Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Tantalum
Pages: 1
#1 2025-09-04 17:24:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,669
Tantalum
Tantalum
Gist
Tantalum (Ta) is a hard, blue-grey, and corrosion-resistant transition metal with a high melting point and an atomic number of 73. Known for its high reliability and biocompatibility, it's used in electronic components like capacitors, as well as in medical implants, cutting tools, and aircraft parts. The element's name comes from the Greek mythological figure Tantalus, reflecting its insolubility in most acids.
Tantalum is widely used in electronics, primarily for capacitors in portable devices like smartphones and computers, due to its high capacitance and reliability. It also has significant applications in the medical field for surgical implants, prosthetics, and imaging equipment, leveraging its biocompatibility. Additionally, its corrosion resistance and high-temperature properties make it valuable in the chemical industry for heat exchangers and reactor components, and in the aerospace industry for high-temperature components in engines and missiles.
Summary
Tantalum is a chemical element; it has symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite, and coltan.
The chemical inertness and very high melting point of tantalum make it valuable for laboratory and industrial equipment such as reaction vessels and vacuum furnaces. It is used in tantalum capacitors for electronic equipment such as computers. It is being investigated for use as a material for high-quality superconducting resonators in quantum processors.
Details
Tantalum (Ta) is a chemical element, bright, very hard, silver-gray metal of Group 5 (Vb) of the periodic table, characterized by its high density, extremely high melting point, and excellent resistance to all acids except hydrofluoric at ordinary temperatures.
Closely associated with niobium in ores and in properties, tantalum was discovered (1802) by the Swedish chemist Anders Gustaf Ekeberg and named after the mythological character Tantalus because of the tantalizing problem of dissolving the oxide in acids. Due to the great chemical similarity of niobium and tantalum, the establishment of the individual identities of the two elements was very difficult. Tantalum was soon identified with niobium (then called columbium), but in 1844 the German chemist Heinrich Rose demonstrated their distinct characters. Although some of the impure metal was isolated earlier, the Russian chemist Werner Bolton prepared (1903) the first ductile tantalum, which was used briefly as incandescent lamp-filament material.
Relatively rare, tantalum is about as abundant as uranium. It occurs, with niobium, in the columbite–tantalite series (in which columbite [FeNb2O6] and tantalite [FeTa2O6] occur in highly variable ratios) and the pyrochlore–microlite series of minerals. Native tantalum metal with some niobium and traces of manganese and gold occurs sparingly in Russia in placers in the Ural Mountains and possibly the Altai Mountains in Central Asia. Rwanda is the world’s largest extractor of tantalum.
Tantalum is separated from niobium compounds by solvent extraction in a liquid-liquid process and is then reduced to metallic tantalum powder. The massive metal is produced by powder metallurgy techniques. It can also be obtained by either electrolysis of fused salts or reduction of fluoro complexes with a very reactive metal such as sodium. The most important uses for tantalum are in electrolytic capacitors and corrosion-resistant chemical equipment. Tantalum capacitors have the highest capacitance per unit volume of any capacitors and are used extensively in miniaturized electrical circuitry. Other uses include getters and components in electron tubes, rectifiers, and prosthetic devices.
Tantalum is chemically much like niobium because both have similar electronic configurations and because the radius of the tantalum ion is nearly the same as that of niobium as a result of the lanthanoid contraction. Tantalum is usually in the +5 oxidation state in its compounds; lower oxidation states, especially from +2 to +4, have been prepared. Tantalum compounds are relatively unimportant commercially, although the carbide TaC is used in cemented-carbide tools for machining hard metals. Nearly all naturally occurring tantalum is in one stable isotope, tantalum-181. However, a small amount, 0.012 percent, is tantalum-180, which has the unusual property of being found in its excited state. The tantalum-180 excited state has a half-life of more than 1.2 × 1015 years; the ground state (the lowest energy state) has a half-life of only 8.154 hours.
Element Properties
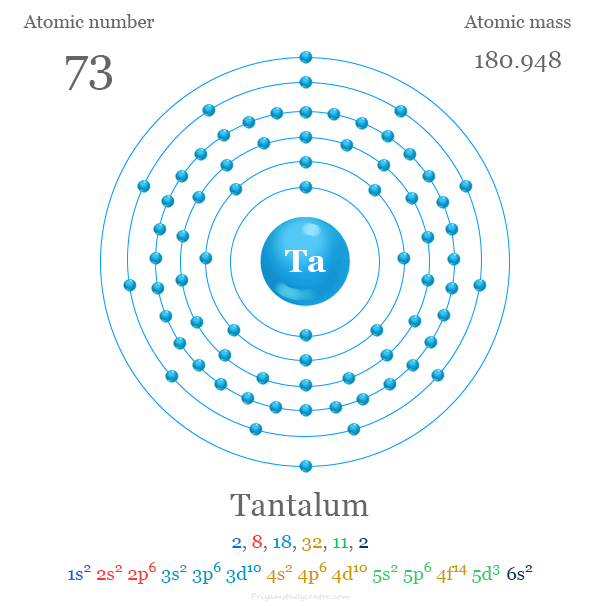
atomic number : 73
atomic weight : 180.94788
melting point : 2,996 °C (5,425 °F)
boiling point : 5,425 °C (9,797 °F)
specific gravity : 16.6 at 20 °C (68 °F)
oxidation states : +2, +3, +4, +5.
Additional Information:
Appearance
A shiny, silvery metal that is very resistant to corrosion.
Uses
One of the main uses of tantalum is in the production of electronic components. An oxide layer which forms on the surface of tantalum can act as an insulating (dielectric) layer. Because tantalum can be used to coat other metals with a very thin layer, a high capacitance can be achieved in a small volume. This makes tantalum capacitors attractive for portable electronics such as mobile phones.
Tantalum causes no immune response in mammals, so has found wide use in the making of surgical implants. It can replace bone, for example in skull plates; as foil or wire it connects torn nerves; and as woven gauze it binds abdominal muscle.
It is very resistant to corrosion and so is used in equipment for handling corrosive materials. It has also found uses as electrodes for neon lights, AC/DC rectifiers and in glass for special lenses.
Tantalum alloys can be extremely strong and have been used for turbine blades, rocket nozzles and nose caps for supersonic aircraft.
Biological role
Tantalum has no known biological role. It is non-toxic.
Natural abundance
Tantalum is sometimes, but only rarely, found uncombined in nature. It occurs mainly in the mineral columbite-tantalite, which also contains other metals including niobium. It is mined in many places including Australia, Canada and Brazil. There are several complicated steps involved in separating the tantalum from the niobium. A lot of tantalum is obtained commercially as a by-product of tin extraction.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Tantalum