Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Actinium
Pages: 1
#1 2025-09-22 16:25:22
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,105
Actinium
Actinium
Gist
Actinium (symbol Ac, atomic number 89) is a highly radioactive, silvery-white metal in the actinide series. Discovered in 1899, it glows with a pale blue light due to its intense radioactivity and is found in trace amounts in uranium ores. Though dangerous, its potent alpha radiation makes Actinium-225 a promising medical isotope for targeted cancer treatment, particularly in clinical trials for leukemias and other cancers.
Actinium's primary uses are in medicine and scientific research, particularly with its isotope Actinium-225 (Ac-225), which is used for targeted alpha therapy to treat certain cancers. It also serves as a neutron source for research in neutron radiography to study material structures. Due to its rarity, high price, and intense radioactivity, actinium has no significant industrial applications.
Summary
Actinium is a chemical element; it has symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. The actinide series, a set of 15 elements between actinium and lawrencium in the periodic table, are named for actinium. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be discovered.
A soft, silvery-white radioactive metal, actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As with most lanthanides and many actinides, actinium assumes oxidation state +3 in nearly all its chemical compounds. Actinium is found only in traces in uranium and thorium ores as the isotope 227Ac, which decays with a half-life of 21.772 years, predominantly emitting beta and sometimes alpha particles, and 228Ac, which is beta active with a half-life of 6.15 hours. One tonne of natural uranium in ore contains about 0.2 milligrams of actinium-227, and one tonne of thorium contains about 5 nanograms of actinium-228. The close similarity of physical and chemical properties of actinium and lanthanum makes separation of actinium from the ore impractical. Instead, the element is prepared, in milligram amounts, by the neutron irradiation of 226Ra in a nuclear reactor. Owing to its scarcity, high price and radioactivity, actinium has no significant industrial use. Its current applications include a neutron source and an agent for radiation therapy.
Details
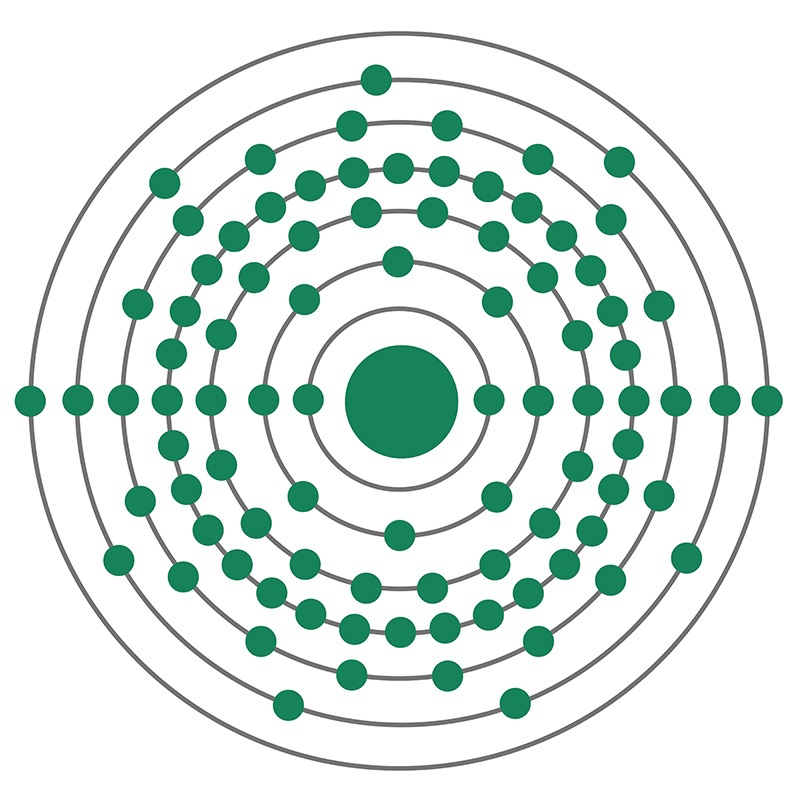
Actinium (Ac) is a radioactive chemical element, in Group 3 (IIIb) of the periodic table, atomic number 89. Actinium was discovered (1899) by French chemist André-Louis Debierne in pitchblende residues left after French physicists Pierre and Marie Curie had extracted radium from them, and it was also discovered (1902) independently by German chemist Friedrich Oskar Giesel. Debierne named the element after the Greek word aktinos (“ray”). A ton of pitchblende ore contains about 0.15 mg of actinium. The rare silvery-white metal is highly radioactive, glowing blue in the dark.
The most common isotope of actinium is actinium-227; the others, natural and artificial, are too short-lived to accumulate in macroscopic quantity. Actinium-227, which is one of the decay products of uranium-235, has a 21.8-year half-life and in turn decays almost entirely to thorium-227, but about 1 percent decays to francium-223. This whole disintegration chain with its branches is called the actinium series.
Actinium-225 has a 10-day half-life, decaying by the emission of alpha particles. Its short-lived daughter isotopes emit only alpha and beta particles with no high-energy gamma rays. This isotope can thus deliver high-energy radiation to a tumour without greatly affecting the surrounding tissue. Complexes of actinium-225 have been studied for their use in nuclear medicine.
Actinium, the ions of which in solution are colourless, exhibits an oxidation state of +3, closely resembling the rare-earth lanthanoid elements in its chemical properties. Actinium is the prototype of a second rare-earth-like series, the actinoid elements.
Element Properties
atomic number : 89
stablest isotope : 227
oxidation state : +3.
Additional Information
Actinium (symbol Ac, atomic number 89) is a highly radioactive, silvery-white metal in the actinide series. Discovered in 1899, it glows with a pale blue light due to its intense radioactivity and is found in trace amounts in uranium ores. Though dangerous, its potent alpha radiation makes Actinium-225 a promising medical isotope for targeted cancer treatment, particularly in clinical trials for leukemias and other cancers.
Appearance
Actinium is a soft, silvery-white radioactive metal. It glows blue in the dark because its intense radioactivity excites the air around it.
Uses
Actinium is a very powerful source of alpha rays, but is rarely used outside research.
Biological role
Actinium has no known biological role. It is toxic due to its radioactivity.
Natural abundance
Actinium used for research purposes is made by the neutron bombardment of radium-226. Actinium also occurs naturally in uranium ores.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Actinium