Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Curium
Pages: 1
#1 2025-09-30 19:46:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,613
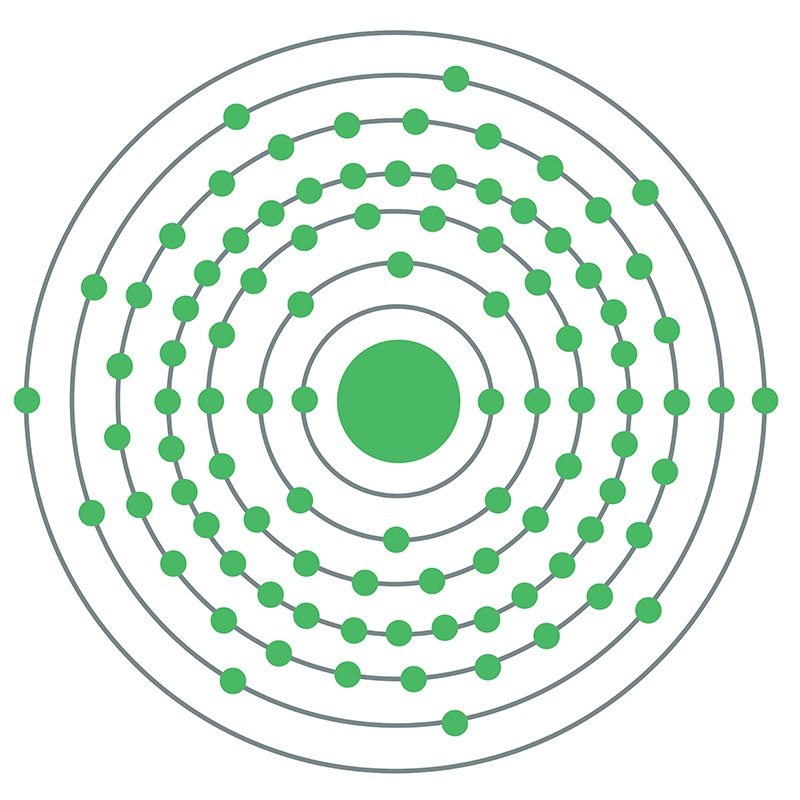
Curium
Curium
Gist
Curium (Cm) is a synthetic, radioactive actinide element with atomic number 96, discovered in 1944 by Glenn Seaborg and his colleagues. Named after Marie and Pierre Curie, it's primarily used as a power source in radioisotope thermoelectric generators (RTGs) for spacecraft and in X-ray spectrometers for analysis. Curium is harmful if inhaled and can cause liver and gastrointestinal problems if ingested.
Curium is used for its radioactive properties, serving as a power source in radioisotope thermoelectric generators (RTGs) for deep space probes and remote scientific instruments, and as an alpha emitter in alpha particle X-ray spectrometers (APXS) to analyze the chemical composition of rocks on other planets, particularly in space applications. It also has limited uses in scientific research to produce heavier elements and for studying the effects of intense radiation.
Summary
Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium (the isotope 239Pu) with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.
Curium is a hard, dense, silvery metal with a high melting and boiling point for an actinide. It is paramagnetic at ambient conditions, but becomes antiferromagnetic upon cooling, and other magnetic transitions are also seen in many curium compounds. In compounds, curium usually has valence +3 and sometimes +4; the +3 valence is predominant in solutions. Curium readily oxidizes, and its oxides are a dominant form of this element. It forms strongly fluorescent complexes with various organic compounds. If it gets into the human body, curium accumulates in bones, lungs, and liver, where it promotes cancer.
All known isotopes of curium are radioactive and have small critical mass for a nuclear chain reaction. The most stable isotope, 247Cm, has a half-life of 15.6 million years; the longest-lived curium isotopes predominantly emit alpha particles. Radioisotope thermoelectric generators can use the heat from this process, but this is hindered by the rarity and high cost of curium. Curium is used in making heavier actinides and the 238Pu radionuclide for power sources in artificial cardiac pacemakers and RTGs for spacecraft. It served as the α-source in the alpha particle X-ray spectrometers of several space probes, including the Sojourner, Spirit, Opportunity, and Curiosity Mars rovers and the Philae lander on comet 67P/Churyumov–Gerasimenko, to analyze the composition and structure of the surface.
Details
Curium (Cm) is a synthetic chemical element of the actinoid series of the periodic table, atomic number 96. Unknown in nature, curium (as the isotope curium-242) was discovered (summer 1944) at the University of Chicago by American chemists Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in a sample of a plutonium isotope, plutonium-239, that had been bombarded by helium ions (alpha particles) in the 152-cm (60-inch) cyclotron at the University of California, Berkeley. It was the third transuranium element to be discovered. The element was named after French physicists Pierre and Marie Curie.
Curium is a silvery metal. All of its isotopes are radioactive. For chemical research, curium-242 (163-day half-life) has been supplanted by curium-244 (18.1-year half-life) and the still longer-lived isotope curium-248, which are built up from plutonium-239 by neutron irradiation. Curium exhibits its common +3 oxidation state as the very faint yellow Cm3+ ion in aqueous solution, as the sesquioxide Cm2O3, and as the trihalides; it is chemically similar to the other tripositive actinoid elements and to the lanthanoid elements. The +4 oxidation state appears in the black dioxide CmO2 and as the Cm4+ ion complexed with the fluoride ion.
Element Properties
atomic number : 96
stablest isotop : 247
melting point : about 1,340 °C (2,444 °F)
specific gravity : about 13.51
oxidation states : +3, +4.
Additional Information:
Appearance
A radioactive metal that is silver in colour. It tarnishes rapidly in air.
Uses
Curium has been used to provide power to electrical equipment used on space missions.
Biological role
Curium has no known biological role. It is toxic due to its radioactivity.
Natural abundance
Curium can be made in very small amounts by the neutron bombardment of plutonium in a nuclear reactor. Minute amounts may exist in natural deposits of uranium. Only a few grams are produced each year.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Curium