Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Hafnium
Pages: 1
#1 Yesterday 17:22:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,563
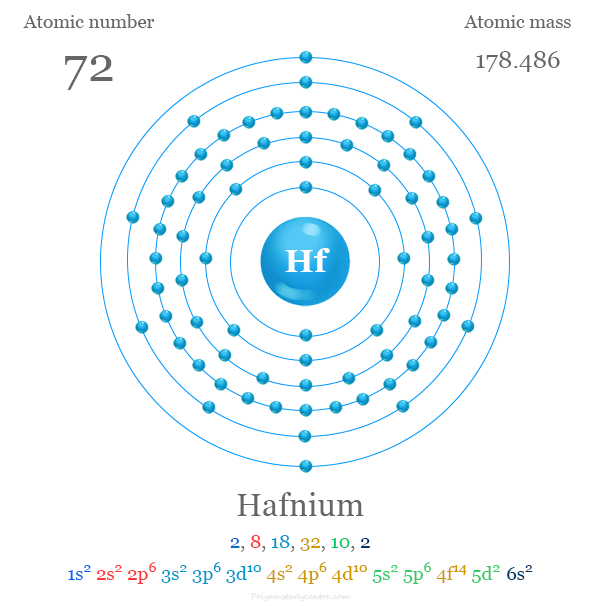
Hafnium
Hafnium
Gist
Hafnium (Hf) is a lustrous, silvery-gray, refractory chemical element with atomic number 72, similar to zirconium, and is used in alloys for high-temperature applications and as control rods in nuclear reactors due to its neutron absorption properties. Discovered in 1923 by George de Hevesy and Dirk Coster, hafnium is obtained from zirconium minerals and is characterized by its high melting point, excellent corrosion resistance, and its use in microprocessors and ceramics.
Hafnium is used in high-performance superalloys for the aerospace industry, as control rods in nuclear reactors due to its neutron-absorbing properties, and in microelectronics for its oxide's use as a high-k dielectric in smaller, more efficient transistors and integrated circuits. Other applications include high-temperature ceramics and refractories, electrodes for plasma cutting torches, and components in incandescent lamps.
Summary
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.
Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten.
Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear reactors.
Details
Hafnium (Hf) is a chemical element (atomic number 72), metal of Group 4 (IVb) of the periodic table. It is a ductile metal with a brilliant silvery lustre. The Dutch physicist Dirk Coster and the Hungarian Swedish chemist George Charles von Hevesy discovered (1923) hafnium in Norwegian and Greenland zircons by analyzing their X-ray spectra. They named the new element for Copenhagen (in New Latin, Hafnia), the city in which it was discovered. Hafnium is dispersed in Earth’s crust to the extent of three parts per million and is invariably found in zirconium minerals up to a few percent compared with zirconium. For example, the minerals zircon, ZrSiO4 (zirconium orthosilicate), and baddeleyite, which is essentially pure zirconium dioxide, ZrO2, generally have a hafnium content that varies from a few tenths of 1 percent to several percent. Altered zircons, like some alvites and cyrtolites, products of residual crystallization, show greater percentages of hafnium (up to 17 percent hafnium oxide in cyrtolite from Rockport, Mass., U.S.). Commercial sources of hafnium-bearing zirconium minerals are found in beach sands and river gravel in the United States (principally Florida), Australia, Brazil, western Africa, and India. Hafnium vapour has been identified in the Sun’s atmosphere.
Ion-exchange and solvent-extraction techniques have supplanted fractional crystallization and distillation as the preferred methods of separating hafnium from zirconium. In the procedure, crude zirconium tetrachloride is dissolved in an aqueous solution of ammonium thiocyanate, and methyl isobutyl ketone is passed countercurrent to the aqueous mixture, with the result that the hafnium tetrachloride is preferentially extracted. The metal itself is prepared by magnesium reduction of hafnium tetrachloride (Kroll process, which is also used for titanium) and by the thermal decomposition of tetraiodide (de Boer–van Arkel process).
For some purposes separation of the two elements is not important; zirconium containing about 1 percent of hafnium is as acceptable as pure zirconium. In the case of the largest single use of zirconium, however, namely, as a structural and cladding material in nuclear reactors, it is essential that the zirconium be essentially free of hafnium, because the usefulness of zirconium in reactors is based on its extremely low absorption cross section for neutrons. Hafnium, on the other hand, has an exceptionally high cross section, and accordingly even slight hafnium contamination nullifies the intrinsic advantage of the zirconium. Because of its high neutron-capture cross section and its excellent mechanical properties, hafnium is used for fabricating nuclear-control rods.
Hafnium produces a protective film of oxide or nitride upon contact with air and thus has high corrosion resistance. Hafnium is fairly resistant to acids and is best dissolved in hydrofluoric acid, in which procedure the formation of anionic fluoro complexes is important in stabilizing the solution. At normal temperatures hafnium is not particularly reactive but becomes quite reactive with a variety of nonmetals at elevated temperatures. It forms alloys with iron, niobium, tantalum, titanium, and other transition metals. The alloy tantalum hafnium carbide (Ta4HfC5), with a melting point of 4,215 °C (7,619 °F), is one of the most refractory substances known.
Hafnium is chemically similar to zirconium. Both transition metals have similar electronic configurations, and their ionic radii (Zr4+, 0.74 Å, and Hf4+, 0.75 Å) and atomic radii (zirconium, 1.45 Å, and hafnium, 1.44 Å) are nearly identical because of the influence of the lanthanoid contraction. In fact, the chemical behaviour of these two elements is more similar than for any other pair of elements known. Although the chemistry of hafnium has been studied less than that of zirconium, the two are so similar that only very small quantitative differences—for example, in solubilities and volatilities of compounds—would be expected in cases that have not actually been investigated. Natural hafnium is a mixture of six stable isotopes: hafnium-174 (0.2 percent), hafnium-176 (5.2 percent), hafnium-177 (18.6 percent), hafnium-178 (27.1 percent), hafnium-179 (13.7 percent), and hafnium-180 (35.2 percent).
The most important respect in which hafnium differs from titanium is that lower oxidation states are of minor importance; there are relatively few compounds of hafnium in other than its tetravalent states. (However, a few trivalent compounds are known.) The increased size of the atoms makes the oxides more basic and the aqueous chemistry somewhat more extensive and permits the attainment of coordination numbers 7 and, quite frequently, 8 in a number of hafnium compounds.
Element Properties
atomic number : 72
atomic weight : 178.486
melting point : 2,227 °C (4,041 °F)
boiling point : 4,603 °C (8,317 °F)
specific gravity : 13.31 (20 °C)
oxidation state : +4.
Additional Information:
Appearance
A shiny, silvery metal that resists corrosion and can be drawn into wires.
Uses
Hafnium is a good absorber of neutrons and is used to make control rods, such as those found in nuclear submarines. It also has a very high melting point and because of this is used in plasma welding torches.
Hafnium has been successfully alloyed with several metals including iron, titanium and niobium.
Hafnium oxide is used as an electrical insulator in microchips, while hafnium catalysts have been used in polymerisation reactions.
Biological role
Hafnium has no known biological role, and it has low toxicity.
Natural abundance
Most zirconium ores contain around 5% hafnium. The metal can be prepared by reducing hafnium tetrachloride with sodium or magnesium.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Hafnium