Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Neodymium
Pages: 1
#1 2025-08-17 15:54:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,439
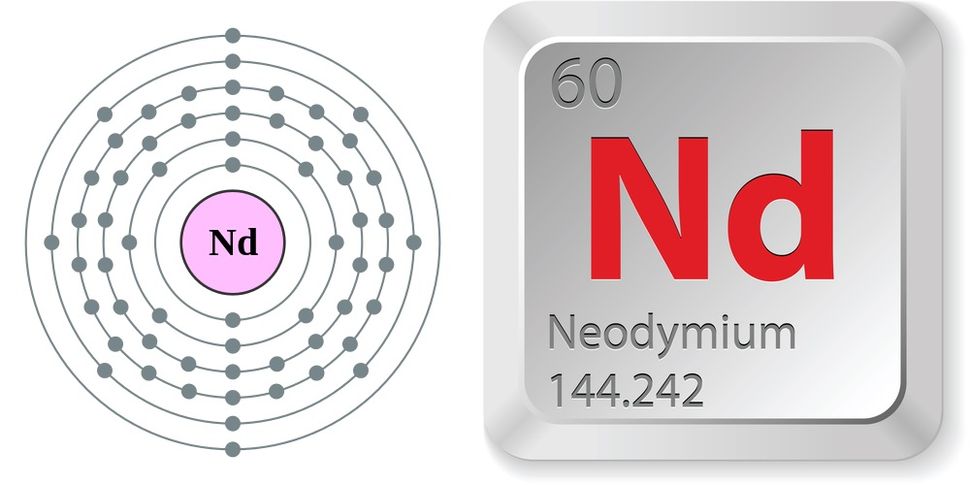
Neodymium
Neodymium
Gist
Neodymium is a chemical element with the symbol Nd and atomic number 60. It's a soft, silvery-white metal that is part of the lanthanide series and is considered a rare earth metal. Neodymium is known for its magnetic properties and is a key component in high-strength permanent magnets. It's also used in various other applications, including glass coloring and lasers.
The most important use for neodymium is in an alloy with iron and boron to make very strong permanent magnets. This discovery, in 1983, made it possible to miniaturise many electronic devices, including mobile phones, microphones, loudspeakers and electronic musical instruments.
Summary
Neodymium is a chemical element; it has symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. Neodymium is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China, as is the case with many other rare-earth metals.
Neodymium compounds were first commercially used as glass dyes in 1927 and remain a popular additive. The color of neodymium compounds comes from the Nd3+ ion and is often a reddish-purple. This color changes with the type of lighting because of the interaction of the sharp light absorption bands of neodymium with ambient light enriched with the sharp visible emission bands of mercury, trivalent europium or terbium. Glasses that have been doped with neodymium are used in lasers that emit infrared with wavelengths between 1047 and 1062 nanometers. These lasers have been used in extremely high-power applications, such as in inertial confinement fusion. Neodymium is also used with various other substrate crystals, such as yttrium aluminium garnet in the Nd:YAG laser.
Neodymium alloys are used to make high-strength neodymium magnets, which are powerful permanent magnets. These magnets are widely used in products like microphones, professional loudspeakers, in-ear headphones, high-performance hobby DC electric motors, and computer hard disks, where low magnet mass (or volume) or strong magnetic fields are required. Larger neodymium magnets are used in electric motors with a high power-to-weight ratio (e.g., in hybrid cars) and generators (e.g., aircraft and wind turbine electric generators).
Details
Neodymium (Nd) is a chemical element, a rare-earth metal of the lanthanide series of the periodic table.
Neodymium is a ductile and malleable silvery white metal. It oxidizes readily in air to form an oxide, Nd2O3, which easily spalls, exposing the metal to further oxidation. The metal must be stored sealed in a plastic covering or kept in vacuum or in an inert atmosphere. It reacts gradually with mineral acids—except hydrofluoric acid (HF), in which it forms a protective layer of trifluoride, NdF3. Neodymium is strongly paramagnetic and orders antiferromagnetically at 7.5 and 19.9 K (−265.7 and −253.3 °C, or −446.2 and −423.9 °F) with spontaneous magnetic moments developing separately on different independent sites, hexagonal and cubic, respectively.
Austrian chemist Carl Auer von Welsbach discovered neodymium in 1885 by separating ammonium didymium nitrate prepared from didymia (a mixture of rare-earth oxides) into a neodymium fraction and a praseodymium fraction by repeated crystallization. Of the rare earths, only yttrium, lanthanum, and cerium are more plentiful than neodymium. In the igneous rocks of Earth’s crust, it is more than twice as abundant as lead and about half as plentiful as copper.
Natural neodymium is a mixture of seven different isotopes. Five of them are stable—neodymium-142 (27.13 percent), neodymium-146 (17.19 percent), neodymium-143 (12.18 percent), neodymium-145 (8.30 percent), and neodymium-148 (5.76 percent)—and two are radioactive, neodymium-144 (23.80 percent) and neodymium-150 (5.64 percent). A total of 31 radioactive isotopes of neodymium (excluding nuclear isomers) have been characterized, ranging in mass from 124 to 161 and in half-life from 0.65 second (neodymium-125) to 7.9 × {10}^{18} years (neodymium-150).
Neodymium occurs in the minerals monazite and bastnasite and is a product of nuclear fission. Liquid-liquid separation or ion-exchange techniques are employed for separation and purification of neodymium. The metal itself is prepared by electrolysis of the fused halides or by metallothermic reduction of the fluoride with calcium. Two allotropes (structural forms) of neodymium exist: the α-phase is double close-packed hexagonal with a = 3.6582 Å and c = 11.7966 Å at room temperature. The β-phase is body-centred cubic with a = 4.13 Å at 883 °C (1,621 °F).
The major application of neodymium is in high-strength permanent magnets based on Nd2Fe14B that are used in high-performance electric motors and generators, as well as in spindle magnets for computer hard drives and wind turbines. The metal is used in the electronics industry, in the manufacture of steel, and as a component in a number of ferrous and nonferrous alloys, among them misch metal (15 percent neodymium), used for lighter flints. The metal itself—and as an alloy with another lanthanide, erbium—has been employed as a regenerator in low-temperature cryocooler applications to provide cooling down to 4.2 K (−269 °C, or −452 °F). Its compounds are used in the ceramics industry for glazes and to colour glass in various shades from pink to purple. Neodymium-stabilized yttrium aluminum garnet (YAG) is a component of many modern lasers, and neodymium glasses are used in fibre optics. A mixture of neodymium and praseodymium absorbs light in the region of the harmful sodium-D spectral lines and therefore is used in the glass of welders’ and glassblowers’ goggles.
The element in its compounds such as the oxide Nd2O3 and the hydroxide Nd(OH)3 is nearly always in the +3 oxidation state; the Nd3+ ion is stable in water. Only a few compounds of neodymium in the +2 state have been prepared, such as the diiodide NdI2 and the dichloride NdCl2; the Nd2+ ion is unstable in aqueous solution.
Element Properties
atomic number : 60
atomic weight : 144.24
melting point : 1,021 °C (1,870 °F)
boiling point : 3,074 °C (5,565 °F)
specific gravity : 7.008 (25 °C, or 77 °F)
oxidation states : +2 (rare, unstable), +3.
Additional Information:
Appearance
A silvery-white metal. It rapidly tarnishes in air.
Uses
The most important use for neodymium is in an alloy with iron and boron to make very strong permanent magnets. This discovery, in 1983, made it possible to miniaturise many electronic devices, including mobile phones, microphones, loudspeakers and electronic musical instruments. These magnets are also used in car windscreen wipers and wind turbines.
Neodymium is a component, along with praseodymium, of didymium glass. This is a special glass for goggles used during glass blowing and welding. The element colours glass delicate shades of violet, wine-red and grey. Neodymium is also used in the glass for tanning booths, since it transmits the tanning UV rays but not the heating infrared rays.
Neodymium glass is used to make lasers. These are used as laser pointers, as well as in eye surgery, cosmetic surgery and for the treatment of skin cancers.
Neodymium oxide and nitrate are used as catalysts in polymerisation reactions.
Biological role
Neodymium has no known biological role. It is moderately toxic and irritating to eyes.
Natural abundance
The main sources of most lanthanide elements are the minerals monazite and bastnaesite. Neodymium can be extracted from these minerals by ion exchange and solvent extraction. The element can also be obtained by reducing anhydrous neodymium chloride or fluoride with calcium.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Neodymium