Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Francium
Pages: 1
#1 2025-09-20 15:59:56
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,105
Francium
Francium
Gist
Francium (Fr) is a synthetic, highly radioactive alkali metal with atomic number 87, known for its extreme rarity and instability, with its most stable isotope having a half-life of just 22 minutes. Discovered by Marguerite Perey in 1939 and named after France, this silvery-white element is the second-most electropositive element, only surpassed by cesium, but its extreme instability prevents any commercial uses, confining it to research purposes.
Francium's use is confined almost entirely to scientific research due to its extreme rarity and instability; it has no commercial or practical applications. Researchers use it to study atomic structure, nuclear decay processes, and the fundamental interactions of heavy elements through spectroscopy experiments.
Summary
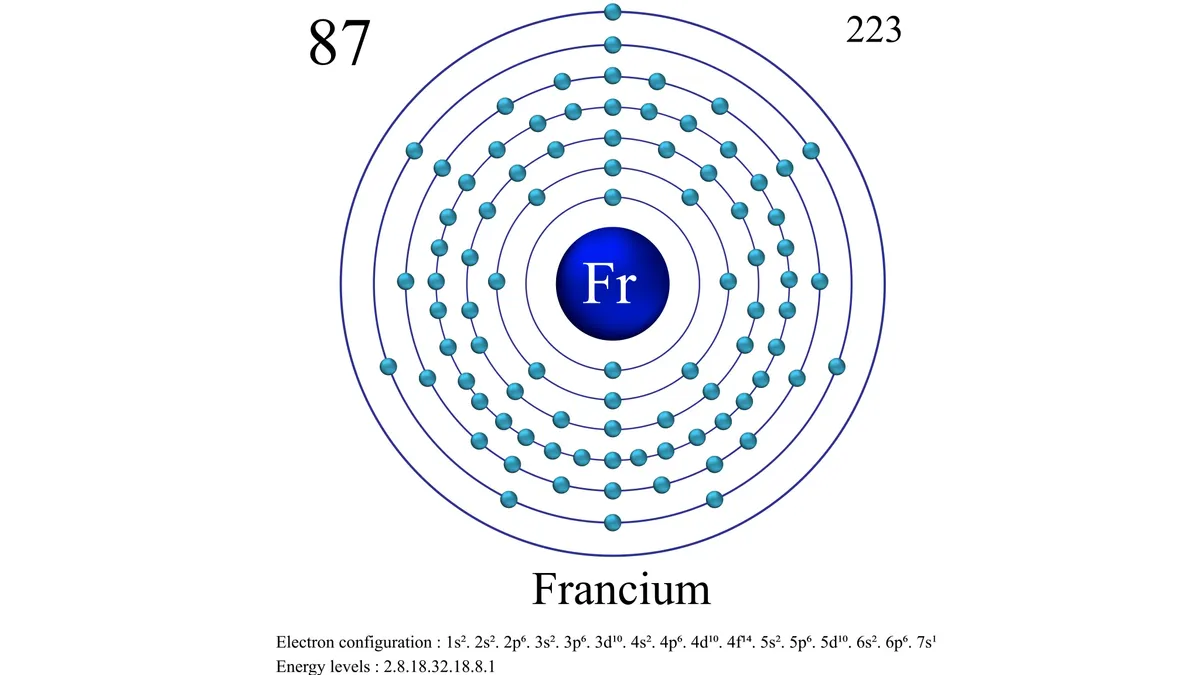
Francium is a chemical element; it has symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain in which it appears), has a half-life of only 22 minutes. It is the second-most electropositive element, behind only caesium, and is the second rarest naturally occurring element (after astatine). Francium's isotopes decay quickly into astatine, radium, and radon. The electronic structure of a francium atom is [Rn] 7s1; thus, the element is classed as an alkali metal.
As a consequence of its extreme instability, bulk francium has never been seen. Because of the general appearance of the other elements in its periodic table column, it is presumed that francium would appear as a highly reactive metal if enough could be collected together to be viewed as a bulk solid or liquid. Obtaining such a sample is highly improbable since the extreme heat of decay resulting from its short half-life would immediately vaporize any viewable quantity of the element.
Francium was discovered by Marguerite Perey in France (from which the element takes its name) on January 7, 1939. Before its discovery, francium was referred to as eka-caesium or ekacaesium because of its conjectured existence below caesium in the periodic table. It was the last element first discovered in nature, rather than by synthesis. Outside the laboratory, francium is extremely rare, with trace amounts found in uranium ores, where the isotope francium-223 (in the family of uranium-235) continually forms and decays. As little as 1 ounce (28 g) exists at any given time throughout the Earth's crust; aside from francium-223 and francium-221, its other isotopes are entirely synthetic. The largest amount produced in the laboratory was a cluster of more than 300,000 atoms.
Details
Francium (Fr) is the heaviest chemical element of Group 1 (Ia) in the periodic table, the alkali metal group. It exists only in short-lived radioactive forms. Natural francium cannot be isolated in visible, weighable amounts, for only 24.5 grams (0.86 ounce) occur at any time in the entire crust of Earth. The existence of francium was predicted by Russian chemist Dmitry I. Mendeleyev in his periodic classification of the elements. French chemist Marguerite Perey discovered francium (1939) while studying actinium-227, which decays by negative beta decay (electron emission) to an isotope of thorium (thorium-227) and by alpha emission (about 1 percent) into an isotope of francium (francium-223) that was formerly called actinium K (AcK) and is a member of the actinium decay series. Though it is the longest-lived isotope of francium, francium-223 has a half-life of only 22 minutes. Thirty-four isotopes of francium with masses between 199 and 232 have been artificially prepared, and, because natural francium cannot be concentrated, it is also prepared by neutron irradiation of radium to produce actinium, which decays to produce traces of francium. The chemistry of francium can be studied only by methods designed for trace quantities. In all respects, its observed behaviour, including the oxidation state of +1, is that to be expected of an alkali element filling a place just below cesium in the periodic table of the elements. There is almost no information on its biological aspects.
Element Properties
atomic number : 87
stablest isotope : (223)
oxidation state : +1.
Additional Information:
Appearance
An intensely radioactive metal.
Uses
Francium has no uses, having a half life of only 22 minutes.
Biological role
Francium has no known biological role. It is toxic due to its radioactivity.
Natural abundance
Francium is obtained by the neutron bombardment of radium in a nuclear reactor. It can also be made by bombarding thorium with protons.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Francium