicarbonate (NaHCO3) as there is in sodium carbonate (Na2CO3). The modern chemical formulas of these compounds now express their precise chemical compositions which were unknown when the name bi-carbonate of potash was coined.
Uses:
Cooking:
In cooking, baking soda is primarily used in baking as a leavening agent. When it reacts with acid or is heated, carbon dioxide is released, which causes expansion of the batter and forms the characteristic texture and grain in cakes, quick breads, soda bread, and other baked and fried foods. When an acid is used, the acid–base reaction can be generically represented as follows:
NaHCO3 + H+ → Na+ + CO2 + H2O
Acidic materials that induce this reaction include hydrogen phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa, and vinegar. Baking soda may be used together with sourdough, which is acidic, making a lighter product with a less acidic taste. Since the reaction occurs slowly at room temperature, mixtures (cake batter, etc.) can be allowed to stand without rising until they are heated in the oven.
Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of thermal decomposition, releasing carbon dioxide at temperatures above 80 °C (180 °F), as follows:
2 NaHCO3 → Na2CO3 + H2O + CO2
When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO2 is released (one CO2 molecule is formed for every two equivalents of NaHCO3). Additionally, in the absence of acid, thermal decomposition of sodium bicarbonate also produces sodium carbonate, which is strongly alkaline and gives the baked product a bitter, soapy taste and a yellow color.
Baking powder
Baking powder, also sold for cooking, contains around 30% of bicarbonate, and various acidic ingredients that are activated by the addition of water, without the need for additional acids in the cooking medium. Many forms of baking powder contain sodium bicarbonate combined with calcium acid phosphate, sodium aluminium phosphate, or cream of tartar. Baking soda is alkaline; the acid used in baking powder avoids a metallic taste when the chemical change during baking creates sodium carbonate.
Food additive
It is often used in conjunction with other bottled water food additives to add taste. Its European Union E number is E500.
Pyrotechnics
Sodium bicarbonate is one of the main components of the common "black snake" firework. The effect is caused by the thermal decomposition, which produces carbon dioxide gas to produce a long snake-like ash as a combustion product of the other main component, sucrose. Sodium bicarbonate also delays combustion reactions through the release of carbon dioxide and water, both of which are flame retardants, when heated.
Mild disinfectant
It has weak disinfectant properties and it may be an effective fungicide against some organisms.
Fire extinguisher
Sodium bicarbonate can be used to extinguish small grease or electrical fires by being thrown over the fire, as heating of sodium bicarbonate releases carbon dioxide. However, it should not be applied to fires in deep fryers; the sudden release of gas may cause the grease to splatter. Sodium bicarbonate is used in BC dry chemical fire extinguishers as an alternative to the more corrosive monoammonium phosphate in ABC extinguishers. The alkaline nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large-scale fire suppression systems installed in commercial kitchens.
Sodium bicarbonate has several fire-extinguishing mechanisms that act simultaneously. It decomposes into water and carbon dioxide when heated, an endothermic reaction that deprives the fire of heat. In addition, it forms intermediates that can scavenge the free radicals which are responsible for the propagation of fire. With grease fires specifically, it also has a mild saponification effect, producing a soapy foam that can help smother the fire.
Neutralization of acids
Sodium bicarbonate reacts spontaneously with acids, releasing CO2 gas as a reaction product. It is commonly used to neutralize unwanted acid solutions or acid spills in chemical laboratories. It is not appropriate to use sodium bicarbonate to neutralize base even though it is amphoteric, reacting with both acids and bases.
Sports supplement
Sodium bicarbonate is taken as a sports supplement to improve muscular endurance. Studies conducted mostly in males have shown that sodium bicarbonate is most effective in enhancing performance in short-term, high-intensity activities.
Agriculture
Sodium bicarbonate can prevent the growth of fungi when applied on leaves, although it will not kill the fungus. Excessive amounts of sodium bicarbonate can cause discolouration of fruits (two percent solution) and chlorosis (one percent solution). Sodium bicarbonate is also commonly used as a free choice dietary supplement in sheep to help prevent bloat.
Medical uses and health
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn. Its reaction with stomach acid produces salt, water, and carbon dioxide:
NaHCO3 + HCl → NaCl + H2O + CO2(g)
A mixture of sodium bicarbonate and polyethylene glycol dissolved in water and taken orally, is an effective gastrointestinal lavage preparation and laxative prior to gastrointestinal surgery, gastroscopy, etc.
Intravenous sodium bicarbonate in an aqueous solution is sometimes used for cases of acidosis, or when insufficient sodium or bicarbonate ions are in the blood. In cases of respiratory acidosis, the infused bicarbonate ion drives the carbonic acid/bicarbonate buffer of plasma to the left, and thus raises the pH. For this reason, sodium bicarbonate is used in medically supervised cardiopulmonary resuscitation. Infusion of bicarbonate is indicated only when the blood pH is markedly low (< 7.1–7.0).
HCO3− is used for treatment of hyperkalemia, as it will drive K+ back into cells during periods of acidosis. Since sodium bicarbonate can cause alkalosis, it is sometimes used to treat aspirin overdoses. Aspirin requires an acidic environment for proper absorption, and a basic environment will diminish aspirin absorption in cases of overdose. Sodium bicarbonate has also been used in the treatment of tricyclic antidepressant overdose. It can also be applied topically as a paste, with three parts baking soda to one part water, to relieve some kinds of insect bites and stings (as well as accompanying swelling).
Some alternative practitioners, such as Tullio Simoncini, have promoted baking soda as a cancer cure, which the American Cancer Society has warned against due to both its unproven effectiveness and potential danger in use. Edzard Ernst has called the promotion of sodium bicarbonate as a cancer cure "one of the more sickening alternative cancer scams I have seen for a long time".
Sodium bicarbonate can be added to local anaesthetics, to speed up the onset of their effects and make their injection less painful. It is also a component of Moffett's solution, used in nasal surgery.
It has been proposed that acidic diets weaken bones. One systematic meta-analysis of the research shows no such effect. Another also finds that there is no evidence that alkaline diets improve bone health, but suggests that there "may be some value" to alkaline diets for other reasons.
Antacid (such as baking soda) solutions have been prepared and used by protesters to alleviate the effects of exposure to tear gas during protests.
Similarly to its use in baking, sodium bicarbonate is used together with a mild acid such as tartaric acid as the excipient in effervescent tablets: when such a tablet is dropped in a glass of water, the carbonate leaves the reaction medium as carbon dioxide gas (HCO3− + H+ → H2O + CO2↑ or, more precisely, HCO3− + H3O+ → 2 H2O + CO2↑). This makes the tablet disintegrate, leaving the medication suspended and/or dissolved in the water together with the resulting salt (in this example, sodium tartrate).
Personal hygiene
Sodium bicarbonate is also used as an ingredient in some mouthwashes. It has anticaries and abrasive properties. It works as a mechanical cleanser on the teeth and gums, neutralizes the production of acid in the mouth, and also acts as an antiseptic to help prevent infections. Sodium bicarbonate in combination with other ingredients can be used to make a dry or wet deodorant. Sodium bicarbonate may be used as a buffering agent, combined with table salt, when creating a solution for nasal irrigation.
It is used in eye hygiene to treat blepharitis. This is done by adding a teaspoon of sodium bicarbonate to cool water that was recently boiled followed by gentle scrubbing of the eyelash base with a cotton swab dipped in the solution.
Veterinary uses
Sodium bicarbonate is used as a cattle feed supplement, in particular as a buffering agent for the rumen.
Cleaning agent
Sodium bicarbonate is used in a process to remove paint and corrosion called sodablasting. As a blasting medium, sodium bicarbonate is used to remove surface contamination from softer and less resilient substrates such as aluminium, copper, or timber that could be damaged by silica sand abrasive media.
A manufacturer recommends a paste made from baking soda with minimal water as a gentle scouring powder. Such a paste can be useful in removing surface rust because the rust forms a water-soluble compound when in a concentrated alkaline solution. Cold water should be used since hot-water solutions can corrode steel. Sodium bicarbonate attacks the thin protective oxide layer that forms on aluminium, making it unsuitable for cleaning this metal.
A solution of baking soda in warm water will remove the tarnish from silver when the silver is in contact with a piece of aluminium foil.
Baking soda is commonly added to washing machines as a replacement for water softener and to remove odors from clothes. When diluted with warm water, it is also almost as effective in removing heavy tea and coffee stains from cups as sodium hydroxide.
During the Manhattan Project to develop the nuclear bomb in the early 1940s, the chemical toxicity of uranium was an issue. Uranium oxides were found to stick very well to cotton cloth and did not wash out with soap or laundry detergent. However, the uranium would wash out with a 2% solution of sodium bicarbonate. Clothing can become contaminated with toxic dust of depleted uranium (DU), which is very dense, hence it is used for counterweights in a civilian context and in armour-piercing projectiles. DU is not removed by normal laundering; washing with about 6 ounces (170 g) of baking soda in 2 gallons (7.5 L) of water will help wash it out.
Additional Information:
Key Points/Overview
Sodium bicarbonate is also called baking soda and is actually a type of salt.
Some popular uses for baking soda are to help baked goods rise, as an antacid to treat indigestion, and as a general household cleaner.
The FDA regards sodium bicarbonate as “Generally Recognized as Safe” (GRAS) for use as a food additive, active ingredient in toothpaste, and antacid.
Uses & Benefits:
Baking & Food Preparation
Baking soda available in the grocery store is pure, food-grade sodium bicarbonate. Bakers add a small amount of baking soda to the mixture of flour, sugar, eggs, butter and other ingredients in cakes, cookies and other baked goods. The resulting chemical reaction then helps batter expand or rise inside a hot oven. Without baking soda and this chemical reaction, muffins, cakes and breads would fall flat.
Personal Care Products & Medicines
In skin care and personal care products like lotions and bath salts, sodium bicarbonate helps control a product’s acid-base balance to keep it from spoiling. In toothpaste, sodium bicarbonate helps to remove stains from teeth by dislodging tiny particles of food or beverages that can blemish tooth enamel. It is also a common ingredient in deodorant because it can help neutralize smelly, acidic scents.
Sodium bicarbonate also is an active ingredient in antacid products used to relieve heartburn and treat acid indigestion. It works by quickly neutralizing stomach acid and temporarily relieving symptoms of acid reflux.
Cleaning Products & Solvents
Sodium bicarbonate is a common ingredient in cleaning, detergent and degreasing products. In cleaning products, sodium bicarbonate can react with vinegar to create a solution that helps unclog drains or remove grime in ovens. Its slight abrasiveness is extremely efficient for eliminating burnt residues or grease.
The chemical properties of sodium bicarbonate can also help enhance the efficiency of laundry detergent by increasing the water’s pH level, which helps to repel dirt from fibers, leading to fresher laundry.
Safety Information
People have been using sodium bicarbonate for thousands of years. Ancient Egyptians used natural deposits of the mineral to clean their teeth and make paints for writing. In the 1830s, New York bakers began adding sodium bicarbonate and sour milk to dough to make bread.
The U.S. Food and Drug Administration (FDA) states that sodium bicarbonate is GRAS (Generally Recognized as Safe) as a direct food additive. It also states that sodium bicarbonate is generally recognized as safe as an antacid and as an anticaries (tooth decay fighting) active ingredient for over-the-counter use within certain conditions.
The Cosmetic Ingredient Review (CIR), an independent expert panel made up of academic researchers and industry scientists, also has evaluated the scientific data and concluded that sodium bicarbonate is safe as a cosmetic ingredient at current levels of use. In 2005, the CIR Expert Panel considered available new data on sodium bicarbonate and reaffirmed its safety conclusion.