Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Carbon
Pages: 1
#1 2025-07-17 16:45:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,563
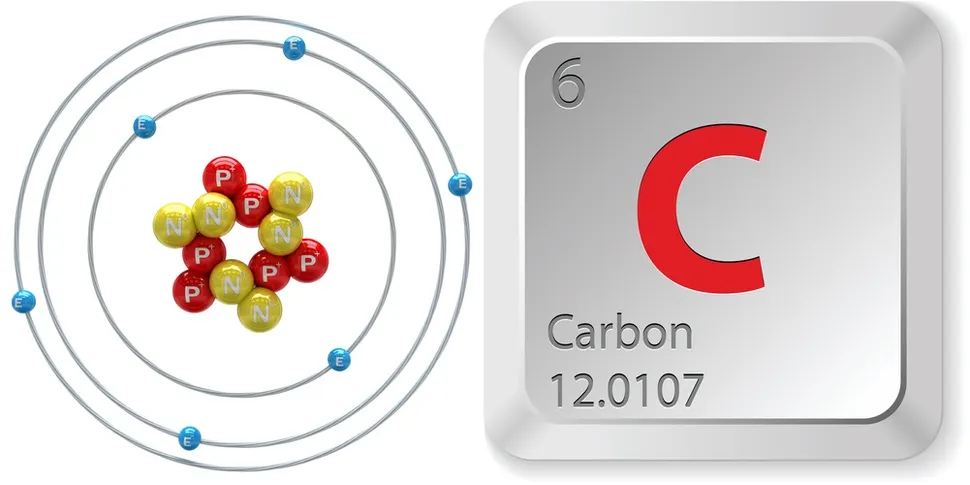
Carbon
Carbon
Gist
Carbon, with the symbol C and atomic number 6, is a nonmetallic chemical element crucial for all known life. It's tetravalent, meaning its atoms can form up to four covalent bonds, and is a key component of organic molecules. Carbon is abundant in the Earth's crust and plays a vital role in the carbon cycle and climate regulation.
Carbon, an essential element for life, is primarily formed in stars through a process called the triple-alpha process. This process involves the fusion of three helium nuclei (alpha particles) to create a carbon nucleus.
Carbon is a fundamental element in life science and biology, serving as the backbone of all living organisms. It is the building block of organic molecules, including DNA, proteins, carbohydrates, and lipids, which are essential for life processes. Carbon's unique ability to form stable bonds with other atoms allows for the creation of diverse and complex molecules necessary for life.
Summary
Carbon (from Latin carbo 'coal') is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the few elements known since antiquity.
Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.
The atoms of carbon can bond together in diverse ways, resulting in various allotropes of carbon. Well-known allotropes include graphite, diamond, amorphous carbon, and fullerenes. The physical properties of carbon vary widely with the allotropic form. For example, graphite is opaque and black, while diamond is highly transparent. Graphite is soft enough to form a streak on paper (hence its name, from the Greek verb which means "to write"), while diamond is the hardest naturally occurring material known. Graphite is a good electrical conductor while diamond has a low electrical conductivity. Under normal conditions, diamond, carbon nanotubes, and graphene have the highest thermal conductivities of all known materials. All carbon allotropes are solids under normal conditions, with graphite being the most thermodynamically stable form at standard temperature and pressure. They are chemically resistant and require high temperature to react even with oxygen.
The most common oxidation state of carbon in inorganic compounds is +4, while +2 is found in carbon monoxide and transition metal carbonyl complexes. The largest sources of inorganic carbon are limestones, dolomites and carbon dioxide, but significant quantities occur in organic deposits of coal, peat, oil, and methane clathrates. Carbon forms a vast number of compounds, with about two hundred million having been described and indexed; and yet that number is but a fraction of the number of theoretically possible compounds under standard conditions.
Details
Carbon (C), nonmetallic chemical element in Group 14 (IVa) of the periodic table. Although widely distributed in nature, carbon is not particularly plentiful—it makes up only about 0.025 percent of Earth’s crust—yet it forms more compounds than all the other elements combined. In 1961 the isotope carbon-12 was selected to replace oxygen as the standard relative to which the atomic weights of all the other elements are measured. Carbon-14, which is radioactive, is the isotope used in radiocarbon dating and radiolabeling.
Element Properties
atomic number : 6
atomic weight : 12.0096 to 12.0116
melting point : 3,550 °C (6,420 °F)
boiling point : 4,827 °C (8,721 °F)
Density
diamond : 3.52 g/{cm}^3
graphite : 2.25 g/{cm}^3
amorphous : 1.9 g/cm3
oxidation states : +2, +3, +4
Properties and uses
On a weight basis, carbon is 19th in order of elemental abundance in Earth’s crust, and there are estimated to be 3.5 times as many carbon atoms as silicon atoms in the universe. Only hydrogen, helium, oxygen, neon, and nitrogen are atomically more abundant in the cosmos than carbon. Carbon is the cosmic product of the “burning” of helium, in which three helium nuclei, atomic weight 4, fuse to produce a carbon nucleus, atomic weight 12.
In the crust of Earth, elemental carbon is a minor component. However, carbon compounds (i.e., carbonates of magnesium and calcium) form common minerals (e.g., magnesite, dolomite, marble, or limestone). Coral and the shells of oysters and clams are primarily calcium carbonate. Carbon is widely distributed as coal and in the organic compounds that constitute petroleum, natural gas, and all plant and animal tissue. A natural sequence of chemical reactions called the carbon cycle—involving conversion of atmospheric carbon dioxide to carbohydrates by photosynthesis in plants, the consumption of these carbohydrates by animals and oxidation of them through metabolism to produce carbon dioxide and other products, and the return of carbon dioxide to the atmosphere—is one of the most important of all biological processes.
Carbon as an element was discovered by the first person to handle charcoal from fire. Thus, together with sulfur, iron, tin, lead, copper, mercury, silver, and gold, carbon was one of the small group of elements well known in the ancient world. Modern carbon chemistry dates from the development of coals, petroleum, and natural gas as fuels and from the elucidation of synthetic organic chemistry, both substantially developed since the 1800s.
Elemental carbon exists in several forms, each of which has its own physical characteristics. Two of its well-defined forms, diamond and graphite, are crystalline in structure, but they differ in physical properties because the arrangements of the atoms in their structures are dissimilar. A third form, called fullerene, consists of a variety of molecules composed entirely of carbon. Spheroidal, closed-cage fullerenes are called buckerminsterfullerenes, or “buckyballs,” and cylindrical fullerenes are called nanotubes. A fourth form, called Q-carbon, is crystalline and magnetic. Yet another form, called amorphous carbon, has no crystalline structure. Other forms—such as carbon black, charcoal, lampblack, coal, and coke—are sometimes called amorphous, but X-ray examination has revealed that these substances do possess a low degree of crystallinity. Diamond and graphite occur naturally on Earth, and they also can be produced synthetically; they are chemically inert but do combine with oxygen at high temperatures, just as amorphous carbon does. Fullerene was serendipitously discovered in 1985 as a synthetic product in the course of laboratory experiments to simulate the chemistry in the atmosphere of giant stars. It was later found to occur naturally in tiny amounts on Earth and in meteorites. Q-carbon is also synthetic, but scientists have speculated that it could form within the hot environments of some planetary cores.
The word carbon probably derives from the Latin carbo, meaning variously “coal,” “charcoal,” “ember.” The term diamond, a corruption of the Greek word adamas, “the invincible,” aptly describes the permanence of this crystallized form of carbon, just as graphite, the name for the other crystal form of carbon, derived from the Greek verb graphein, “to write,” reflects its property of leaving a dark mark when rubbed on a surface. Before the discovery in 1779 that graphite when burned in air forms carbon dioxide, graphite was confused with both the metal lead and a superficially similar substance, the mineral molybdenite.
Pure diamond is the hardest naturally occurring substance known and is a poor conductor of electricity. Graphite, on the other hand, is a soft slippery solid that is a good conductor of both heat and electricity. Carbon as diamond is the most expensive and brilliant of all the natural gemstones and the hardest of the naturally occurring abrasives. Graphite is used as a lubricant. In microcrystalline and nearly amorphous form, it is used as a black pigment, as an adsorbent, as a fuel, as a filler for rubber, and, mixed with clay, as the “lead” of pencils. Because it conducts electricity but does not melt, graphite is also used for electrodes in electric furnaces and dry cells as well as for making crucibles in which metals are melted. Molecules of fullerene show promise in a range of applications, including high-tensile-strength materials, unique electronic and energy-storage devices, and safe encapsulation of flammable gases, such as hydrogen. Q-carbon, which is created by rapidly cooling a sample of elemental carbon whose temperature has been raised to 4,000 K (3,727 °C [6,740 °F]), is harder than diamond, and it can be used to manufacture diamond structures (such as diamond films and microneedles) within its matrix. Elemental carbon is nontoxic.
Each of the “amorphous” forms of carbon has its own specific character, and, hence, each has its own particular applications. All are products of oxidation and other forms of decomposition of organic compounds. Coal and coke, for example, are used extensively as fuels. Charcoal is used as an absorptive and filtering agent and as a fuel and was once widely used as an ingredient in gunpowder. (Coals are elemental carbon mixed with varying amounts of carbon compounds. Coke and charcoal are nearly pure carbon.) In addition to its uses in making inks and paints, carbon black is added to the rubber used in tires to improve its wearing qualities. Bone black, or animal charcoal, can adsorb gases and colouring matter from many other materials.
Carbon, either elemental or combined, is usually determined quantitatively by conversion to carbon dioxide gas, which can then be absorbed by other chemicals to give either a weighable product or a solution with acidic properties that can be titrated.
Additional Information:
Appearance
There are a number of pure forms of this element including graphite, diamond, fullerenes and graphene.
Diamond is a colourless, transparent, crystalline solid and the hardest known material. Graphite is black and shiny but soft. The nano-forms, fullerenes and graphene, appear as black or dark brown, soot-like powders.
Uses
Carbon is unique among the elements in its ability to form strongly bonded chains, sealed off by hydrogen atoms. These hydrocarbons, extracted naturally as fossil fuels (coal, oil and natural gas), are mostly used as fuels. A small but important fraction is used as a feedstock for the petrochemical industries producing polymers, fibres, paints, solvents and plastics etc.
Impure carbon in the form of charcoal (from wood) and coke (from coal) is used in metal smelting. It is particularly important in the iron and steel industries.
Graphite is used in pencils, to make brushes in electric motors and in furnace linings. Activated charcoal is used for purification and filtration. It is found in respirators and kitchen extractor hoods.
Carbon fibre is finding many uses as a very strong, yet lightweight, material. It is currently used in tennis rackets, skis, fishing rods, rockets and aeroplanes.
Industrial diamonds are used for cutting rocks and drilling. Diamond films are used to protect surfaces such as razor blades.
The more recent discovery of carbon nanotubes, other fullerenes and atom-thin sheets of graphene has revolutionised hardware developments in the electronics industry and in nanotechnology generally.
150 years ago the natural concentration of carbon dioxide in the Earth’s atmosphere was 280 ppm. In 2013, as a result of combusting fossil fuels with oxygen, there was 390 ppm. Atmospheric carbon dioxide allows visible light in but prevents some infrared escaping (the natural greenhouse effect). This keeps the Earth warm enough to sustain life. However, an enhanced greenhouse effect is underway, due to a human-induced rise in atmospheric carbon dioxide. This is affecting living things as our climate changes.
Biological role
Carbon is essential to life. This is because it is able to form a huge variety of chains of different lengths. It was once thought that the carbon-based molecules of life could only be obtained from living things. They were thought to contain a ‘spark of life’. However, in 1828, urea was synthesised from inorganic reagents and the branches of organic and inorganic chemistry were united.
Living things get almost all their carbon from carbon dioxide, either from the atmosphere or dissolved in water. Photosynthesis by green plants and photosynthetic plankton uses energy from the sun to split water into oxygen and hydrogen. The oxygen is released to the atmosphere, fresh water and seas, and the hydrogen joins with carbon dioxide to produce carbohydrates.
Some of the carbohydrates are used, along with nitrogen, phosphorus and other elements, to form the other monomer molecules of life. These include bases and sugars for RNA and DNA, and amino acids for proteins.
Living things that do not photosynthesise have to rely on consuming other living things for their source of carbon molecules. Their digestive systems break carbohydrates into monomers that they can use to build their own cellular structures. Respiration provides the energy needed for these reactions. In respiration oxygen rejoins carbohydrates, to form carbon dioxide and water again. The energy released in this reaction is made available for the cells.
Natural abundance
Carbon is found in the sun and other stars, formed from the debris of a previous supernova. It is built up by nuclear fusion in bigger stars.
It is present in the atmospheres of many planets, usually as carbon dioxide. On Earth, the concentration of carbon dioxide in the atmosphere is currently 390 ppm and rising.
Graphite is found naturally in many locations. Diamond is found in the form of microscopic crystals in some meteorites. Natural diamonds are found in the mineral kimberlite, sources of which are in Russia, Botswana, DR Congo, Canada and South Africa.
In combination, carbon is found in all living things. It is also found in fossilised remains in the form of hydrocarbons (natural gas, crude oil, oil shales, coal etc) and carbonates (chalk, limestone, dolomite etc).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Carbon