Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#76 Science HQ » Tantalum » 2025-09-04 17:24:28
- Jai Ganesh
- Replies: 0
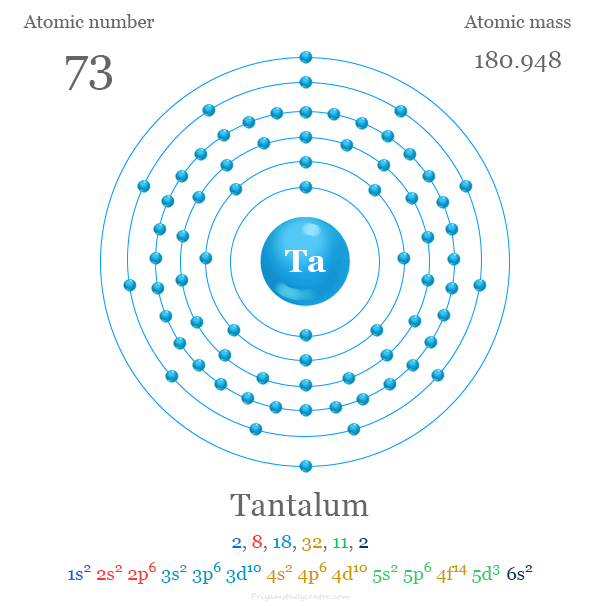
Tantalum
Gist
Tantalum (Ta) is a hard, blue-grey, and corrosion-resistant transition metal with a high melting point and an atomic number of 73. Known for its high reliability and biocompatibility, it's used in electronic components like capacitors, as well as in medical implants, cutting tools, and aircraft parts. The element's name comes from the Greek mythological figure Tantalus, reflecting its insolubility in most acids.
Tantalum is widely used in electronics, primarily for capacitors in portable devices like smartphones and computers, due to its high capacitance and reliability. It also has significant applications in the medical field for surgical implants, prosthetics, and imaging equipment, leveraging its biocompatibility. Additionally, its corrosion resistance and high-temperature properties make it valuable in the chemical industry for heat exchangers and reactor components, and in the aerospace industry for high-temperature components in engines and missiles.
Summary
Tantalum is a chemical element; it has symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite, and coltan.
The chemical inertness and very high melting point of tantalum make it valuable for laboratory and industrial equipment such as reaction vessels and vacuum furnaces. It is used in tantalum capacitors for electronic equipment such as computers. It is being investigated for use as a material for high-quality superconducting resonators in quantum processors.
Details
Tantalum (Ta) is a chemical element, bright, very hard, silver-gray metal of Group 5 (Vb) of the periodic table, characterized by its high density, extremely high melting point, and excellent resistance to all acids except hydrofluoric at ordinary temperatures.
Closely associated with niobium in ores and in properties, tantalum was discovered (1802) by the Swedish chemist Anders Gustaf Ekeberg and named after the mythological character Tantalus because of the tantalizing problem of dissolving the oxide in acids. Due to the great chemical similarity of niobium and tantalum, the establishment of the individual identities of the two elements was very difficult. Tantalum was soon identified with niobium (then called columbium), but in 1844 the German chemist Heinrich Rose demonstrated their distinct characters. Although some of the impure metal was isolated earlier, the Russian chemist Werner Bolton prepared (1903) the first ductile tantalum, which was used briefly as incandescent lamp-filament material.
Relatively rare, tantalum is about as abundant as uranium. It occurs, with niobium, in the columbite–tantalite series (in which columbite [FeNb2O6] and tantalite [FeTa2O6] occur in highly variable ratios) and the pyrochlore–microlite series of minerals. Native tantalum metal with some niobium and traces of manganese and gold occurs sparingly in Russia in placers in the Ural Mountains and possibly the Altai Mountains in Central Asia. Rwanda is the world’s largest extractor of tantalum.
Tantalum is separated from niobium compounds by solvent extraction in a liquid-liquid process and is then reduced to metallic tantalum powder. The massive metal is produced by powder metallurgy techniques. It can also be obtained by either electrolysis of fused salts or reduction of fluoro complexes with a very reactive metal such as sodium. The most important uses for tantalum are in electrolytic capacitors and corrosion-resistant chemical equipment. Tantalum capacitors have the highest capacitance per unit volume of any capacitors and are used extensively in miniaturized electrical circuitry. Other uses include getters and components in electron tubes, rectifiers, and prosthetic devices.
Tantalum is chemically much like niobium because both have similar electronic configurations and because the radius of the tantalum ion is nearly the same as that of niobium as a result of the lanthanoid contraction. Tantalum is usually in the +5 oxidation state in its compounds; lower oxidation states, especially from +2 to +4, have been prepared. Tantalum compounds are relatively unimportant commercially, although the carbide TaC is used in cemented-carbide tools for machining hard metals. Nearly all naturally occurring tantalum is in one stable isotope, tantalum-181. However, a small amount, 0.012 percent, is tantalum-180, which has the unusual property of being found in its excited state. The tantalum-180 excited state has a half-life of more than 1.2 × 1015 years; the ground state (the lowest energy state) has a half-life of only 8.154 hours.
Element Properties
atomic number : 73
atomic weight : 180.94788
melting point : 2,996 °C (5,425 °F)
boiling point : 5,425 °C (9,797 °F)
specific gravity : 16.6 at 20 °C (68 °F)
oxidation states : +2, +3, +4, +5.
Additional Information:
Appearance
A shiny, silvery metal that is very resistant to corrosion.
Uses
One of the main uses of tantalum is in the production of electronic components. An oxide layer which forms on the surface of tantalum can act as an insulating (dielectric) layer. Because tantalum can be used to coat other metals with a very thin layer, a high capacitance can be achieved in a small volume. This makes tantalum capacitors attractive for portable electronics such as mobile phones.
Tantalum causes no immune response in mammals, so has found wide use in the making of surgical implants. It can replace bone, for example in skull plates; as foil or wire it connects torn nerves; and as woven gauze it binds abdominal muscle.
It is very resistant to corrosion and so is used in equipment for handling corrosive materials. It has also found uses as electrodes for neon lights, AC/DC rectifiers and in glass for special lenses.
Tantalum alloys can be extremely strong and have been used for turbine blades, rocket nozzles and nose caps for supersonic aircraft.
Biological role
Tantalum has no known biological role. It is non-toxic.
Natural abundance
Tantalum is sometimes, but only rarely, found uncombined in nature. It occurs mainly in the mineral columbite-tantalite, which also contains other metals including niobium. It is mined in many places including Australia, Canada and Brazil. There are several complicated steps involved in separating the tantalum from the niobium. A lot of tantalum is obtained commercially as a by-product of tin extraction.
#77 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-04 16:53:56
Hi,
#10545. What does the term in Biology Cell wall mean?
#10546. What does the term in Biology Centriole mean?
#78 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-04 16:38:36
Hi,
#5735. What does the noun manifestation mean?
#5736. What does the noun manifesto mean?
#79 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-04 16:04:31
Hi,
#2459. What does the medical term Landau reflex mean?
#80 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-04 15:49:14
Hi,
#9726.
#81 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-04 15:18:37
Hi,
#6232.
#83 Re: This is Cool » Miscellany » 2025-09-04 14:33:58
2380) Penguin
Gist
A penguin is a flightless, black-and-white seabird found almost exclusively in the Southern Hemisphere, known for its adaptations to marine life, including flippers for swimming, dense feathers for warmth, and a diet of fish and krill. While most live in cold Antarctic or sub-Antarctic regions, the Galápagos penguin lives near the equator. Penguins are excellent swimmers and divers, using their short, paddle-like wings to propel themselves through the water, and their contrasting dark backs and white bellies provide camouflage.
Penguins primarily live in the Southern Hemisphere, inhabiting cold environments like Antarctica, subantarctic islands, and temperate coasts in countries such as Australia, New Zealand, and South Africa. While most species are found in these southern regions, the Galápagos penguin is an exception, living on islands near the equator in the warm waters off the coast of Ecuador. All penguins require proximity to the ocean for food and to raise their young on land.
Summary
Penguins are a group of aquatic flightless birds from the family Spheniscidae of the order Sphenisciformes. They live almost exclusively in the Southern Hemisphere. Only one species, the Galápagos penguin, is equatorial, with a small portion of its population extending slightly north of the equator (within a quarter degree of latitude). Highly adapted for life in the ocean water, penguins have countershaded dark and white plumage and flippers for swimming. Most penguins feed on krill, fish, squid and other forms of sea life which they catch with their bills and swallow whole while swimming. A penguin has a spiny tongue and powerful jaws to grip slippery prey.
They spend about half of their lives on land and the other half in the sea. The largest living species is the emperor penguin (Aptenodytes forsteri): on average, adults are about 1.1 m (3 ft 7 in) tall and weigh 35 kg (77 lb). The smallest penguin species is the little blue penguin (Eudyptula minor), also known as the fairy penguin, which stands around 30–33 cm (12–13 in) tall and weighs 1.2–1.3 kg (2.6–2.9 lb). Today, larger penguins generally inhabit colder regions, and smaller penguins inhabit regions with temperate or tropical climates. Some prehistoric penguin species were enormous: as tall or heavy as an adult human. There was a great diversity of species in subantarctic regions, and at least one giant species in a region around 2,000 km south of the equator 35 mya, during the Late Eocene, a climate decidedly warmer than today.
Details
A penguin is (order Sphenisciformes), any of 18–21 species of flightless marine birds that live only in the Southern Hemisphere. The majority of species live not in Antarctica but rather between latitudes 45° and 60° S, where they breed on islands. A few penguins inhabit temperate regions, and one, the Galapagos penguin (Spheniscus mendiculus), lives at the Equator.
General features:
The world's deepest-diving birds
The world's deepest-diving birdsEmperor penguins (Aptenodytes forsteri) live in colonies along the coastline of Antarctica. They are capable of diving to depths of about 550 metres (1,800 feet) in search of food, which makes them Earth's deepest-diving birds.
The stocky, short-legged appearance of penguins has endeared them to people worldwide. They range from about 35 cm (14 inches) in height and approximately 1 kg (about 2 pounds) in weight in the blue, or fairy, penguin (Eudyptula minor) to 115 cm (45 inches) and 25 to 40 kg (55 to 90 pounds) in the emperor penguin (Aptenodytes forsteri). Most are black on the back and white below, often with lines of black across the upper breast or spots of white on the head. Colour is rare, being limited to red or yellow irises of the eye in some species; red beaks or feet in a few; yellow brow tufts in the three species of Eudyptes; and orange and yellow on the head, neck, and breast in the emperor and king (A. patagonica) penguins.
The total populations of some species, such as the emperor, are estimated in the hundreds of thousands, but most species of smaller penguins certainly run into the millions. Immense island breeding colonies, some teeming with hundreds of thousands of nesting pairs, represent a large potential food resource, but the economic importance of penguins is negligible. Nineteenth-century whalers and seal hunters visited some colonies for meat and eggs, and a penguin oil industry once took large numbers of the birds. By the early 20th century, however, this exploitation was no longer profitable, and most colonies were left alone or actively protected. Some species are now increasing in numbers, apparently as a result of the mid-20th century’s decimation of Antarctic whales, which compete with penguins for the krill (minute crustaceans) on which both feed. Penguin populations, however, are highly vulnerable to changes in climate and ocean temperature, including recent global warming. Penguins also are very sensitive to depletion of local fish populations by humans.
Natural history:
Reproduction
Many features of the penguin life cycle vary with body size and geographic distribution; the chronology of breeding may also vary within a species in relation to latitude. The majority of species breed only once each year. Certain species, such as the African penguin (Spheniscus demersus), probably other members of this genus, and the blue penguin, breed twice a year. The king penguin breeds twice in three years. One egg is laid by the emperor and king penguins; all others lay two or occasionally three. Most penguins begin breeding in the austral (southern) spring or summer. King penguins are on a 14- to 18-month cycle, and the timing of an individual pair depends on the success or failure of the previous breeding attempt. Some populations of the gentoo penguin (Pygoscelis papua) also breed in winter. The breeding of the emperor penguin begins in autumn, apparently timed so that the long developmental period will produce the young in midsummer, when their chances of survival are greatest.
The gentoo, which has a circumpolar distribution, is notable for its lack of synchrony among populations, but otherwise its breeding schedule is essentially comparable to that of most other species. In the Crozet Islands off southern Africa, for example, egg laying takes place in July. The two eggs are incubated for 35 or 36 days, and the rearing of the chick takes two months. The last immature birds go to sea in January.
Many types of visual and vocal displays are employed between the arrival of the birds at the colony and their departure. Courtship calls are used during pairing and to a lesser degree during the succeeding phases of breeding. There are marked vocal differences between sexes in the emperor penguin and the king penguin and less-marked dimorphisms in some other species. Upon arrival at the colony each bird returns to the nest that it left the previous year and generally rejoins its mate of the previous year, unless the death of the latter forces it to choose another partner. This applies even to the emperor penguin, which is capable of finding its mate despite the absence of a nest and the large size of the colony.
The displays that occur with the reassembly of the colony and the finding of mates, as well as those preceding copulation, are quite similar among the majority of species, but the accompanying vocalizations are more diverse. Various species have been described as trumpeting, croaking, cackling, and cooing; members of the genus Spheniscus are called jackass penguins for the braying sounds they make. The behaviour of experienced older birds is more elaborate and more effective than that of younger individuals. For example, Adélie penguins (Pygoscelis adeliae) may return to the reproductive colony from their third year onward but do not breed successfully until their fifth or sixth year.
Incubation of eggs is performed by both sexes in all species except the emperor penguin, in which it is done exclusively by the male, and it is begun immediately after egg laying. With the advent of incubation, the bustle and myriad cries that characterized mating give way to quiet and inactivity. Faulty incubation behaviour by inexperienced birds frequently results in the abandonment or breakage of eggs. The mortality rate (eggs and chicks) is very important at the egg stage, varying from year to year depending on climatic conditions, the percentage of young birds in the reproductive population, and the pressure of predation. In general, mortality (eggs and chicks) is from 40 to 80 percent of the eggs laid. In coastal colonies predators include, in order of importance: skuas, sheathbills, and the giant petrel. On the Australian, African, and South American continents, the nocturnal habits of certain penguins and the fact that they nest in burrows substantially limit predation, which is mostly by gulls and man.
Following egg laying, the female usually departs for the sea to feed, returning to relieve her mate after about 10 to 20 days. Thereafter, father and mother alternate in periods of a week or two. The female emperor penguin, however, must often walk 80 to 160 km (50 to 100 miles) from the colony to the sea and does not return until the end of the incubation period. During the 64-day incubation period, which extends through the height of the Antarctic winter, the male emperor penguin incubates the egg, holding it on his feet and living on stored fat reserves. During violent winter storms, members of the colony gather for mutual protection from wind and cold in tightly packed crowds called huddles.
Emergence from the shell takes 24 to 48 hours, during which the brooding parent is particularly irritable. The chick shows feeding behaviour immediately on hatching, receiving a regurgitated “soup” of crustaceans or fish by inserting its bill into the open mouth of the parent. During its early days the young bird is sheltered under the body of one of its parents, who take turns foraging and brooding. Growing larger, the young bird remains at a parent’s side, although the fledgling is able to maintain its body heat and move about alone. The chick then joins 100 or more of its contemporaries in a nursery group, or crèche, sometimes guarded by a few adults, while both its parents forage at sea. Upon returning with food, the parent calls its chick from the crèche and is able to distinguish it from other chicks (which frequently respond) by voice and appearance.
During the breeding season the number of “unemployed” adults in the colony increases with the addition of those who have lost eggs or chicks. In emperor penguin colonies, these unemployed birds often interfere with parents that have young and cause increased mortality. During the crèche stage the fuzzy down that has covered the chick since hatching is replaced by a coat of short stiff feathers, which are similar to those of the adult but usually somewhat different in colour. Once this molt is complete, the juvenile leaves the colony to seek its own food at sea.
The period of growth of the young bird from hatching to complete independence varies from two months, in the smallest species of the genus Eudyptula, to 51/2 months in the emperor and 12 to 14 months in the king penguin.
Adult penguins molt all of their feathers once a year following the breeding period. While in molt the bird is unable to enter the water and instead withdraws to a communal molting site usually situated in a sheltered area away from the colony. The duration of the molt varies from about two weeks in small species to more than a month in the larger ones.
The principal enemies of penguins at sea are the leopard seal and the killer whale (orca). Seals also take penguins near Australia, New Zealand, and other subantarctic regions.
Locomotion and orientation
Penguins are adapted for rapid locomotion in water, in which the wings, or flippers, are used for propulsion; the birds “fly” underwater. When moving at high speed, they frequently leave the water in leaps that may carry them a metre or more through the air; it is during this time that they breathe.
On land, penguins are much more awkward, even amusing, as they rock from side to side as they walk. Despite their short legs, however, penguins can run with surprising speed. Some, such as the northern rockhopper (Eudyptes moseleyi), the southern rockhopper (E. chrysocome), and Adélie penguins, move among rocks with agility, using the flippers for balance. On snow or ice, many penguins “toboggan,” sliding on the belly as they propel themselves with the feet and flippers. The flippers, along with the beak, are the prime weapons in defense and attack.
Scientists have long wondered how penguins are able to find their way back to their colonies from far out at sea, where currents may have carried them great distances. Also perplexing is how they are able to direct themselves correctly on land in the absence of clear-cut landmarks. Studies of penguins transported to the interior of Antarctica have found that they are able to find their way back to the ocean by using the sun as a directional aid. It is probable that the same means of orientation is used at sea. Upon approaching the coast they are able to recognize features of the shoreline and ocean bottom.
Food habits
The type of food utilized varies with the species, the geographic region, and the time of year. Most of the smaller southern penguins feed primarily upon krill, which attain high densities in the rich, well-oxygenated Antarctic waters. Cephalopods (squid and cuttlefish) and small fishes may form substantial fractions of the food, and in a few, such as the African penguin, fish is the basic element of the diet. The total weight of food consumed by a large penguin colony is prodigious, often exceeding several tons per day.
Form and function
The penguins are highly specialized for their flightless aquatic existence. The feet are located much farther back than those of other birds, with the result that the bird carries itself mostly upright; its walk can thus be described as plantigrade (i.e., on the soles). The sole comprises the whole foot instead of just the toes, as in other birds. The most notable characteristic of the group is the transformation of the forelimb into a paddle. This is accompanied by a body morphology particularly adapted to movement in a liquid medium. The thoracic (rib) cage is well developed, and the sternum bears a pronounced keel for the attachment of the pectoral muscles, which move the flippers. The flipper has the same skeletal base as the wing of flying birds but with its elements shortened and flattened, producing a relatively rigid limb covered with very short feathers—an ideal organ for rapid propulsion. The body plumage likewise consists of very short feathers, which minimize friction and turbulence. The density of the plumage and the layer of air that it retains provide almost complete insulation of the body.
Insulation of the bird’s body is particularly important for Antarctic species that live in water that is always below 0 °C (32 °F). The cooling power of seawater at −1.9 °C (28.6 °F) is equal to that of a temperature of −20 °C (−4 °F) with a wind of 110 km (70 miles) per hour. The skin is insulated by a layer of air trapped under the plumage, and the only bare skin in direct contact with the water is that of the feet. In the case of the emperor penguin on land, the feet are in almost constant contact with ice. The skin temperature is in the neighbourhood of 0 °C, and snow does not melt upon contact. This is possible because of remarkable anatomical arrangements in the lower limb, whereby closely adjacent arteries and veins form a system of heat exchange between opposing flows of blood. This arrangement permits cooled blood from the feet to absorb heat from outflowing blood, providing maximum economy of heat consistent with the functioning of the foot.
Like other seabirds, penguins have salt glands that enable them to ingest salt from seawater. Excess chloride is excreted in the form of a solution the concentration of which is greater than that of seawater. These glands are located above the eyes and are already functional in the young chick, which begins to consume food of marine origin from its first day of life.
Recent research has shown that the species most isolated geographically, such as the emperor penguin, can be subject to diseases. Some, such as the Adélie penguin, carry in their bodies trace amounts of pollutants, albeit in lower quantities than are found in many birds that live closer to humans.
Evidence from paleontology indicates that the penguins and the order Procellariiformes (albatrosses, shearwaters, and petrels) had a common origin. Both groups are represented by well-defined fossils dating to about 50 million years ago. The flightless sphenisciform line produced a number of distinctive side branches, all recognizably penguins, some giant in size. All of the fossil remains of penguins have been collected within the zone of the present-day distribution of the Sphenisciformes. Some apparently lived in warmer regions than do most of today’s penguins.
Phylogenetic analysis of living and fossil penguins shows that the group evolved a large body size early in its history. For example, two of the largest fossil penguins known—Icadyptes, which stood some 1.5 metres (about 5 feet) tall, and math, which stood about 1.8 metres (6 feet) tall—date to the Eocene Epoch (56 million to 33.9 million years ago). Living penguins make up a separate lineage characterized by smaller, highly aquatic species that began about 8 million years ago. The comparatively small size of living penguins is thus a geologically recent phenomenon that postdates the original radiation of giant penguins.
Additional Information
Penguins are a family of 17 to 19 species of birds that live primarily in the Southern Hemisphere. They include the tiny blue penguins of Australia and New Zealand, the majestic emperor penguins of Antarctica and king penguins found on many sub- Antarctic islands, the endangered African penguin and the Galápagos penguin—the only penguin to be found north of the equator.
Though they are birds, penguins have flippers instead of wings. They cannot fly and on land they waddle walking upright—though when snow conditions are right they will slide on their bellies. In the water they are expert swimmers and divers, and some species can reach speeds of up to 15 miles per hour. The penguin’s distinctive coloring—black body with white belly—helps camouflage the bird in the water as it searches for meals of small shrimp, fish, crabs and squid.

#84 Re: Exercises » Compute the solution: » 2025-09-04 13:49:05
Hi,
2574.
#85 Re: Exercises » Compute the solution: » 2025-09-03 23:14:17
Hi,
You are correct! Good work!
Post once a day in 'Compute the solution'.
2573.
#86 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-09-03 22:55:46
2232) John Hopfield
Gist:
Work
When we talk about artificial intelligence, we often mean machine learning using artificial neural networks. This technology was originally inspired by the structure of the brain. In an artificial neural network, the brain’s neurons are represented by nodes that have different values. In 1982, John Hopfield invented a network that uses a method for saving and recreating patterns. He found inspiration in physics' models of how many small parts in a system affect the system as a whole. The invention became important in, for example, image analysis.
Summary
John J. Hopfield (born July 15, 1933, Chicago, Illinois, U.S.) is an American physicist who was awarded the 2024 Nobel Prize in Physics for his work with neural networks. He shared the prize with British-Canadian cognitive psychologist Geoffrey Hinton. At age 91, he became the third oldest person to receive a Nobel Prize, after John B. Goodenough (in chemistry in 2019, at age 97) and Arthur Ashkin (in physics in 2018, at age 96).
Hopfield, born to parents who were physicists, took an interest in the subject from an early age; he would later describe physics as not just a subject matter but rather a way in which to view the physical world. His parents encouraged his inquisitive nature, and he was allowed to take apart various items in his home, which would sometimes necessitate his father’s help in piecing them back together.
“The central idea was that the world is understandable, that you should be able to take anything apart, understand the relationships between its constituents, do experiments, and on that basis be able to develop a quantitative understanding of its behavior.” —Hopfield describing how he viewed physics.
In 1954 Hopfield received a bachelor’s degree in physics at Swarthmore College. Four years later he received a Ph.D. in physics from Cornell University. Hopfield then joined AT&T Bell Laboratories, where he worked on solid-state physics research. Starting in 1964, he was a professor of physics at Princeton University. By the end of his time there, Hopfield had moved away from physics to problems in chemistry and biology, and he then became a professor of those subjects at the California Institute of Technology (Caltech) in 1980.
Hopfield became interested in how the brain works and, specifically, how neurons work together. In 1982 he proposed a simple network that would explain how memories are stored in the brain. Neurons could be in either one of two states: 0, “not firing,” or 1, “firing at maximum rate.” The connections between the neurons had a certain strength. By analogy with the mathematics that describes magnetic systems, Hopfield was able to describe an “energy” for the system that was −1 times a sum over all the pairs of neurons with the terms of the sum being the strength of the connection between two neurons times the value of the state of each neuron. The strength of the connection term was set so that memories would be in the lowest energy state of the system.
Hopfield extended his model (which came to be called the Hopfield network) to have more complex features. For example, the neurons could be in any state, not just 0 and 1. Thus, the model could store more complex information. Hopfield and American neuroscientist David Tank used such a network to solve the traveling salesman problem.
At Caltech, Hopfield and his colleague Carver Andress Mead created a new interdisciplinary program in computation and neural systems in 1986. Hopfield returned to Princeton, as a professor of molecular biology, in 1997 and there helped create the Princeton Neuroscience Institute. He retired and became a professor emeritus in 2008.
Hopfield has won multiple awards and held many prestigious positions during his career. He won the Buckley Prize (with D.G. Thomas), awarded by the American Physical Society, in 1969 for his work on light-emitting diodes (LEDs). He won a MacArthur grant in 1983. He was named California Scientist of the Year in 1991 and won the Albert Einstein Award of Science in 2005. Hopfield also was awarded a Guggenheim fellowship (just as his father had been, 40 years earlier) in 1968.
Details
John Joseph Hopfield (born July 15, 1933) is an American physicist and emeritus professor of Princeton University, most widely known for his study of associative neural networks in 1982. He is known for the development of the Hopfield network. Before its invention, research in artificial intelligence (AI) was in a decay period or AI winter, Hopfield's work revitalized large-scale interest in this field.
In 2024 Hopfield, along with Geoffrey Hinton, was awarded the Nobel Prize in Physics for "foundational discoveries and inventions that enable machine learning with artificial neural networks." He has been awarded various major physics awards for his work in multidisciplinary fields including condensed matter physics, statistical physics and biophysics.
Biography:
Early life and education
John Joseph Hopfield was born in 1933 in Chicago to physicists John Joseph Hopfield (born in Poland as Jan Józef Chmielewski) and Helen Hopfield (née Staff).
Hopfield received a Bachelor of Arts with a major in physics from Swarthmore College in Pennsylvania in 1954 and a Doctor of Philosophy in physics from Cornell University in 1958. His doctoral dissertation was titled "A quantum-mechanical theory of the contribution of excitons to the complex dielectric constant of crystals". His doctoral advisor was Albert Overhauser.
Career
He spent two years in the theory group at Bell Laboratories working on optical properties of semiconductors working with David Gilbert Thomas and later on a quantitative model to describe the cooperative behavior of hemoglobin in collaboration with Robert G. Shulman. Subsequently he became a faculty member at University of California, Berkeley (physics, 1961–1964), Princeton University (physics, 1964–1980), California Institute of Technology (Caltech, chemistry and biology, 1980–1997) and again at Princeton (1997–), where he is the Howard A. Prior Professor of Molecular Biology, emeritus.
In 1976, he participated in a science short film on the structure of the hemoglobin, featuring Linus Pauling.
From 1981 to 1983 Richard Feynman, Carver Mead and Hopfield gave a one-year course at Caltech called "The Physics of Computation". This collaboration inspired the Computation and Neural Systems PhD program at Caltech in 1986, co-founded by Hopfield.
His former PhD students include Gerald Mahan (PhD in 1964), Bertrand Halperin (1965), Steven Girvin (1977), Terry Sejnowski (1978), Erik Winfree (1998), José Onuchic (1987), Li Zhaoping (1990) and David J. C. MacKay (1992).
Work
In his doctoral work of 1958, he wrote on the interaction of excitons in crystals, coining the term polariton for a quasiparticle that appears in solid-state physics. He wrote: "The polarization field 'particles' analogous to photons will be called 'polaritons'." His polariton model is sometimes known as the Hopfield dielectric.
From 1959 to 1963, Hopfield and David G. Thomas investigated the exciton structure of cadmium sulfide from its reflection spectra. Their experiments and theoretical models allowed to understand the optical spectroscopy of II-VI semiconductor compounds.
Condensed matter physicist Philip W. Anderson reported that John Hopfield was his "hidden collaborator" for his 1961–1970 works on the Anderson impurity model which explained the Kondo effect. Hopfield was not included as a co-author in the papers but Anderson admitted the importance of Hopfield's contribution in various of his writings.
William C. Topp and Hopfield introduced the concept of norm-conserving pseudopotentials in 1973.
In 1974 he introduced a mechanism for error correction in biochemical reactions known as kinetic proofreading to explain the accuracy of DNA replication.
Hopfield published his first paper in neuroscience in 1982, titled "Neural networks and physical systems with emergent collective computational abilities" where he introduced what is now known as Hopfield network, a type of artificial network that can serve as a content-addressable memory, made of binary neurons that can be 'on' or 'off'. He extended his formalism to continuous activation functions in 1984. The 1982 and 1984 papers represent his two most cited works. Hopfield has said that the inspiration came from his knowledge of spin glasses from his collaborations with P. W. Anderson.
Together with David W. Tank, Hopfield developed a method in 1985–1986 for solving discrete optimization problems based on the continuous-time dynamics using a Hopfield network with continuous activation function. The optimization problem was encoded in the interaction parameters (weights) of the network. The effective temperature of the analog system was gradually decreased, as in global optimization with simulated annealing.
Hopfield is one of the pioneers of the critical brain hypothesis, he was the first to link neural networks with self-organized criticality in reference to the Olami–Feder–Christensen model for earthquakes in 1994. In 1995, Hopfield and Andreas V. Herz showed that avalanches in neural activity follow power law distribution associated to earthquakes.
The original Hopfield networks had a limited memory, this problem was addressed by Hopfield and Dimitry Krotov in 2016. Large memory storage Hopfield networks are now known as modern Hopfield networks.
Views on artificial intelligence
In March 2023, Hopfield signed an open letter titled "Pause Giant AI Experiments", calling for a pause on the training of artificial intelligence (AI) systems more powerful than GPT-4. The letter, signed by over 30,000 individuals including AI researchers Yoshua Bengio and Stuart Russell, cited risks such as human obsolescence and society-wide loss of control.
Upon being jointly awarded the 2024 Nobel Prize in Physics, Hopfield revealed he was very unnerved by recent advances in AI capabilities, and said "as a physicist, I'm very unnerved by something which has no control". In a followup press conference in Princeton University, Hopfield compared AI with discovery of nuclear fission, which led to nuclear weapons and nuclear power.

#87 Dark Discussions at Cafe Infinity » Climbing Quotes - II » 2025-09-03 17:47:59
- Jai Ganesh
- Replies: 0
Climbing Quotes - II
1. When you go to the mountains, you see them and you admire them. In a sense, they give you a challenge, and you try to express that challenge by climbing them. - Edmund Hillary
2. When you're climbing at high altitudes, life can get pretty miserable. - Edmund Hillary
3. I've always hated the danger part of climbing, and it's great to come down again because it's safe. - Edmund Hillary
4. When I was climbing, I built up a close relationship with the Sherpa people. - Edmund Hillary
5. Many people have been getting too casual about climbing Everest. I forecast a disaster many times. - Edmund Hillary
6. I enjoyed climbing with other people, good friends, but I did quite a lot of solo climbing, too. - Edmund Hillary
7. If I wished to do something, even if I couldn't find anyone who wanted to make the effort with me, I would go out solo climbing. I did find solo climbing very challenging and a little frightening. You knew that you were completely on your own, and you had to overcome all the problems and possible dangers. - Edmund Hillary.
#88 Science HQ » Hafnium » 2025-09-03 17:22:31
- Jai Ganesh
- Replies: 0
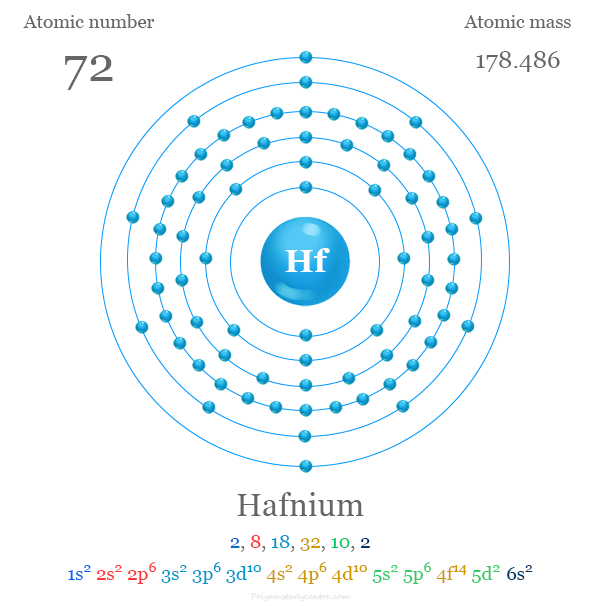
Hafnium
Gist
Hafnium (Hf) is a lustrous, silvery-gray, refractory chemical element with atomic number 72, similar to zirconium, and is used in alloys for high-temperature applications and as control rods in nuclear reactors due to its neutron absorption properties. Discovered in 1923 by George de Hevesy and Dirk Coster, hafnium is obtained from zirconium minerals and is characterized by its high melting point, excellent corrosion resistance, and its use in microprocessors and ceramics.
Hafnium is used in high-performance superalloys for the aerospace industry, as control rods in nuclear reactors due to its neutron-absorbing properties, and in microelectronics for its oxide's use as a high-k dielectric in smaller, more efficient transistors and integrated circuits. Other applications include high-temperature ceramics and refractories, electrodes for plasma cutting torches, and components in incandescent lamps.
Summary
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.
Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten.
Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear reactors.
Details
Hafnium (Hf) is a chemical element (atomic number 72), metal of Group 4 (IVb) of the periodic table. It is a ductile metal with a brilliant silvery lustre. The Dutch physicist Dirk Coster and the Hungarian Swedish chemist George Charles von Hevesy discovered (1923) hafnium in Norwegian and Greenland zircons by analyzing their X-ray spectra. They named the new element for Copenhagen (in New Latin, Hafnia), the city in which it was discovered. Hafnium is dispersed in Earth’s crust to the extent of three parts per million and is invariably found in zirconium minerals up to a few percent compared with zirconium. For example, the minerals zircon, ZrSiO4 (zirconium orthosilicate), and baddeleyite, which is essentially pure zirconium dioxide, ZrO2, generally have a hafnium content that varies from a few tenths of 1 percent to several percent. Altered zircons, like some alvites and cyrtolites, products of residual crystallization, show greater percentages of hafnium (up to 17 percent hafnium oxide in cyrtolite from Rockport, Mass., U.S.). Commercial sources of hafnium-bearing zirconium minerals are found in beach sands and river gravel in the United States (principally Florida), Australia, Brazil, western Africa, and India. Hafnium vapour has been identified in the Sun’s atmosphere.
Ion-exchange and solvent-extraction techniques have supplanted fractional crystallization and distillation as the preferred methods of separating hafnium from zirconium. In the procedure, crude zirconium tetrachloride is dissolved in an aqueous solution of ammonium thiocyanate, and methyl isobutyl ketone is passed countercurrent to the aqueous mixture, with the result that the hafnium tetrachloride is preferentially extracted. The metal itself is prepared by magnesium reduction of hafnium tetrachloride (Kroll process, which is also used for titanium) and by the thermal decomposition of tetraiodide (de Boer–van Arkel process).
For some purposes separation of the two elements is not important; zirconium containing about 1 percent of hafnium is as acceptable as pure zirconium. In the case of the largest single use of zirconium, however, namely, as a structural and cladding material in nuclear reactors, it is essential that the zirconium be essentially free of hafnium, because the usefulness of zirconium in reactors is based on its extremely low absorption cross section for neutrons. Hafnium, on the other hand, has an exceptionally high cross section, and accordingly even slight hafnium contamination nullifies the intrinsic advantage of the zirconium. Because of its high neutron-capture cross section and its excellent mechanical properties, hafnium is used for fabricating nuclear-control rods.
Hafnium produces a protective film of oxide or nitride upon contact with air and thus has high corrosion resistance. Hafnium is fairly resistant to acids and is best dissolved in hydrofluoric acid, in which procedure the formation of anionic fluoro complexes is important in stabilizing the solution. At normal temperatures hafnium is not particularly reactive but becomes quite reactive with a variety of nonmetals at elevated temperatures. It forms alloys with iron, niobium, tantalum, titanium, and other transition metals. The alloy tantalum hafnium carbide (Ta4HfC5), with a melting point of 4,215 °C (7,619 °F), is one of the most refractory substances known.
Hafnium is chemically similar to zirconium. Both transition metals have similar electronic configurations, and their ionic radii (Zr4+, 0.74 Å, and Hf4+, 0.75 Å) and atomic radii (zirconium, 1.45 Å, and hafnium, 1.44 Å) are nearly identical because of the influence of the lanthanoid contraction. In fact, the chemical behaviour of these two elements is more similar than for any other pair of elements known. Although the chemistry of hafnium has been studied less than that of zirconium, the two are so similar that only very small quantitative differences—for example, in solubilities and volatilities of compounds—would be expected in cases that have not actually been investigated. Natural hafnium is a mixture of six stable isotopes: hafnium-174 (0.2 percent), hafnium-176 (5.2 percent), hafnium-177 (18.6 percent), hafnium-178 (27.1 percent), hafnium-179 (13.7 percent), and hafnium-180 (35.2 percent).
The most important respect in which hafnium differs from titanium is that lower oxidation states are of minor importance; there are relatively few compounds of hafnium in other than its tetravalent states. (However, a few trivalent compounds are known.) The increased size of the atoms makes the oxides more basic and the aqueous chemistry somewhat more extensive and permits the attainment of coordination numbers 7 and, quite frequently, 8 in a number of hafnium compounds.
Element Properties
atomic number : 72
atomic weight : 178.486
melting point : 2,227 °C (4,041 °F)
boiling point : 4,603 °C (8,317 °F)
specific gravity : 13.31 (20 °C)
oxidation state : +4.
Additional Information:
Appearance
A shiny, silvery metal that resists corrosion and can be drawn into wires.
Uses
Hafnium is a good absorber of neutrons and is used to make control rods, such as those found in nuclear submarines. It also has a very high melting point and because of this is used in plasma welding torches.
Hafnium has been successfully alloyed with several metals including iron, titanium and niobium.
Hafnium oxide is used as an electrical insulator in microchips, while hafnium catalysts have been used in polymerisation reactions.
Biological role
Hafnium has no known biological role, and it has low toxicity.
Natural abundance
Most zirconium ores contain around 5% hafnium. The metal can be prepared by reducing hafnium tetrachloride with sodium or magnesium.
#89 Re: This is Cool » Miscellany » 2025-09-03 16:53:36
2379) Bluff (Geography)
Gist
In geography, a bluff is a high, steep slope or cliff that typically overlooks a body of water, formed by the erosion of the outer bank of a river's meander or along a coastline. The term can also refer to a clump of trees, particularly willows or poplars, found on the flat Canadian prairies, which is a different meaning from the geological landform.
Although any cliff with a broad, steep face may be called a bluff, the term bluff refers to a high bank or bold headland with a broad, precipitous, sometimes rounded cliff face. Bluffs can overlook either a plain or a body of water, especially on the outside curve of a stream meander.
Summary
In geography and geology, a cliff or rock face is an area of rock which has a general angle defined by the vertical, or nearly vertical. Cliffs are formed by the processes of weathering and erosion, with the effect of gravity. Cliffs are common on coasts, in mountainous areas, escarpments and along rivers. Cliffs are usually composed of rock that is resistant to weathering and erosion. The sedimentary rocks that are most likely to form cliffs include sandstone, limestone, chalk, and dolomite. Igneous rocks such as granite and basalt also often form cliffs.
An escarpment (or scarp) is a type of cliff formed by the movement of a geologic fault, a landslide, or sometimes by rock slides or falling rocks which change the differential erosion of the rock layers.
Most cliffs have some form of scree slope at their base. In arid areas or under high cliffs, they are generally exposed jumbles of fallen rock. In areas of higher moisture, a soil slope may obscure the talus. Many cliffs also feature tributary waterfalls or rock shelters. Sometimes a cliff peters out at the end of a ridge, with mushroom rocks or other types of rock columns remaining. Coastal erosion may lead to the formation of sea cliffs along a receding coastline.
The British Ordnance Survey distinguishes between cliffs (continuous line along the topper edge with projections down the face) and outcrops (continuous lines along lower edge).
Details:
What Is A Bluff In Geography?
A bluff is a ridge of land that extends into the air. The term is commonly used in geography to refer to high ground. Bluffs are often situated at the foot of steep cliffs and mountains.
A bluff is a steep hill or cliff, especially one that appears close to its base. It can also refer to an area where the slope is so steep that it appears to be an unbroken cliff.
A typical bluff is situated at the foot of steep cliffs and mountains.
Why Is A Bluff Called A Bluff?
A bluff is a cliff, small hill, or other elevation on the side of a mountain or hill, that rises steeply from the ground, usually without trees or shrubs.
Bluffs are called bluff because they appear to be higher than they actually are. From the distance, they seem to be steeper than they actually are.
How Is A Bluff Formed?
How a bluff is formed in geography is a question that has no clear answer. It all depends on the environment that the bluff was formed in and other factors like how deep it is and how much erosion takes place.
When a bluff is created naturally, it forms by the force of wind and water along with the effect of gravity on the earth’s surface. This natural process mainly happens in steep places where there are high elevation changes.
As the slope becomes too steep for the water or wind to erode it, it builds up and forms a bluff.
Bluffs are also formed by the slow movement of glaciers, ice sheets, and permafrost. Glaciers push large amounts of rock off of mountainside through grinding and crushing – this produces large boulders that act as rubble dams blocking the flow of streams.
How To Identify A Bluff In Geography?
Bluffs are natural formations that can be found near water and mountain ranges. They take many forms and typically look like a hill reaching the sky or a mound of rock.
There are many ways to identify a bluff. A bluff is usually steep enough to have a significant decline or incline on both sides of the top.
The bluffs are not just steep but have uneven, sharp sawtooth edges that provide cover for any assailant. The bluff is also made up of large stones that have become cemented together over time and are covered with moss and vegetation, which further provides cover from above.
Bluff is not higher than its surroundings but it grants an advantage to those who use it as cover from above due to the uneven, sharp edges.
Bluffs are also characterized by their lack of vegetation and the presence of large rock formations jutting out from them.
In order to identify a bluff, you need to look at the shape and size. The bluffs should be steep, irregularly shaped.
What Is A Bluff On A Lake Or Beach?
A bluff is a shoreline that plunges abruptly into the water, as seen in some lakes and rivers. In order to prevent erosion from the water, the land behind a bluff is often protected by planting shrubs and trees along the steep slope of the bank.
A bluff can be created naturally or artificially through human efforts such as earth-moving projects, filling of river beds, building dams, or installing rock protections.
Cliff vs Bluff [What Is A Bluff In Geography]
Here are some of the differences between a bluff and a cliff:
Cliff
A cliff is a sharp change in elevation with little or no horizontal level between two points of different elevations. A cliff face is usually vertical or nearly so but can also be almost flat at low elevations.
Such features are typically found in mountainous terrain and may be formed either by erosion or by other types of geological activity.
Cliffs can be considered natural features that stabilize a slope, preventing further erosion and landslides from occurring on the mountainside, but they have also been used for building retaining walls and even as places to live.
Bluff
A bluff is a high point of land that appears to be very steep, but it is actually not.
Bluffs are generally found on the side of a mountain or hill, but sometimes they are found on level ground or even on the seashore.
Bluffs are defined by their steepness, meaning the difference in elevation between the highest point on top of the bluff and its base.
Conclusion: What Is A Bluff In Geography
When you are talking about geography, bluff can be a natural feature, a geological formation, or an archaeological site. These things that are difficult to see from one perspective are easy to spot from another perspective.
Bluff is the name given to a low-lying area of land that is surrounded by steep cliffs, steep slopes, or water. It’s the place you hide in when you don’t want to be seen.
Additional Information
A bluff is a type of broad, rounded cliff. Most bluffs border a river, beach, or other coastal area.
Bluffs may form along a river where it meanders, or curves from side to side. River currents on the outside of the curve erode, or wear away, the lower part of a river bank. No longer supported, the upper part of the bank breaks off, leaving the high wall of a bluff. The 150-meter (500-foot) Great River Bluffs in the U.S. state of Minnesota, for example, were carved by the meanderings of the mighty Mississippi River.
Erosion also produces bluffs along the wider floodplain of a river. Over thousands of years, a meandering river gradually shifts from side to side across its floodplain. Where the meanders, or loops, of the river reach valley walls, the water may carve bluffs. In fact, a “bluff line” defines the outer limits of a river’s floodplain, and is often another name for valley wall. A floodplain’s bluff lines may be steep and narrow, or they may be wide and gentle.
Coastal bluffs are formed through a combination of erosion from wind, sea spray, and crashing waves. These bluffs are often more rugged than their inland counterparts, and are more vulnerable to major erosion. Coastal bluffs, especially those in the Puget Sound region of the U.S. state of Washington, are sometimes called feeder bluffs. The constant erosion of feeder bluffs supplies (feeds) sediment to the beaches and seashore downstream below.
Another sort of coastal bluff is the beach ridge. Beach ridges are formed entirely by waves lapping onshore, pushing sand and sediment up and away from the body of water. Beach ridges run parallel to the shoreline and are often associated with sand dunes. The Indiana Dunes, for example, are extensions of beach ridges formed by the waters of Lake Michigan. At this national lakeshore, tiny bluffs give way to larger dunes, and the ecosystem eventually creates the ideal conditions for an oak forest through the process of plant succession.
Like many types of cliffs, bluffs provide important information about how Earth developed. Scotts Bluff, for instance, rises more than 1,400 meters (4,600 feet) above the North Platte River in the U.S. state of Nebraska. The exposed rock of this national monument allows geologists to peer more than 30 million years into North America’s past. Rock formations at Scotts Bluff preserve the history of ancient volcanic activity nearby, as well as the fossilized presence of prehistoric North American rhinos, tapirs, and even camels.
Life on the Bluff
The summits of many bluffs are bare, rocky outcroppings. However, the harsh environment of bluffs are often vital ecosystems. Tiny organisms called lichen often colonize rocky bluffs, providing vital nutrients for many insects and birds.
Hardy grasses and shrubs can also take root on rocky bluffs. The shallow roots of these plants slow erosion of the bluff, even helping to secure valuable topsoil in some places.
Bluffs provide an ideal nesting spot for fishing birds such as cormorants and kingfishers. Cormorants build their nests on the bare ground of bluffs, bringing sticks and seaweed for construction. Cormorant colonies can grow so large that they take over the entire bluff. Kingfishers don’t nest on top the bluff, but dig a burrow directly in it. They prefer burrows on bluffs with little vegetation, as root systems get in the way of digging.
Bluffs are even home to endangered species. The El Segundo blue butterfly (Euphilotes battoides allyni), for example, is found only in a small bluff ecosystem near the giant Los Angeles International Airport in Southern California.

#90 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-03 16:24:30
Hi,
#10543. What does the term in Geography Blowout mean?
#10544. What does the term in Geography Bocage mean?
#91 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-03 15:49:28
Hi,
#5733. What does the verb (used with object) intersperse mean?
#5734. What does the noun interstice mean?
#92 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-03 15:36:44
Hi,
#2458. What does the medical term Free nerve ending mean?
#93 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-03 14:57:46
Hi,
#9725.
#94 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-03 14:22:27
Hi,
#6231.
#95 Jokes » Lawyer Jokes - X » 2025-09-03 13:50:45
- Jai Ganesh
- Replies: 0
Q: How many personal injury attorneys does it take to change a light bulb?
A: Three--one to turn the bulb, one to shake him off the ladder, and the third to sue the ladder company.
* * *
Q: Why does California have the most attorneys, and New Jersey have the most toxic waste dumps?
A: New Jersey got first pick.
* * *
Q: What's the difference between an attorney and a pit bull?
A: Jewelry.
* * *
Q: What do lawyers use for birth control?
A: Their personalities.
* * *
Q: What's the definition of mixed emotions?
A: Watching your attorney drive over a cliff in your new Ferrari.
* * *
#96 Re: Exercises » Compute the solution: » 2025-09-03 13:41:15
Hi,
2572.
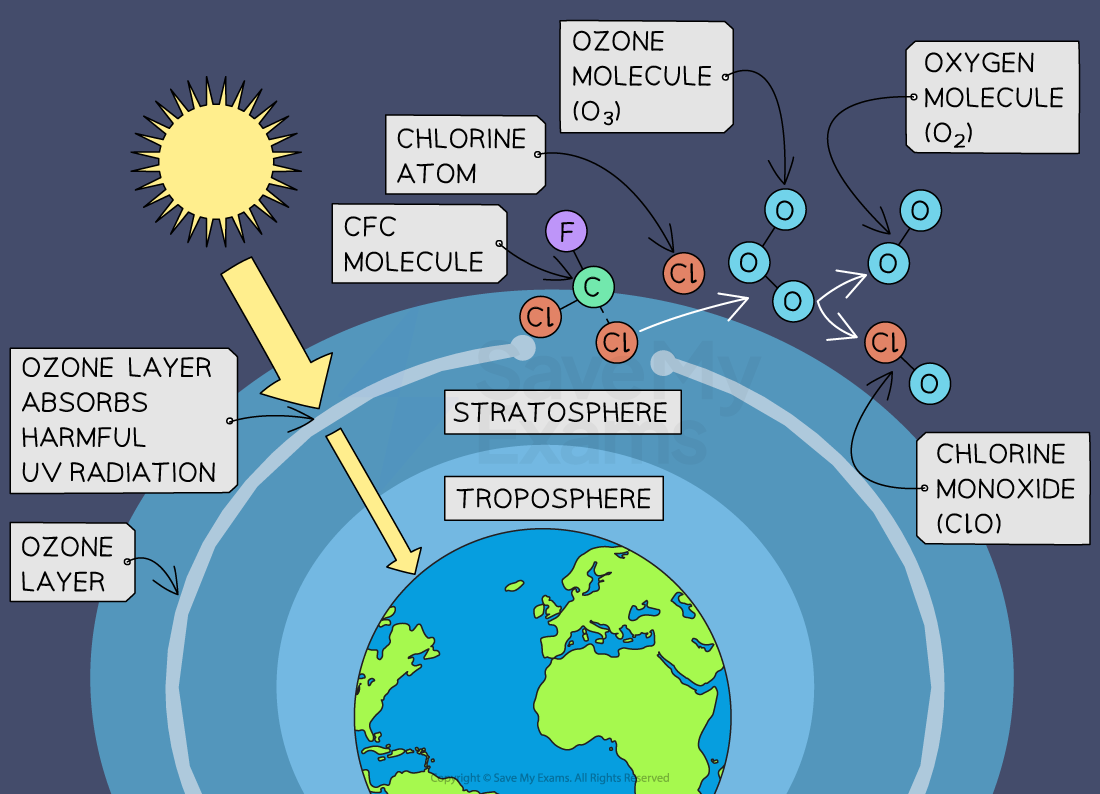
#97 This is Cool » Ozone » 2025-09-02 23:09:32
- Jai Ganesh
- Replies: 0
Ozone
Gist
Ozone (O3) is an unstable, blue gas composed of three oxygen atoms that can be beneficial or harmful depending on its location in the atmosphere. In the stratosphere (high atmosphere), the ozone layer naturally shields Earth from harmful ultraviolet (UV) radiation, while at ground level in the troposphere, it's a pollutant formed from nitrogen oxides (NOx) and volatile organic compounds (VOCs), posing health risks and contributing to smog.
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere : (the stratosphere) and lower atmosphere (the troposphere).
Summary
Ozone, also called trioxygen, is an inorganic molecule with the chemical formula O3. It is a pale-blue gas with a distinctively pungent odor. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O2, breaking down in the lower atmosphere to O2 (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.
Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as 0.1 ppm in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black solid. Ozone's instability with regard to more common dioxygen is such that both concentrated gas and liquid ozone may decompose explosively at elevated temperatures, physical shock, or fast warming to the boiling point. It is therefore used commercially only in low concentrations.
Ozone is a powerful oxidizing agent (far more so than dioxygen) and has many industrial and consumer applications related to oxidation. This same high oxidizing potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about 0.1 ppm. While this makes ozone a potent respiratory hazard and pollutant near ground level, a higher concentration in the ozone layer (from two to eight ppm) is beneficial, preventing damaging UV light from reaching the Earth's surface.
Details
Ozone, (O3), is triatomic allotrope of oxygen (a form of oxygen in which the molecule contains three atoms instead of two as in the common form) that accounts for the distinctive odor of the air after a thunderstorm or around electrical equipment. The odor of ozone around electrical machines was reported as early as 1785; ozone’s chemical constitution was established in 1872. Ozone is an irritating pale blue gas that is explosive and toxic, even at low concentrations. It occurs naturally in small amounts in Earth’s stratosphere, where it absorbs solar ultraviolet radiation, which otherwise could cause severe damage to living organisms on Earth’s surface. Under certain conditions, photochemical reactions between nitrogen oxides and hydrocarbons in the lower atmosphere can produce ozone in concentrations high enough to cause irritation of the eyes and mucous membranes. Such ground-level ozone is considered a major air pollutant.
Ozone usually is manufactured by passing an electric discharge through a current of oxygen or dry air. The resulting mixtures of ozone and original gases are suitable for most industrial purposes, although purer ozone may be obtained from them by various methods; for example, upon liquefaction, an oxygen-ozone mixture separates into two layers, of which the denser one contains about 75 percent ozone. The extreme instability and reactivity of concentrated ozone makes its preparation both difficult and hazardous.
Ozone is 1.5 times as dense as oxygen; at −112 °C (−170 °F) it condenses to a dark blue liquid, which freezes at −251.4 °C (−420 °F). The gas decomposes rapidly at temperatures above 100 °C (212 °F) or, in the presence of certain catalysts, at room temperatures. Although it resembles oxygen in many respects, ozone is much more reactive; hence, it is an extremely powerful oxidizing agent, particularly useful in converting olefins into aldehydes, ketones, or carboxylic acids. Because it can decolorize many substances, it is used commercially as a bleaching agent for organic compounds; as a strong germicide it is used to sterilize drinking water as well as to remove objectionable odors and flavors.
Additional Information:
What is ozone and where is it in the atmosphere?
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere ozone molecule (the stratosphere) and lower atmosphere (the troposphere). Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways.
Stratospheric ozone is formed naturally through the interaction of solar ultraviolet (UV) radiation with molecular oxygen (O2). The "ozone layer," approximately 6 through 30 miles above the Earth's surface, reduces the amount of harmful UV radiation reaching the Earth's surface.
Tropospheric or ground-level ozone – what we breathe – is formed primarily from photochemical reactions between two major classes of air pollutants, volatile organic compounds (VOC) and nitrogen oxides (NOx). These reactions have traditionally been viewed as depending upon the presence of heat and sunlight, resulting in higher ambient ozone concentrations in summer months. Within the last decade, however, high ozone concentrations have also been observed under specific circumstances in cold months, where a few high elevation areas in the Western U.S. with high levels of local VOC and NOx emissions have formed ozone when snow is on the ground and temperatures are near or below freezing. Ozone contributes to what we typically experience as "smog" or haze, which still occurs most frequently in the summertime, but can occur throughout the year in some southern and mountain regions.
Although some stratospheric ozone is transported into the troposphere, and some VOC and NOx occur naturally, the majority of ground-level ozone is the result of reactions of man-made VOC and NOx. Significant sources of VOC are chemical plants, gasoline pumps, oil-based paints, autobody shops, and print shops. Nitrogen oxides result primarily from high temperature combustion. Significant sources are power plants, industrial furnaces and boilers, and motor vehicles.
How does atmospheric ozone affect human health?
Ozone has two properties of interest to human health. First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin cancer and cataracts. Second, when inhaled, it reacts chemically with many biological molecules in the respiratory tract, leading to a number of adverse health effects.
Meet the Ozone Molecule
The ozone molecule (O3) is formed in the Earth’s atmosphere primarily through a series of photochemical reactions involving oxygen molecules (O2) and ultraviolet (UV) radiation from the Sun.
i) Ozone is a gas made up of three (3) atoms of oxygen:
O + O + O = O3
ii) Oxygen (we breathe) is made up of two (2) atoms of oxygen:
O + O = O2
The atoms in O2 are stable – each atom “holds on” to the other.
The atoms in O3 consist of a stable pair (O2) and a third, unstable atom.
It is the unstable atom that gives ozone its power! Ozone is generated when energy “splits” the stable O2 bond.
Ozone Can be Generated in Three Ways:
Ozone, a molecule composed of three oxygen atoms (O3), can be generated through various methods. Here are three common ways ozone can be produced:
i) Ultraviolet (UV) Radiation: Ozone can be generated naturally in the Earth’s stratosphere through the interaction of UV radiation from the sun and oxygen molecules (O2). In this process, high-energy UV radiation breaks apart oxygen molecules, forming oxygen radicals (O). These radicals then react with other oxygen molecules to form ozone (O3).
ii) Corona Discharge (Electric Discharge): This method involves passing a high-voltage electric discharge through oxygen gas (O2) or dry air containing oxygen. The electric discharge creates a plasma, which leads to the dissociation of oxygen molecules into oxygen atoms. These oxygen atoms then combine with other oxygen molecules to form ozone.
iii) Cold Plasma (Dielectric Barrier Discharge): In this method, oxygen gas (O2) or dry air containing oxygen is passed through a dielectric barrier discharge (DBD), which is essentially a gap between two electrodes with a dielectric material in between. When a high-voltage alternating current is applied to the electrodes, it creates a plasma that facilitates the dissociation of oxygen molecules into oxygen atoms. These oxygen atoms then combine with other oxygen molecules to produce ozone.
These methods are commonly employed in various industries and applications, such as water treatment, air purification, and industrial processes. Each method has its advantages and limitations depending on the specific requirements of the application.
#98 Science HQ » Lutetium » 2025-09-02 22:01:37
- Jai Ganesh
- Replies: 0
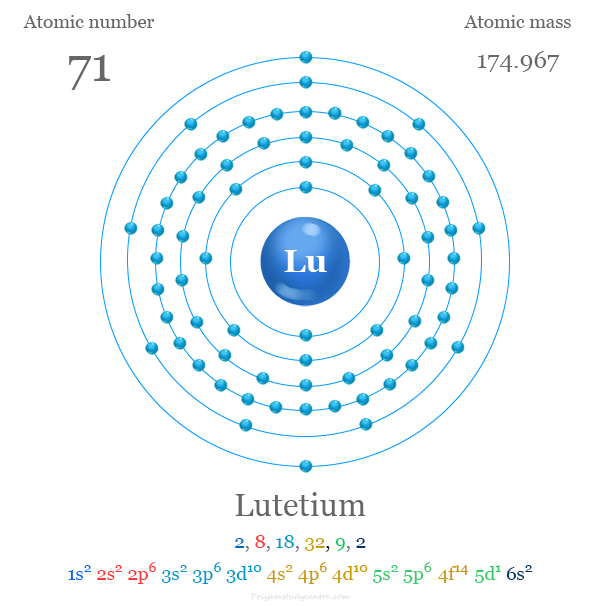
Lutetium
Gist
Lutetium (Lu) is the element with atomic number 71, a rare earth metal that is silvery-white, hard, dense, and expensive. It is found in trace amounts in minerals like monazite and is used as a catalyst in the petroleum industry for processes like polymerization and alkylation. Lutetium-177 is a radioactive isotope used in targeted cancer therapies, such as in the treatment of prostate cancer.
Lutetium, outside of scientific research, is primarily used in the medical, petroleum, and geological industries. In medicine, it is used in cancer research, and geologically it is used to date meteorites. In petroleum, it is used to help crack hydrocarbons.
Lutetium (Lu) is the element with atomic number 71, a rare earth metal that is silvery-white, hard, dense, and expensive. It is found in trace amounts in minerals like monazite and is used as a catalyst in the petroleum industry for processes like polymerization and alkylation. Lutetium-177 is a radioactive isotope used in targeted cancer therapies, such as in the treatment of prostate cancer.
Summary
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements; it can also be classified as the first element of the 6th-period transition metals.
Lutetium was independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James. All of these researchers found lutetium as an impurity in ytterbium. The dispute on the priority of the discovery occurred shortly after, with Urbain and Welsbach accusing each other of publishing results influenced by the published research of the other; the naming honor went to Urbain, as he had published his results earlier. He chose the name lutecium for the new element, but in 1949 the spelling was changed to lutetium. In 1909, the priority was finally granted to Urbain and his names were adopted as official ones; however, the name cassiopeium (or later cassiopium) for element 71 proposed by Welsbach was used by many German scientists until the 1950s.
Lutetium is not a particularly abundant element, although it is significantly more common than silver in the Earth's crust. It has few specific uses. Lutetium-176 is a relatively abundant (2.5%) radioactive isotope with a half-life of about 38 billion years, used to determine the age of minerals and meteorites. Lutetium usually occurs in association with the element yttrium and is sometimes used in metal alloys and as a catalyst in various chemical reactions. 177Lu-DOTA-TATE is used for radionuclide therapy on neuroendocrine tumours. Lutetium has the highest Brinell hardness of any lanthanide, at 890–1300 MPa.
Details
Lutetium (Lu) is a chemical element, a rare-earth metal of the lanthanide series of the periodic table, that is the densest and the highest-melting rare-earth element and the last member of the lanthanide series.
In its pure form, lutetium metal is silvery white and stable in air. The metal is easily dissolved in diluted acids—except hydrofluoric acid (HF), in which a protective layer of LuF3 forms on the surface and prevents the metal from further dissolution. The metal is paramagnetic from 0 K (−273 °C, or −460 °F) to its melting point at 1,936 K (1,663 °C, or 3,025 °F) with a temperature-independent magnetic susceptibility between approximately 4 and 300 K (−269 and 27 °C, or −452 and 80 °F). It becomes superconducting at 0.022 K (−273.128 °C, or −459.63 °F) and pressures exceeding 45 kilobars.
Lutetium was discovered in 1907–08 by Austrian chemist Carl Auer von Welsbach and Georges Urbain, working independently. Urbain derived the name for the element from Lutetia, the ancient Roman name for Paris, to honour his native city. The name lutetium became widely accepted except in Germany, where it was commonly called cassiopeium until the 1950s. One of the rarest of the rare earths, lutetium occurs in rare-earth minerals such as laterite clays, xenotime, and euxenite. Though lutetium composes only trace mounts (less than 0.1 percent by weight) of the commercially important minerals bastnasite and monazite, it has proved feasible to extract the metal as a by-product. Lutetium is also found in the products of nuclear fission.
Natural lutetium consists of two isotopes: stable lutetium-175 (97.4 percent) and radioactive lutetium-176 (2.6 percent, 3.76 × {10}^{10}-year half-life). The radioactive isotope is used to determine the age of meteorites relative to that of Earth. In addition to lutetium-176, and not counting nuclear isomers, 33 more radioactive isotopes of lutetium are known. They range in mass from 150 to 184; the least stable isotope (lutetium-150) has a half-life of 45 milliseconds, and the most stable isotope is lutetium-176.
Separation and purification are accomplished by liquid-liquid extraction or ion-exchange techniques. The metal is prepared by metallothermic reduction of the anhydrous halides by alkali or alkaline-earth metals. Lutetium is monomorphic and has a close-packed hexagonal structure with a = 3.5052 Å and c = 5.5494 Å at room temperature.
Lutetium is used in research. Its compounds are used as hosts for scintillators and X-ray phosphors, and the oxide is used in optical lenses. The element behaves as a typical rare earth, forming a series of compounds in oxidation state +3, such as lutetium sesquioxide, sulfate, and chloride.
Element Properties
atomic number : 71
atomic weight : 174.967
melting point : 1,663 °C (3,025 °F)
boiling point : 3,402 °C (6,156 °F)
specific gravity : 9.841 (24 °C, or 75 °F)
oxidation state : +3.
Additional Information:
Appearance
A silvery-white, hard, dense metal.
Uses
Lutetium is little used outside research. One of its few commercial uses is as a catalyst for cracking hydrocarbons in oil refineries.
Biological role
Lutetium has no known biological role. It has low toxicity.
Natural abundance
In common with many other lanthanides, the main source of lutetium is the mineral monazite. It is extracted, with difficulty, by reducing the anhydrous fluoride with calcium metal.
#99 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-02 21:32:49
Hi,
#10541. What does the term in Geography Blockfield mean
#10542. What does the term in Geography Blowhole (geology) mean?
#100 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-02 21:14:45
Hi,
#5731. What does the adjective interpretive mean?
#5732. What does the adjective interrogative mean?