Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-11-18 16:41:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,200
Electrovalent Bond
Electrovalent Bond
Gist
An electrovalent bond, also known as an ionic bond, is a type of chemical bond formed by the transfer of one or more electrons from one atom to another, resulting in the formation of oppositely charged ions that are held together by strong electrostatic attraction. This process typically occurs between a metal and a non-metal, where the metal atom loses electrons to become a positive ion (cation) and the non-metal atom gains electrons to become a negative ion (anion).
There is no difference between ionic and electrovalent bonds; the terms are synonymous and both describe a bond formed by the complete transfer of electrons between atoms, typically a metal and a non-metal. This transfer creates oppositely charged ions (a cation and an anion) that are then held together by a strong electrostatic force of attraction.
Summary
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding, along with covalent bonding and metallic bonding. Ions are atoms (or groups of atoms) with an electrostatic charge. Atoms that gain electrons make negatively charged ions (called anions). Atoms that lose electrons make positively charged ions (called cations).
Clean ionic bonding – in which one atom or molecule completely transfers an electron to another – cannot exist: all ionic compounds have some degree of covalent bonding or electron sharing. Thus, the term "ionic bonding" is given when the ionic character is greater than the covalent character – that is, a bond in which there is a large difference in electronegativity between the cation and anion, causing the bonding to be more polar (ionic) than in covalent bonding where electrons are shared more equally. Bonds with partially ionic and partially covalent characters are called polar covalent bonds.
Ionic compounds conduct electricity when molten or in solution, typically not when solid. Ionic compounds generally have a high melting point, depending on the charge of the ions they consist of. The higher the charges the stronger the cohesive forces and the higher the melting point. They also tend to be soluble in water; the stronger the cohesive forces, the lower the solubility.
Overview
Atoms that have an almost full or almost empty valence shell tend to be very reactive. Strongly electronegative atoms (such as halogens) often have only one or two empty electron states in their valence shell, and frequently bond with other atoms or gain electrons to form anions. Weakly electronegative atoms (such as alkali metals) have relatively few valence electrons, which can easily be lost to strongly electronegative atoms. As a result, weakly electronegative atoms tend to distort their electron cloud and form cations.
Properties of ionic bonds
* They are considered to be among the strongest of all types of chemical bonds. This often causes ionic compounds to be very stable.
* Ionic bonds have high bond energy. Bond energy is the mean amount of energy required to break the bond in the gaseous state.
* Most ionic compounds exist in the form of a crystal structure, in which the ions occupy the corners of the crystal. Such a structure is called a crystal lattice.
* Ionic compounds lose their crystal lattice structure and break up into ions when dissolved in water or any other polar solvent. This process is called solvation. The presence of these free ions makes aqueous ionic compound solutions good conductors of electricity. The same occurs when the compounds are heated above their melting point in a process known as melting.
Details
Ionic bond is the type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. The atom that loses the electrons becomes a positively charged ion (cation), while the one that gains them becomes a negatively charged ion (anion). A brief treatment of ionic bonds follows. For full treatment, see chemical bonding: The formation of ionic bonds.
Ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals and the alkali and alkaline-earth metals. In ionic crystalline solids of this kind, the electrostatic forces of attraction between opposite charges and repulsion between similar charges orient the ions in such a manner that every positive ion becomes surrounded by negative ions and vice versa. In short, the ions are so arranged that the positive and negative charges alternate and balance one another, the overall charge of the entire substance being zero. The magnitude of the electrostatic forces in ionic crystals is considerable. Accordingly, these substances tend to be hard and nonvolatile.
An ionic bond is actually the extreme case of a polar covalent bond, the latter resulting from unequal sharing of electrons rather than complete electron transfer. Ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar.
Additional Information
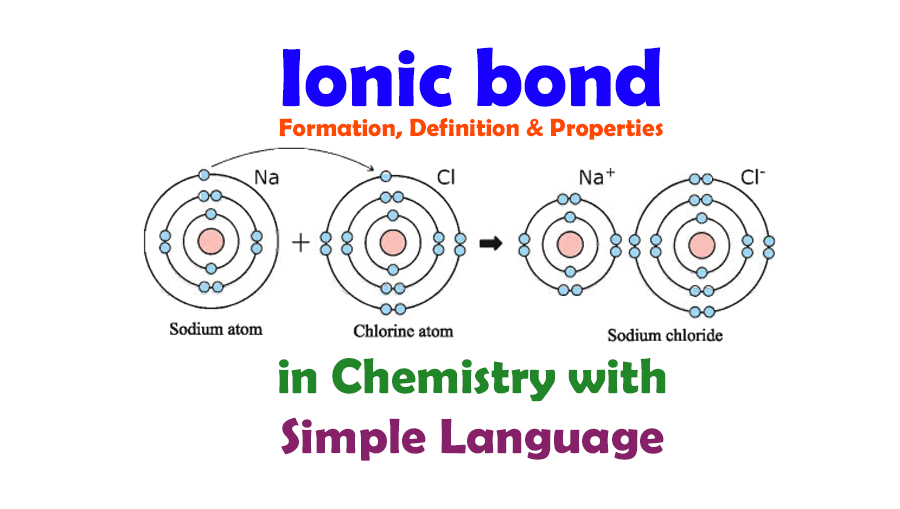
An electrovalent bond, also known as an ionic bond, is a type of chemical bond that occurs between two atoms when one atom transfers one or more of its electrons to another atom. This transfer of electrons leads to the formation of ions: the atom that loses electrons becomes a positively charged ion (cation), while the atom that gains electrons becomes a negatively charged ion (anion). The electrostatic attraction between these oppositely charged ions results in the formation of the electrovalent bond. Electrovalent bonds typically form between metals and non-metals, such as sodium (Na) and chlorine (Cl), where sodium donates an electron to chlorine, resulting in the formation of sodium chloride (NaCl).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1