Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Osmium
Pages: 1
#1 2025-04-10 17:17:26
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,460
Osmium
Osmium
Gist
Osmium (Os), a hard, brittle, bluish-white transition metal, is the densest naturally occurring element, belonging to the platinum group and having atomic number 76.
Osmium (Os), a chemical element, is one of the platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table and the densest naturally occurring element. A gray-white metal, osmium is very hard, brittle, and difficult to work, even at high temperatures
Osmium is primarily used in hard alloys, especially when alloyed with other platinum group metals like platinum, for applications like surgical implants, fountain pen tips, and electrical contacts. Osmium tetroxide, a compound of osmium, is also used as a stain in microscopy and for fingerprint detection.
Summary
Osmium (Os), chemical element, one of the platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table and the densest naturally occurring element. A gray-white metal, osmium is very hard, brittle, and difficult to work, even at high temperatures. Of the platinum metals, it has the highest melting point, so fusing and casting are difficult. Osmium wires were used for filaments of early incandescent lamps before the introduction of tungsten. It has been used chiefly as a hardener in alloys of the platinum metals, though ruthenium has generally replaced it. A hard alloy of osmium and iridium was used for tips of fountain pens and phonograph needles, and osmium tetroxide is used in certain organic syntheses.
Pure osmium metal does not occur in nature. Osmium has a low crustal abundance of about 0.001 part per million. Though rare, osmium is found in native alloys with other platinum metals: in siserskite (up to 80 percent), in iridosmine, in aurosmiridium (25 percent), and in slight amounts in native platinum. Processes for isolating it are an integral part of the metallurgical art that applies to all platinum metals.
The English chemist Smithson Tennant discovered the element together with iridium in the residues of platinum ores not soluble in aqua regia. He announced its isolation (1804) and named it for the unpleasant odour of some of its compounds (Greek osme, odour).
Osmium exhibits oxidation states from 0 to +8 in its compounds, with the exception of +1; well-characterized and stable compounds contain the element in +2, +3, +4, +6, and +8 states. There are also carbonyl and organometallic compounds in the low oxidation states −2, 0, and +1. Ruthenium is the only other element known to have an oxidation state of 8. (The chemistries of ruthenium and osmium are generally similar.) All compounds of osmium are easily reduced or decomposed by heating to form the free element as a powder or sponge. There is an extensive chemistry of the tetroxides, oxohalides, and oxo anions. There is little, if any, evidence that simple aquo ions exist, and virtually all their aqueous solutions, whatever the anions present, may be considered to contain complexes.
Natural osmium consists of a mixture of seven stable isotopes: osmium-184 (0.02 percent), osmium-186 (1.59 percent), osmium-187 (1.96 percent), osmium-188 (13.24 percent), osmium-189 (16.15 percent), osmium-190 (26.26 percent), and osmium-192 (40.78 percent).
Element Properties
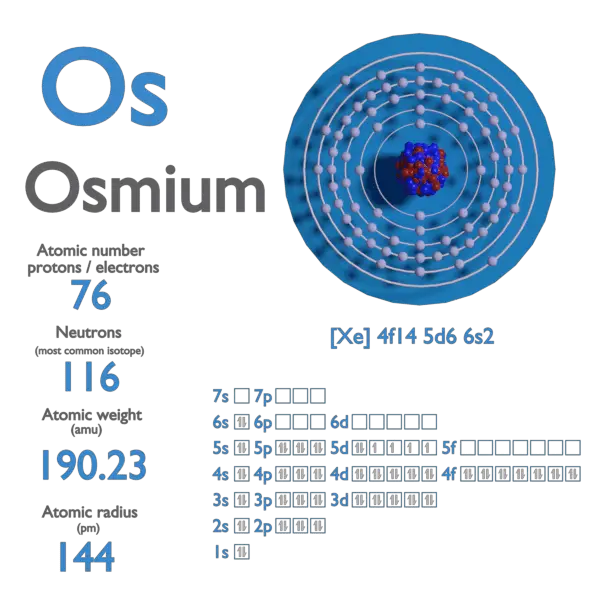
atomic number : 76
atomic weight : 190.23
melting point : 3,000 °C (5,432 °F)
boiling point : about 5,000 °C (9,032 °F)
specific gravity : 22.59 (20 °C).
Details
Osmium is a chemical element; it has symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element. When experimentally measured using X-ray crystallography, it has a density of 22.59 g/{cm}^{3}. Manufacturers use its alloys with platinum, iridium, and other platinum-group metals to make fountain pen nib tipping, electrical contacts, and in other applications that require extreme durability and hardness.
Osmium is among the rarest elements in the Earth's crust, making up only 50 parts per trillion (ppt).
Characteristics:
Physical properties
Osmium is a hard, brittle, blue-gray metal, and the densest stable element—about twice as dense as lead. The density of osmium is slightly greater than that of iridium; the two are so similar (22.587 versus 22.562 g/{cm}^{3} at 20 °C) that each was at one time considered to be the densest element. Only in the 1990s were measurements made accurately enough (by means of X-ray crystallography) to be certain that osmium is the denser of the two.
Osmium has a blue-gray tint. The reflectivity of single crystals of osmium is complex and strongly direction-dependent, with light in the red and near-infrared wavelengths being more strongly absorbed when polarized parallel to the c crystal axis than when polarized perpendicular to the c axis; the c-parallel polarization is also slightly more reflected in the mid-ultraviolet range. Reflectivity reaches a sharp minimum at around 1.5 eV (near-infrared) for the c-parallel polarization and at 2.0 eV (orange) for the c-perpendicular polarization, and peaks for both in the visible spectrum at around 3.0 eV (blue-violet).
Osmium is a hard but brittle metal that remains lustrous even at high temperatures. It has a very low compressibility. Correspondingly, its bulk modulus is extremely high, reported between 395 and 462 GPa, which rivals that of diamond (443 GPa). The hardness of osmium is moderately high at 4 GPa. Because of its hardness, brittleness, low vapor pressure (the lowest of the platinum-group metals), and very high melting point (the fourth highest of all elements, after carbon, tungsten, and rhenium), solid osmium is difficult to machine, form, or work.
Chemical properties
Osmium forms compounds with oxidation states ranging from −4 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium.[
Only two osmium compounds have major applications: osmium tetroxide for staining tissue in electron microscopy and for the oxidation of alkenes in organic synthesis, and the non-volatile osmates for organic oxidation reactions.
Osmium pentafluoride (OsF5) is known, but osmium trifluoride (OsF3) has not yet been synthesized. The lower oxidation states are stabilized by the larger halogens, so that the trichloride, tribromide, triiodide, and even diiodide are known.
Additional Information
Osmium is a hard, brittle, blue-gray transition metal belonging to the platinum family and is the densest natural element. It possesses a hexagonal close-packed structure. Osmium can be applied as electrical contact material or diffusion barrier in microelectronic devices because it possesses a high melting point (3050 °C) and metallic conductivity. It is mechanically strong and chemically inert. Next to electronic devices, osmium is also of interest in catalysis as well as fuel cell and sensor technologies.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
Pages: 1
- Index
- » Science HQ
- » Osmium