Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Mercury
Pages: 1
#1 2025-09-12 15:35:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,459
Mercury
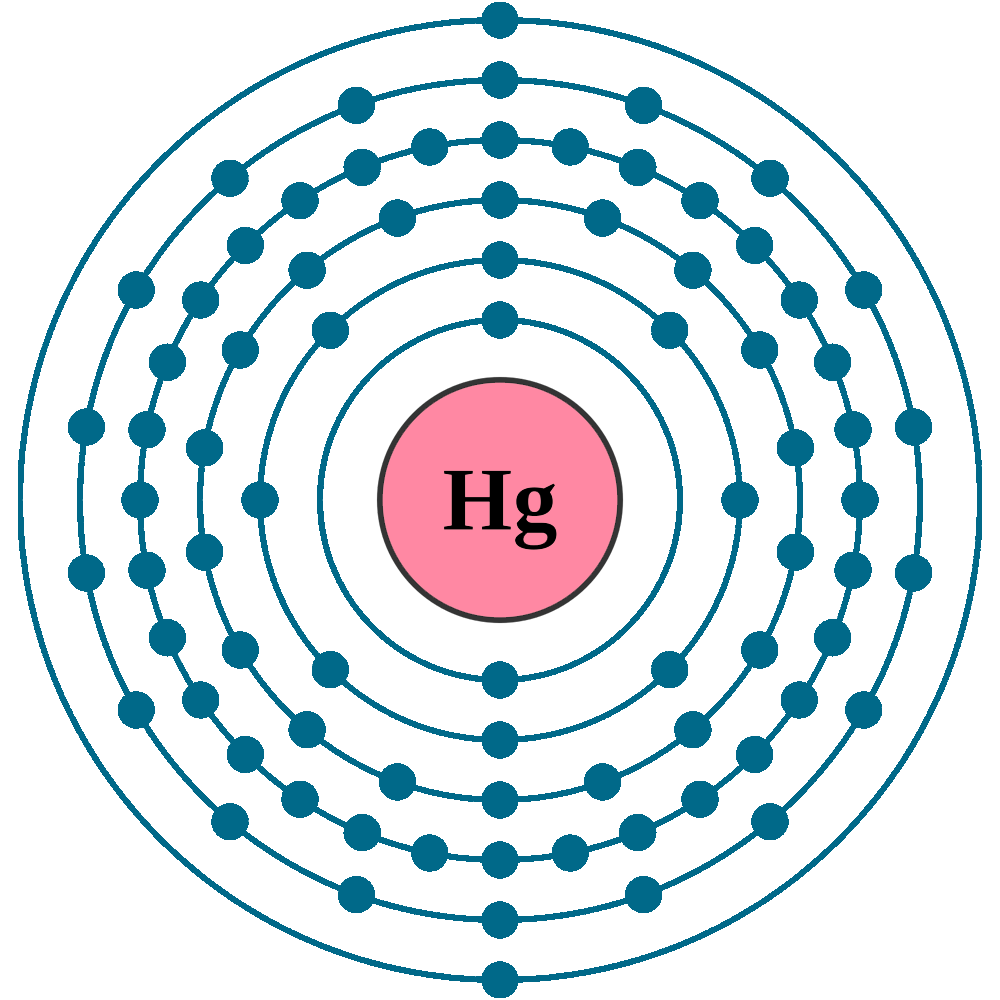
Mercury (Element)
Gist
Mercury is a chemical element with the symbol Hg and atomic number 80. It is a heavy, silvery-white metal that is unique for being the only metallic element that remains liquid at standard temperature and pressure, though other metals like gallium melt just above room temperature. Also known as quicksilver, it forms alloys called amalgams with other metals and is found in nature in deposits of cinnabar. Mercury is toxic and can cause serious health problems, particularly to the nervous system, and bioaccumulates in the environment and food chains.
Mercury has historically been used in thermometers, barometers, electrical switches, fluorescent lamps, dental amalgams, and some batteries, pharmaceuticals, and cosmetics. However, due to its toxicity, many of these uses are being phased out in favor of alternatives, with the Minamata Convention on Mercury working to eliminate its use in products and industries globally.
Summary
Mercury is a chemical element; it has symbol Hg and atomic number 80. It is commonly known as quicksilver. A heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide. Exposure to mercury and mercury-containing organic compounds is toxic to the nervous system, immune system and kidneys of humans and other animals; mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury) either directly or through mechanisms of biomagnification.
Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, although concerns about the element's toxicity have led to the phasing out of such mercury-containing instruments. It remains in use in scientific research applications and in amalgam for dental restoration in some locales. It is also used in fluorescent lighting. Electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light, which then causes the phosphor in the tube to fluoresce, making visible light.
Details
Mercury (Hg) is a chemical element, liquid metal of Group 12 (IIb, or zinc group) of the periodic table.
Element Properties
atomic number : 80
atomic weight : 200.592
melting point : -38.83 °C (-37.89 °F)
boiling point : 356.62 °C (673.91 °F)
specific gravity : 13.5 at 20 °C (68 °F)
valence : 1, 2.
Properties, uses, and occurrence
Mercury was known in Egypt and also probably in the East as early as 1500 bce. The name mercury originated in 6th-century alchemy, in which the symbol of the planet was used to represent the metal; the chemical symbol Hg derives from the Latin hydrargyrum, “liquid silver.” Although its toxicity was recognized at an early date, its main application was for medical purposes.
Mercury is the only elemental metal that is liquid at room temperature. (Cesium melts at about 28.5 °C [83 °F], gallium at about 30 °C [86 °F], and rubidium at about 39 °C [102 °F].) Mercury is silvery white, slowly tarnishes in moist air, and freezes into a soft solid like tin or lead at −38.83 °C (−37.89 °F). It boils at 356.62 °C (673.91 °F).
It alloys with copper, tin, and zinc to form amalgams, or liquid alloys. An amalgam with silver is used as a filling in dentistry. Mercury does not wet glass or cling to it, and this property, coupled with its rapid and uniform volume expansion throughout its liquid range, made it useful in thermometers. (Mercury thermometers were supplanted by more accurate electronic digital thermometers in the early 21st century.) Barometers and manometers also used its high density and low vapour pressure. However, mercury’s toxicity has led to its replacement in these instruments. Gold and silver dissolve readily in mercury, and in the past this property was used in the extraction of these metals from their ores.
The good electrical conductivity of mercury makes it exceptionally useful in sealed electrical switches and relays. An electrical discharge through mercury vapour contained in a fused silica tube or bulb produces a bluish glow rich in ultraviolet light, a phenomenon exploited in ultraviolet, fluorescent, and high-pressure mercury-vapour lamps. Some mercury is used in the preparation of pharmaceuticals and agricultural and industrial fungicides.
In the 20th century the use of mercury in the manufacture of chlorine and sodium hydroxide by electrolysis of brine depended upon the fact that mercury employed as the negative pole, or cathode, dissolves the sodium liberated to form a liquid amalgam. In the early 21st century, however, mercury-cell plants for manufacturing chlorine and sodium hydroxide have mostly been phased out.
Mercury occurs in Earth’s crust on the average of about 0.08 gram (0.003 ounce) per ton of rock. The principal ore is the red sulfide, cinnabar. Native mercury occurs in isolated drops and occasionally in larger fluid masses, usually with cinnabar, near volcanoes or hot springs. Extremely rare natural alloys of mercury have also been found: moschellandsbergite (with silver), potarite (with palladium), and gold amalgam. Over 90 percent of the world’s supply of mercury comes from China; it is often a by-product of gold mining.
Cinnabar is mined in shaft or open-pit operations and refined by flotation. Most of the methods of extraction of mercury rely on the volatility of the metal and the fact that cinnabar is readily decomposed by air or by lime to yield the free metal. Mercury is extracted from cinnabar by roasting it in air, followed by condensation of the mercury vapour. Because of the toxicity of mercury and the threat of rigid pollution control, attention is being directed toward safer methods of extracting mercury. These generally rely on the fact that cinnabar is readily soluble in solutions of sodium hypochlorite or sulfide, from which the mercury can be recovered by precipitation with zinc or aluminum or by electrolysis.
Mercury is toxic. Poisoning may result from inhalation of the vapour, ingestion of soluble compounds, or absorption of mercury through the skin.
Natural mercury is a mixture of seven stable isotopes: 196Hg (0.15 percent), 198Hg (9.97 percent), 199Hg (16.87 percent), 200Hg (23.10 percent), 201Hg (13.18 percent), 202Hg (29.86 percent), and 204Hg (6.87 percent). Isotopically pure mercury consisting of only mercury-198 prepared by neutron bombardment of natural gold, gold-197, has been used as a wavelength standard and for other precise work.
Principal compounds
The compounds of mercury are either of +1 or +2 oxidation state. Mercury(II) or mercuric compounds predominate. Mercury does not combine with oxygen to produce mercury(II) oxide, HgO, at a useful rate until heated to the range of 300 to 350 °C (572 to 662 °F). At temperatures of about 400 °C (752 °F) and above, the reaction reverses with the compound decomposing into its elements. Antoine-Laurent Lavoisier and Joseph Priestley used this reaction in their study of oxygen.
There are relatively few mercury(I) or mercurous compounds. The mercury(I) ion, Hg22+, is diatomic and stable. Mercury(I) chloride, Hg2Cl2 (commonly known as calomel), is probably the most important univalent compound. It was used in antiseptic salves. Mercury(II) chloride, HgCl2 (also called bichloride of mercury or corrosive sublimate), is perhaps the commonest bivalent compound. Although extremely toxic, this odourless, colourless substance has a wide variety of applications. In agriculture it is used as a fungicide, in medicine it was sometimes employed as a topical antiseptic in concentrations of one part per 2,000 parts of water, and in the chemical industry it serves as a catalyst in the manufacture of vinyl chloride and as a starting material in the production of other mercury compounds. Mercury(II) oxide, HgO, provides elemental mercury for the preparation of various organic mercury compounds and certain inorganic mercury salts. This red or yellow crystalline solid is also used as an electrode (mixed with graphite) in zinc-mercuric oxide electric cells and in mercury batteries. Mercury(II) sulfide, HgS, is a black or red crystalline solid used chiefly as a pigment in paints, rubber, and plastics.
Additional Information:
Appearance
A liquid, silvery metal.
Uses
Mercury has fascinated people for millennia, as a heavy liquid metal. However, because of its toxicity, many uses of mercury are being phased out or are under review.
It is now mainly used in the chemical industry as catalysts. It is also used in some electrical switches and rectifiers.
Previously its major use was in the manufacture of sodium hydroxide and chlorine by electrolysis of brine. These plants will all be phased out by 2020. It was also commonly used in batteries, fluorescent lights, felt production, thermometers and barometers. Again, these uses have been phased out.
Mercury easily forms alloys, called amalgams, with other metals such as gold, silver and tin. The ease with which it amalgamates with gold made it useful in recovering gold from its ores. Mercury amalgams were also used in dental fillings.
Mercuric sulfide (vermilion) is a high-grade, bright-red paint pigment, but is very toxic so is now only used with great care.
Biological role
Mercury has no known biological role, but is present in every living thing and widespread in the environment. Every mouthful of food we eat contains a little mercury.
Our daily intake is less than 0.01 milligrams (about 0.3 grams in a lifetime), and this we can cope with easily. However, in much higher doses it is toxic and one form of mercury – methylmercury – is particularly dangerous. It can accumulate in the flesh of fish and be eaten by people, making them ill.
Natural abundance
Mercury rarely occurs uncombined in nature, but can be found as droplets in cinnabar (mercury sulfide) ores. China and Kyrgyzstan are the main producers of mercury. The metal is obtained by heating cinnabar in a current of air and condensing the vapour.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Mercury