Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#201 Re: This is Cool » Behind the scenes of proof by contradiction II » 2025-08-25 02:33:18
Please hide tags for hiding the information, and use URL.
Hide hereURL here.[hide]Hidden text[/ hide][url = Here][/url]#202 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-08-24 22:02:22
2229) Alexey Ekimov
Gist:
Work
When grains of matter become very small – when they shrink to nano-dimensions – quantum phenomena arise. Such particles are called quantum dots. In 1981, Aleksey Yekimov succeeded in creating size-dependent quantum effects in coloured glass. The colour came from nanoparticles of copper chloride and Yekimov demonstrated that the particle size affected the colour of the glass via quantum effects. Quantum dots are used in electronics, including computer and television screens as well as LED lamps. They can also be used to map biological tissue.
Summary
Alexei Ekimov (born 1945, U.S.S.R.) is a Soviet-born American physicist who was awarded the 2023 Nobel Prize in Chemistry for his work in producing quantum dots, which are very small particles whose unusual quantum properties depend on their size. He shared the prize with American chemist Louis Brus and French-born American chemist Moungi Bawendi.
Ekimov graduated with a bachelor’s degree in physics from Leningrad State University (now St. Petersburg University) in 1967. He received a Ph.D. in physics in 1974 from the Ioffe Physical-Technical Institute of the Russian Academy of Sciences, also in Leningrad (now St. Petersburg). Following this, Ekimov conducted research at the Vavilov State Optical Institute in Leningrad.
Since the 1930s physicists and chemists have known that a material’s size has a significant effect on its properties. That is, in particles of matter a few nanometers in size (1 nanometer = 10−9 meter), quantum mechanical effects become significant. Particles of this size are called nanoparticles.
In the late 1970s Ekimov became interested in colored glass and how adding different substances to glass produced the colors. He worked with glass to which he added copper chloride (CuCl). He found tiny CuCl crystals in the glass ranging in size from about 2 to 30 nanometers, the size of the crystals depending on the temperature and length of the glassmaking process.
Ekimov scattered X-rays off the glass and found that the wavelengths of the absorption lines depended on the size of the CuCl crystals in the glass. He realized that this wavelength shift was a quantum mechanical effect. He had discovered quantum dots and showed that they could be produced in the well-known glassmaking process. Ekimov and his collaborators published their work in 1981 in a Soviet journal that was not well known in the West; thus Brus did not know about Ekimov’s work until 1984, a year after Brus independently discovered quantum dots. Today quantum dots are used in many applications, including QLED (quantum-dot light-emitting diode) screens and solar cells, and as markers in biomedical imaging.
Ekimov became chief scientist at the American company Nanocrystals Technology in 1999. Among his other honors are the U.S.S.R. State Prize in Science and Engineering (1975) for his work with electron spin orientation in semiconductors and the R.W. Wood Prize (2006, with Brus).
Details
Alexey Ekimov or Aleksey Yekimov (born 1945) is a Russian solid state physicist and a pioneer in nanomaterials research. He discovered the semiconductor nanocrystals known as quantum dots in 1981, while working at the Vavilov State Optical Institute. In 2023, he was awarded the Nobel Prize in Chemistry for this discovery.
Life:
Early years and education
Ekimov was born in Leningrad, Soviet Union in 1945. In 1967, he graduated from the Faculty of Physics, Leningrad State University. He went on to receive his PhD in physics at the Ioffe Institute of the Russian Academy of Sciences in 1974.
Research and career
After graduation, Ekimov moved to the Vavilov State Optical Institute to conduct research. He began studying semiconductor-activated glasses, known as Schott glasses, and developing theories to explain their color. When the glasses were heated and then cooled, copper chloride crystals formed, as revealed by X-rays, creating blue colors. Smaller crystals produced bluer glass.
In 1981, Ekimov, along with Alexei A. Onushchenko, reported the discovery of quantum size effects in copper chloride nanocrystals in glass, a phenomenon known now known as quantum dots. During his time at the institute he further investigated these system and developed the theory of quantum confinement with Alexander Efros.
Since 1999, Ekimov has been living and working in the United States as a scientist for Nanocrystals Technology, a company based in New York State.
Honors and awards
Ekimov was awarded the 1975 USSR State Prize in Science and Engineering for work on electron spin orientation in semiconductors. He is co-recipient of the 2006 R. W. Wood Prize of the Optical Society of America for "discovery of nanocrystal quantum dots and pioneering studies of their electronic and optical properties" shared with Alexander Efros and Louis E. Brus.
Ekimov, Brus and Moungi Bawendi were the recipients of the 2023 Nobel Prize in Chemistry "for the discovery and synthesis of quantum dots".

#203 Re: Dark Discussions at Cafe Infinity » Greatest Mathematicians from 1 CE ... » 2025-08-24 21:32:05
21) Nicole Oresme
Nicole Oresme (1 January 1325 – 11 July 1382), also known as Nicolas Oresme, Nicholas Oresme, or Nicolas d'Oresme, was a French philosopher of the later Middle Ages. He wrote influential works on economics, mathematics, physics, astrology, astronomy, philosophy, and theology. He served as Bishop of Lisieux, translated Aristotelian texts for King Charles V of France, and was a prominent scholar of 14th-century Europe.
Life
Nicole Oresme was born c. 1320–1325 in the village of Allemagnes (today's Fleury-sur-Orne) in the vicinity of Caen, Normandy, in the diocese of Bayeux. Little is known about his family background, but his attendance at the royally sponsored College of Navarre in Paris, which supported students of modest means, suggests he likely came from a peasant or modest family.
Oresme studied the "arts" in Paris, together with Jean Buridan (the so-called founder of the French school of natural philosophy), Albert of Saxony and perhaps Marsilius of Inghen, and there received the Magister Artium. By 1342, he was a regent master in arts during debates over William of Ockham's natural philosophy.
In 1348, he was a student of theology in Paris.
In 1356 he received his doctorate and in the same year he became grand master (grand-maître) of the College of Navarre.
In 1364 he was appointed dean of the Cathedral of Rouen. From 1369, at the request of Charles V, he translated Aristotelian works into French, receiving a pension in 1371. In 1377, with royal support, he became bishop of Lisieux, where he died in 1382.
Mathematics
Oresme's most important contributions to mathematics are contained in Tractatus de configurationibus qualitatum et motuum. In a quality, or accidental form, such as heat, he distinguished the intensio (the degree of heat at each point) and the extensio (as the length of the heated rod). These two terms were often replaced by latitudo and longitudo. For the sake of clarity, Oresme conceived the idea of visualizing these concepts by plane figures, approaching what we would now call rectangular coordinates. The intensity of the quality was represented by a length or latitudo proportional to the intensity erected perpendicular to the base at a given point on the base line, which represents the longitudo. Oresme proposed that the geometrical form of such a figure could be regarded as corresponding to a characteristic of the quality itself. Oresme defined a uniform quality as that which is represented by a line parallel to the longitude, and any other quality as difform. Uniformly varying qualities are represented by a straight line inclined to the axis of the longitude, while he described many cases of nonuniformly varying qualities. Oresme extended this doctrine to figures of three dimensions. He considered this analysis applicable to many different qualities such as hotness, whiteness, and sweetness. Significantly for later developments, Oresme applied this concept to the analysis of local motion where the latitudo or intensity represented the speed, the longitudo represented the time, and the area of the figure represented the distance travelled. He formulated a theorem for uniformly accelerated motion, showing distance traveled as the area under a velocity-time graph, predating Galileo. have been cited to credit Oresme with the discovery of "proto bar charts". He also proved the divergence of the harmonic series and introduced early concepts of curvature. Oresme was the first mathematician to prove this fact, and (after his proof was lost) it was not proven again until the 17th century by Pietro Mengoli. He explored fractional powers and probability over infinite sequences, concepts developed centuries later.
#204 This is Cool » Khone Phapheng Falls » 2025-08-24 19:02:05
- Jai Ganesh
- Replies: 0
Khone Phephang Falls
Gist
The widest waterfalls in the world are the Khône Falls on the Mekong River in Laos, with a total width of 10,783 m (35,376 ft). The falls measure 15–21 m (50–70 ft) in height and have a flood flow of 42,500 {m}^{3}/sec (1,500,000 cusec).
It is the widest waterfall in the world at 10,783 metres (35,376 feet or 6.7 miles) in width from one edge of its multiple channels to the other. The Khone Falls are the largest in southeast Asia, and are the main reason that the Mekong is not fully navigable into China.
Summary
The Khone Falls and Pha Pheng Falls form a waterfall located in Champasak Province on the Mekong River in southern Laos, near the border with Cambodia. It is the widest waterfall in the world at 10,783 metres (35,376 feet or 6.7 miles) in width from one edge of its multiple channels to the other.
The Khone Falls are the largest in southeast Asia, and are the main reason that the Mekong is not fully navigable into China. The falls are characterised by thousands of islands and countless waterways, giving the area its name Si Phan Don or 'the 4,000 islands'.
The highest falls reach to 21 metres (69 ft); the succession of rapids stretch 9.7 km (6.0 mi) of the river's length. The average discharge of the cataract is nearly 11,000 {m}^{3}/s (390,000 cu ft/s), with the highest flow on record at over 49,000 {m}^{3}/s (1,700,000 cu ft/s).
Navigable efforts
Because the Khone Falls stop the Mekong river from carrying boat traffic to and from China, French colonialists in the late 19th century made repeated attempts to navigate the falls. Their efforts failed, which led to the construction of the Don Det–Don Khon railway on Don Det and Don Khon islands.
Wildlife
Hemimyzon khonensis, a species of hillstream loach, is known from a single specimen collected in the Mekong at the Khone Falls. The falls are home to the plabuck, an endangered species of catfish said to be the largest freshwater fish in the world. The plabuck is alleged to reach lengths of 3 m (10 ft) and weights of up to 293 kilograms (646 lb).
Details:
Khone Phapheng Falls in Laos: World's Biggest Waterfall
If you want to see the biggest waterfall in all the world by volume and by width, it is in southern Laos where the mighty Mekong River falls in cataracts into a pool. It goes by several names such as Khone Phapheng in Laotian and Khone Falls, and in French, it is called Chutes De Khone.
This waterfall might be what kept the civilization of the Laotian Mekong basin distinctive because though it was very useful for native people to float around on over its broad placid face in Laos, it kept travel, trade and naval invasion from the sea at bay.
Most people never see it. It is out of the way of most tourist travel. There are no large cities or airports right by it, and the transportation infrastructure there is primitive. So it is a good idea to arrange for a private driver who can take you there, take you around, and take you back for meals and hotel. Here is important information about the waterfall, what to do around it, and prices for entry and transport.
Highlights of Khone Phapheng Falls
A huge crashing volume of white water: Hear it roar! Many rivers have a greater volume of water. However, those rivers don't have big falls like this.
Good for: people who love natural places, waterfalls, and majestic scenery
Not overly touristy: The whole area is off the beaten track in the extreme south of Laos where it is more quiet, sleepy, and lazy. The island towns are good for relaxation, and you'll you find many foreigners simply doing that. The main international travelers are Thais, Vietnamese and Westerners. There are no large Chinese and Korean tour groups as in the north.
Lots of other things to do nearby: It is in Champasak Province that is rich in things to do without crowds. You can find awesome places such as 4,000 small islands and the waterfall. You could kayak on the broad Mekong River, stay and eat in laid back native towns, go to other waterfalls, and kayak out to see dolphins!
Khone Phapheng Falls Quick Facts
* Height, breadth and volume: The main drop is 14 meters (45 feet), and the stair of cataracts have a total drop of 22 meters.
* Width: It is almost 11 kilometers wide. At any one point though, you'll only glimpse a small portion of the falls.
* Volume: The flow is 9,500,000 liters (2,500,000 gallons) per second. This is almost double the volume of Niagara Falls.
* Suitable for: nature lovers and photography
Additional Information
Khone Falls, series of cataracts on the Mekong River, extreme southern Laos, on the Cambodian border. The falls are the principal impediment to navigation of the river and have impeded economic use of the Mekong by the peoples of the Cambodian plain to the south and those of Laos to the north; a narrow-gauge railway was once built for transport around the falls. The double series of cataracts is caused by a resistant bed of basalt over which the river tumbles 45 feet (14 m) to a pool 269 feet (82 m) above sea level. The strata causing the falls are also responsible for several islands, the largest of which, Không, has a small port based on the portaging of goods around the falls. The Khone has the greatest volume of the world’s waterfalls, its 2,500,000 gallons (9,500,000 litres) per second being nearly double that of Niagara Falls.
More a glorified set of rapids than a waterfall, but oh, how glorious it is. The largest and by far the most awesome waterfall anywhere along the Mekong, Khon Phapheng is pure, unrestrained aggression, as millions of litres of water crash over the rocks. While pricier than the similar Tat Somphamit, this place, with its gardens and walking paths, is more attractive. You can also get down closer to the rapids.

#205 Dark Discussions at Cafe Infinity » Climate Quotes - VIII » 2025-08-24 18:07:29
- Jai Ganesh
- Replies: 0
Climate Quotes - VIII
1. I think the climate has been changing for billions of years. - Buzz Aldrin
2. First, how do we give everyone a fair shot at opportunity and security in this new economy? Second, how do we make technology work for us, and not against us - especially when it comes to solving urgent challenges like climate change? Third, how do we keep America safe and lead the world without becoming its policeman? - Barack Obama
3. Climate change has been going on as long as the planet is here, and there will always be a little bit of it. - Rupert Murdoch
4. The practical importance of the preservation of our forests is augmented by their relations to climate, soil and streams. - John Muir
5. We have shared responsibility for global climate; we have to reduce climate change below 2 degrees Celsius. - Angela Merkel
6. Industrialised countries must take the responsibility of helping poorer countries in the climate change action plan. - Angela Merkel
7. I've fallen in love with Waikiki; the beach, the climate, the people, and the hotel - it really is a paradise. - Ivanka Trump
8. Despite widely differing perspectives and agendas, there seems to be a remarkable global consensus that has built up over a fairly short period of time that climate change and ecology is one of the truly defining issues for humanity. - Azim Premji.
#206 Re: This is Cool » Miscellany » 2025-08-24 17:37:19
2373) Tristan da Cunha
Gist
Tristan da Cunha is a British Overseas Territory : one of the remaining former colonies which have not yet asked for independence, and wish specifically to retain their link with the United Kingdom.
English is spoken on Tristan da Cunha. There is a distinct local dialect with words derived from the many cultures (Scottish, English, St Helenian, South African, American, Dutch, Italian, and Irish of the original settlers and visitors).
Tristan is not an easy place to get to, but is an unforgetable experience for people fortunate enough to make it, either on scheduled ships or on expedition cruises. There is no airport.
Summary
Tristan da Cunha, colloquially Tristan, is a remote group of volcanic islands in the South Atlantic Ocean. It is one of three constituent parts of the British Overseas Territory of Saint Helena, Ascension and Tristan da Cunha, with its own constitution.
The territory consists of the inhabited island Tristan da Cunha, which has a diameter of roughly 11 kilometres (6.8 mi) and an area of 98 square kilometres (38 sq mi); the wildlife reserves of Gough Island and Inaccessible Island; and the smaller, uninhabited Nightingale Islands. As of October 2018, the main island had 250 permanent inhabitants, who all hold British Overseas Territories citizenship. The other islands are uninhabited, except for the South African personnel of a weather station on Gough Island.
As there is no airstrip on the island, the only way of travelling to or from Tristan is by ship. There are six-day journeys from Cape Town, South Africa, and some cruises offered departing from Ushuaia, Argentina.
Details
Tristan da Cunha is an island and group of islands in the South Atlantic Ocean, about midway between southern Africa and South America. The island group is a constituent part of the British overseas territory of St. Helena, Ascension and Tristan da Cunha. The six small islands of the Tristan da Cunha group are administered collectively. Five of them—Tristan da Cunha, Inaccessible, Nightingale, Middle, and Stoltenhoff—are located within 25 miles (40 km) of one another, and the sixth, Gough, lies about 200 miles (320 km) south-southeast of the group. The territory is located approximately 1,300 miles (2,100 km) to the south of St. Helena. Inaccessible, Nightingale, Middle, and Stoltenhoff are uninhabited, while a weather station is manned on Gough Island.
Tristan da Cunha island, the largest and northernmost of the group, is roughly circular, with a coastline of 21 miles (34 km) and a central volcanic cone (6,760 feet [2,060 metres]) that is usually cloud-covered. The climate is wet, windy, and mild. About 66 inches (1,675 mm) of rain falls annually on the north coast at Edinburgh of the Seven Seas (frequently shortened to Edinburgh), the only permanent settlement. Plant and animal life includes elephant seals and other species not found elsewhere in the world.
Gough and Inaccessible islands together constitute a wildlife reserve, designated a UNESCO World Heritage site in 1995. Inaccessible is about 20 miles (32 km) west-southwest of Tristan da Cunha. It is ringed by cliffs some 1,000 feet (300 metres) high, and beneath the cliffs are occasional narrow beaches. A bird endemic to Inaccessible is the small, flightless land rail. Nightingale Island, the southernmost of the group, is 12 miles (19 km) southeast of Inaccessible and 20 miles (32 km) south-southwest of Tristan da Cunha. Its coasts have low cliffs where millions of seabirds nest. The tiny islands of Middle and Stoltenhoff abut the north coast of Nightingale. Gough Island is about 8 miles (13 km) long and 4 miles (6 km) wide and is of volcanic origin.
Executive authority is vested in a governor, who is also the governor of St. Helena and resides on that island. The governor appoints an administrator to represent him or her in Tristan da Cunha. An Island Council advises the administrator; it has three ex officio members and eight elected members. The administrator serves as president of the Island Council.
The island group was discovered in 1506 by a Portuguese admiral, Tristão da Cunha. Two unsuccessful attempts to settle the islands during the 17th century and one in 1810 preceded the stationing of a British garrison on Tristan da Cunha in 1816, when the island group was formally annexed by the United Kingdom. When the garrison was withdrawn in 1817, three of its members chose to stay, and over the years they were joined by shipwrecked sailors, settlers of European extraction, and women from St. Helena. By 1886 there were 97 inhabitants. The settlement, named Edinburgh of the Seven Seas, was located on the largest lowland strip, about 0.5 mile (0.8 km) wide and 5 miles (8 km) long. In 1938 the six islands were made dependencies of St. Helena. During World War II a naval meteorological and radio station was set up on Tristan da Cunha; afterward a South African weather station was also established there.
A volcanic eruption on the island in October 1961 directly threatened the settlement, and the inhabitants were evacuated to England via Nightingale Island. The main body of 198 islanders returned to the island in November 1963. A new harbour was built in 1965–67. Roads and a hospital, as well as electric, water, and sewerage facilities, were later constructed. After a hurricane severely damaged Edinburgh in May 2001, aid flowed in from abroad to pay for rebuilding. According to the terms of the 2009 constitution drawn up for the islands, Tristan da Cunha was no longer a dependency of St. Helena but an equal part of the territory of St. Helena, Ascension and Tristan da Cunha,
Potatoes are the main crop and shore-based shellfish fishing the main industry; lobster and crawfish are exported. Livestock is raised for domestic consumption. Sales of the island’s postage stamps and coins also contribute to revenue. Area Tristan da Cunha island, 38 square miles (98 square km). Pop. (2014 est.) Tristan da Cunha island, 269.
Additional Information
Tristan da Cunha, the most remote inhabited island in the world, is over 2,700 kilometers from South Africa and 3,700 kilometers from the nearest shores of South America. Sitting between the South Atlantic Current to the north and the Antarctic Circumpolar Current to the south, the volcanic island and its archipelago are a hotspot of endemic biodiversity both on land and at sea.
Among the wildlife found here are seven-gill sharks, blue sharks, shortfin mako sharks, southern right whales, fin whales, humpback whales, sperm whales, dolphins, elephant seals, and albatrosses, as well as 200,000 rockhopper penguins, more than five million shearwaters, and 300,000 sub-Antarctic fur seals.
An overseas territory of the U.K., the Tristan da Cunha island group comprises Tristan da Cunha, Nightingale, Inaccessible, and Gough Islands.
The Mission
In collaboration with the Royal Society for the Protection of Birds (RSPB) and the Tristan da Cunha government, Pristine Seas launched an expedition to Tristan da Cunha in January 2017. Spending 20 days at the archipelago, the team carried out quantitative surveys of shallow flora and fauna, open-water communities, and deep-sea habitats to determine the health of its largely unknown marine environment.
The team obtained their data during scuba dives and from baited stereo cameras and deep-water drop cams. They used satellite tags to examine the movements of apex predators, such as sharks, and conducted botanical work and bird and seal studies.
Among their findings: Migratory blue sharks—the most heavily fished sharks in the world, highly prized for their fins—may have found a refuge in Tristan da Cunha’s waters. The team saw more blue sharks here than in any other location they’ve sampled.
"Tristan de Cunha really is one of the key global hotspots of life— there is a most wonderful sense of the power of nature in this perfectly balanced ecosystem."
The Result
In November 2020, the Government and people of Tristan da Cunha announced the creation of a Marine Protection Zone, which will be the largest in the Atlantic at almost 700,000 square kilometers. Scientific data and imagery from the expedition were used to support the Tristan-led, science-based process.
The Highlights
Aboard the S.V.S. Grenville, suspicious albatrosses eye the team’s bird tags, and passengers ready themselves for Tristan’s heavy biosecurity. An exciting passage to Gough Island is interrupted when the ship’s engine stops dead. Trespassing birds force the ship to go dark. This is one of the world’s most desirable lobsters. Intrepid team members achieve a first for the surfing world.

#207 Jokes » Lawyer Jokes - IV » 2025-08-24 16:55:54
- Jai Ganesh
- Replies: 0
Q: What do you get when you cross a lawyer with a demon from hell?
A: No changes occur.
* * *
Q: What's the difference between God and an attorney?
A: God doesn't think he's an attorney.
* * *
Q: Why is going to a meeting of the Bar Association like going into a bait shop?
A: Because of the abundance of suckers, leeches, maggots and nightcrawlers.
* * *
Q: Why are lawyers like nuclear weapons?
A: When they land, they prevent anything from functioning for the next hundred years.
* * *
Q. What's the difference between a lawyer and a vampire?
A. A vampire only drags blood at night.
* * *
#208 Science HQ » Holmium » 2025-08-24 16:44:51
- Jai Ganesh
- Replies: 0
Holmium
Gist
Holmium (Ho) is a soft, silvery-white, rare-earth element with atomic number 67, known for having the greatest magnetic strength of any element. It is used in applications requiring strong magnetic fields, such as nuclear reactor control rods and components in high-strength magnets, as well as in lasers, glass coloring, and as a calibration standard for spectrometers.
Holmium (Ho) is a rare earth element with diverse applications, primarily due to its strong magnetic properties and ability to absorb neutrons. It's used in powerful magnets, nuclear reactor control rods, and specialized lasers for medical procedures. It also finds use in coloring glass and cubic zirconia, and as a dopant in certain materials.
Summary
Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.
In nature, holmium occurs together with the other rare-earth metals (like thulium). It is a relatively rare lanthanide, making up 1.4 parts per million of the Earth's crust, an abundance similar to tungsten. Holmium was discovered through isolation by Swedish chemist Per Theodor Cleve. It was also independently discovered by Jacques-Louis Soret and Marc Delafontaine, who together observed it spectroscopically in 1878. Its oxide was first isolated from rare-earth ores by Cleve in 1878. The element's name comes from Holmia, the Latin name for the city of Stockholm.
Like many other lanthanides, holmium is found in the minerals monazite and gadolinite and is usually commercially extracted from monazite using ion-exchange techniques. Its compounds in nature and in nearly all of its laboratory chemistry are trivalently oxidized, containing Ho(III) ions. Trivalent holmium ions have fluorescent properties similar to many other rare-earth ions (while yielding their own set of unique emission light lines), and thus are used in the same way as some other rare earths in certain laser and glass-colorant applications.
Holmium has the highest magnetic permeability and magnetic saturation of any element and is thus used for the pole pieces of the strongest static magnets. Because holmium strongly absorbs neutrons, it is also used as a burnable poison in nuclear reactors.
Details
Holmium (Ho) is a chemical element, a rare-earth metal of the lanthanide series of the periodic table.
Holmium is a moderately hard, silvery white metal that is relatively stable in air. It readily reacts with diluted acids but does not react with either diluted or concentrated hydrofluoric acid (HF), due to formation of a protective surface layer of HoF3. Holmium is a very strong paramagnet above 133 K (−140 °C, or −220 °F). At that temperature the metal orders antiferromagnetically, forming a basal plane spiral structure. At 19 K (−254 °C, or −425 °F) the magnetic moments tilt along the c-axis lifting out of the basal plane by some 10°, forming a conical ferrimagnetic structure.
Holmium was discovered spectroscopically (1878) by Swiss chemists Jacques-Louis Soret and Marc Delafontaine and independently (1879) by Swedish chemist Per Teodor Cleve, who separated it chemically from erbium and thulium. Cleve named the element for his native city of Stockholm, its Latinized name being Holmia. Holmium occurs associated with other rare earths in laterite clays and in the minerals xenotime, euxenite, and many others; it also occurs in the products of nuclear fission.
The one naturally occurring isotope, holmium-165, is stable. There are numerous radioactive isotopes (a total of 35, not counting nuclear isomers), ranging from holmium-140 to holmium-175 and having half-lives from 4.1 milliseconds (holmium-141) to 4,570 years (holmium-163). Holmium is one of the least abundant rare earths; its abundance in Earth’s crust is comparable to that of thallium.
The classical methods of separating and purifying the element were fractional crystallization and precipitation, but solvent-solvent extraction and ion-exchange technologies have made available kilogram quantities of highly pure holmium oxide. The metal is produced by metallothermic reduction of the anhydrous fluoride HoF3 with calcium. Only one allotropic (structural) form is known for holmium. The metal adopts a close-packed hexagonal structure with a = 3.5778 Å and c = 5.6178 Å at room temperature.
Holmium and its compounds have limited applications except for research. Holmium has been used as a component of some electronic devices; the ion Ho3+ has been used as a catalyst for ortho-para hydrogen conversion; and the oxide has been used as a special refractory.
Holmium behaves as a typical rare earth. It forms a series of yellow-brown salts, many of which are obtained in solution by dissolving the oxide Ho2O3 in the appropriate acid.
Element Properties
atomic number : 67
atomic weight : 164.930328
melting point : 1,474 °C (2,685 °F)
boiling point : 2,700 °C (4,892 °F)
specific gravity : 8.795 (24 °C, or 75 °F)
oxidation state : +3.
Additional Information:
Appearance
A bright, silvery metal.
Uses
Holmium can absorb neutrons, so it is used in nuclear reactors to keep a chain reaction under control. Its alloys are used in some magnets.
Biological role
Holmium has no known biological role, and is non-toxic.
Natural abundance
Holmium is found as a minor component of the minerals monazite and bastnaesite. It is extracted from those ores that are processed to extract yttrium. It is obtained by ion exchange and solvent extraction.

#209 Re: Jai Ganesh's Puzzles » General Quiz » 2025-08-24 15:59:26
Hi,
#10531. What does the term in Geography Biological diversity mean?
#10532. What does the term in Geogaphy Biome mean?
#210 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-08-24 15:28:06
Hi,
#5721. What does the verb (used with object) overawe mean?
#5722. What does the adjective overcast mean?
#211 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-08-24 14:57:30
Hi,
#2452. What does the medical term Internuclear ophthalmoplegia mean?
#212 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-08-24 14:45:25
Hi,
#9720.
#213 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-08-24 14:35:21
Hi,
#6225.
#214 Re: Exercises » Compute the solution: » 2025-08-24 13:59:00
Hi,
2566.
#215 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-08-24 00:21:51
2228) Louis E. Brus
Gist
Professor Louis Brus, a longtime professor and an alumnus of Columbia University, has been awarded the Nobel Prize in Chemistry. Brus was recognized along with two other scientists—Moungi G. Bawendi of MIT and Alexei I. Ekimov of Nanocrystals Technology Inc.—for his work on the “discovery and development of quantum dots, nanoparticles so tiny that their size determines their properties.”
Brus is currently the Samuel Latham Mitchill Professor Emeritus, and Special Research Scientist at Columbia. He is one of 87 Columbians—alumni, faculty, adjunct faculty, researchers, and administrators—to win a Nobel Prize.
Summary
Louis Brus (born August 10, 1943, Cleveland, Ohio, U.S.) is an American physical chemist who was awarded the 2023 Nobel Prize in Chemistry for his work in discovering and producing quantum dots, which are very small particles whose unusual quantum properties depend on their size. He shared the prize with Russian-born American physicist Alexei Ekimov and French-born American chemist Moungi Bawendi.
Brus spent his early life in Cleveland, but his father, an insurance executive, moved the family several times throughout the Midwest. He attended Shawnee Mission North High School in Roeland Park, Kansas, before enrolling at Rice University in Houston on a Naval Reserve Officers Training Corps (NROTC) scholarship. He completed his bachelor’s degree in chemical physics in 1965. After graduation, he was able to delay his active service in the United States Navy to complete a Ph.D. degree in chemical physics. Upon his graduation in 1969, he was made a lieutenant and stationed in Washington, D.C., as a science staff officer at the United States Naval Research Laboratory. After his service was completed in 1973, he served as a research technician at AT&T Bell Laboratories in Murray Hill, New Jersey, where he began to experiment with nanocrystals and semiconducting materials.
Since the 1930s, physicists and chemists have known that a material’s size has a significant effect on its properties. That is, in particles of matter a few nanometers in size (1 nanometer = 10−9 meter), quantum mechanical effects become significant. Particles of this size are called nanoparticles.
Brus’s discovery of quantum dots in late 1982 was accidental. He and his collaborators were interested in using semiconductors to drive chemical reactions. They worked with nanoparticles of cadmium sulfide (CdS) in a solution. Brus observed that the absorption spectra of newly produced CdS nanoparticles was different from those of particles that had been allowed to sit for a day. He suspected this happened because the nanoparticles grew in size. Upon measurement, new CdS nanoparticles proved to be about 4.5 nanometers across, and older (or “ripened”) CdS nanoparticles were about 12.5 nanometers across. (Ekimov had discovered quantum dots independently and had published his work in 1981, but, because Ekimov had published in a Soviet journal, Brus did not learn about his discovery until 1984.)
Brus and his group continued working on quantum dots. Bawendi was a postdoctoral fellow under Brus in the late 1980s, and later, as a professor at MIT (Massachusetts Institute of Technology), he developed a method for producing high-quality quantum dots of a consistent size. Today quantum dots are used in many applications, including in QLED (quantum-dot light-emitting diode) screens, in solar cells, and as markers in biomedical imaging.
Brus remained at Bell Laboratories until he joined the chemistry faculty at Columbia University in 1996. There he served as the scientific head of the complex films research group at the National Science Foundation’s Materials Research Science and Engineering Center from 1998 to 2008 and as codirector of the U.S. Department of Energy’s Energy Frontiers Research Center from 2009 to 2014.
Brus became an elected fellow of the American Physical Society in 1980 and of the American Academy of Arts and Sciences in 1998, and he was elected to the United States National Academy of Sciences in 2004. He was the recipient of the American Chemical Society’s Chemistry of Materials Prize in 2005 and of the Kavli Prize in Nanoscience in 2008. Brus shared the R.W. Wood Prize of the Optical Society of America (OSA, now Optica) with Russian scientist Alexander L. Efros and Ekimov in 2006 for their work with quantum dots. In addition, Brus received the National Academy of Sciences Award in Chemical Sciences in 2010 for his ongoing work with nanocrystals and investigations into the quantum effects that govern their optical properties.
Details
Louis Eugene Brus (born August 10, 1943) is an American chemist, and currently the Samuel Latham Mitchell Professor of Chemistry at Columbia University. He is the co-discoverer of the colloidal semi-conductor nanocrystals known as quantum dots. In 2023, he was awarded the Nobel Prize in Chemistry.
Early life and education
Louis Eugene Brus was born in 1943 in Cleveland, Ohio, United States of America. During high school in Roeland Park, Kansas, he developed an interest for chemistry and physics.
He entered Rice University in 1961 with a Naval Reserve Officers Training Corps (NROTC) college scholarship, which required him to participate in NROTC activities at sea as a midshipman. In 1965, he graduated at Rice with a B.S. degree in chemical physics, and then moved to Columbia University for his doctoral research. For his dissertation, he worked on the photodissociation of sodium iodide vapor, under the supervision of Richard Bersohn. After obtaining his Ph.D. degree in chemical physics in 1969, Brus returned to the Navy as a lieutenant and served as a scientific staff officer in collaboration with Lin Ming-chang, at the United States Naval Research Laboratory in Washington, D.C.
Under the recommendation of Bersohn, Brus left the Navy permanently and joined AT&T Bell Laboratories in 1973, where he did the work that led to the discovery of quantum dots. In 1996, Brus left Bell Labs and joined the faculty in the Department of Chemistry at Columbia University.
Work on quantum dots
Brus is a foundational figure in the research and development of quantum dots. Quantum dots are tiny semiconducting crystals whose nanoscale size gives them unique optical and electronic properties.
Brus was independently the first to synthesize them in a solution in 1982. At the time, he was studying organic photochemistry on cadmium sulfide particle surfaces using pump–probe Raman spectroscopy, looking for possible applications for solar-energy. He noticed that the optical properties of the crystals changed after leaving them for 24 hours. He attributed this change in band gap energy to Ostwald ripening when the crystal increased size.
Brus provided the theoretical framework for understanding the behavior of quantum dots in terms of quantum size effects. He identified the connection between the particle size of semiconductors and the wavelength of the light they emit, now known as the Brus equation.
Brus tried to contact researchers in the Soviet Union. It was in 1990, that he finally met Alexey Ekimov and Alexander Efros, who had first developed the semiconductor nanocrystals in glass in 1981 under more rudimentary conditions, however their research was not available in the United States.
At Bell Labs, Brus worked with postdoc researchers Paul Alivisatos and Moungi Bawendi in a research project with organometallic synthetic chemist Michael L. Steigerwald on reducing the size of the quantum dots.
Awards and honors
Brus was elected a fellow of the American Academy of Arts and Sciences in 1998, a member of the United States National Academy of Sciences in 2004, and is a member of the Norwegian Academy of Science and Letters.
He received the Distinguished Alumni Award from the Association of Rice University Alumni in 2010. He was co-recipient of the 2006 R. W. Wood Prize of the Optical Society of America "for the discovery of nanocrystal quantum dots and pioneering studies of their electronic and optical properties" shared with Alexander Efros and Alexey Ekimov. He also received the inaugural Kavli Prize for nanoscience along with Sumio Iijima in 2008 for "for their large impact in the development of the nanoscience field of the zero and one dimensional nanostructures in physics, chemistry and biology". In 2009 he was awarded the Willard Gibbs Award "for his leading role in the creation of chemical quantum dots". Brus was chosen for the 2010 NAS Award in Chemical Sciences. In 2012 he received the Franklin Institute's Bower Award and Prize for Achievement in Science, and was selected as a Clarivate Citation laureate in Chemistry "for discovery of colloidal semiconductor nanocrystals (quantum dots)".
In 2023, Brus was awarded the Nobel Prize in Chemistry jointly with Ekimov and Moungi Bawendi "for the discovery and synthesis of quantum dots". Bawendi had worked as a postdoc with Brus, when they were in Bell Labs.

#216 This is Cool » Lesotho » 2025-08-23 20:11:57
- Jai Ganesh
- Replies: 0
Lesotho
Gist
Lesotho is a landlocked, high-altitude kingdom in Southern Africa, entirely surrounded by South Africa. Also known as the "Kingdom of Lesotho," it is one of the world's few sovereign enclaves. Its official motto is "Khotso, Pula, Nala," which translates to "Peace, Rain, Prosperity".
The Kingdom of Lesotho is made up mostly of highlands, where many villages can only be reached on horseback, by foot or light aircraft. It is known as the "Kingdom in the Sky" and is the only independent state in the world that lies entirely above 1,000m (3,281ft) in elevation, according to Encyclopaedia Britannica.
The best time to visit Lesotho depends on your activity preferences: October to April is ideal for hiking and outdoor activities, with drier conditions in March/April and October/November. The summer months offer more sunlight and pleasant, although sometimes wet, conditions. For skiing, July and August are the prime months, thanks to the snow and cold.
Summary
Lesotho, formally the Kingdom of Lesotho and formerly known as Basutoland, is a landlocked country in Southern Africa. Entirely surrounded by South Africa, it is the largest of only three sovereign enclaves in the world, the others being San Marino and Vatican City, which are surrounded by Italy. Lesotho is situated in the Maloti Mountains and contains the highest peak in Southern Africa.[9] It has an area of over 30,000 sq km (11,600 sq mi) and has a population of about 2.3 million. Its capital and largest city is Maseru.
Lesotho was formed in 1824 by King Moshoeshoe I. Continuous encroachments by Dutch settlers made the King enter into an agreement with the United Kingdom to become a protectorate in 1868 and, in 1884, a crown colony. It achieved independence in 1966, and was subsequently ruled by the Basotho National Party (BNP) for two decades. Its constitutional government was restored in 1993 after seven years of military rule. King Moshoeshoe II was exiled in 1990 but returned in 1992 and was reinstated in 1995. One year later, Moshoeshoe II died and his son Letsie III took the throne, which he still holds.
The Sotho ethnic group (also known as Basotho), from which the country derives its name, comprises 99.7% of the country's current population, making it one of the most ethnically homogeneous in the world. Their native language, Sesotho, is an official language along with English, IsiXhosa, and SiPhuthi.
Lesotho is considered a lower middle income country with significant socioeconomic challenges. Almost half of its population is below the poverty line, and the country's HIV/AIDS prevalence rate is the second-highest in the world. However, it also targets a high rate of universal primary education and has one of the highest rates of literacy in Africa (81% as of 2021). Lesotho is a member of the United Nations, the Non-Aligned Movement, the Commonwealth of Nations, the African Union, and the Southern African Development Community.
Details
Lesotho is a landlocked country in Southern Africa. A scenic land of tall mountains and narrow valleys, Lesotho owes a long history of political autonomy to the mountains that surround it and protect it from encroachment. Since the Neolithic Period, the mountain kingdom was the domain of Khoisan-speaking hunter-gatherers. In the 19th century the Sotho, led by Moshoeshoe I, took control of the region. It remained independent until it became a British protectorate, one of three British High Commission Territories (the others being Bechuanaland [now Botswana] and Swaziland [now Eswatini]).
Completely encircled by the Republic of South Africa but separated from it by forbidding mountain ranges, Lesotho has endured decades of turbulent politics, periodic economic crises, and grinding poverty since gaining its independence from Great Britain in 1966. Though culturally conservative in the main, the people of the country welcomed the modernization programs begun in the 1990s, which have brought new wealth to the country but at the cost of much environmental damage. Tourism and revenues from the country’s diamond industry have also helped to improve material conditions, and the capital, Maseru, has grown to become one of Southern Africa’s most attractive cities. Of these changes, Sotho writer Mpho ’M’Atsepo Nthunya remarks,
Land:
The country forms an enclave within South Africa, bordering on three of the latter’s provinces—KwaZulu-Natal, Free State, and Eastern Cape. Like only two other independent states in the world (Vatican City and the Republic of San Marino), Lesotho is completely encircled by another country, on which it must depend for access to the outside world.
Relief, drainage, and soils
Two-thirds of Lesotho consists of mountains. The highest peak, Mount Ntlenyana, is 11,424 feet (3,482 metres) above sea level. The Drakensberg range forms the eastern boundary with KwaZulu-Natal. The Maloti spurs of the Drakensberg, running north and south, join the main range in the north, forming a plateau from 9,000 to 10,500 feet (2,700 to 3,200 metres) in elevation. This plateau, the center of the cattle-raising and agricultural industries, is the source of South Africa’s two largest rivers—the eastward-flowing Tugela and the westward-flowing Orange—as well as tributaries of the Caledon (Mohokare). Three other important rivers in Lesotho are the Senqunyane in the center of the country, the Kometspruit in the southwest, and the Matsoku in the northeast. The foothills, with elevations averaging between 6,000 and 7,000 feet (1,800 and 2,100 metres), descend in undulating slopes to the west, where the lowlands bordering Free State rise to elevations of 5,000 to 6,000 feet (1,500 to 1,800 metres). The mountain soils are of basaltic origin and are shallow but rich. The soils of the lowlands derive mainly from the underlying sandstone. Extensive erosion has severely damaged soils throughout the country.
Climate
Precipitation, brought by the prevailing winds, occurs mostly between October and April and is variable; the annual average is about 28 inches (710 mm), with amounts decreasing from east to west. Hail is a frequent summer hazard. Temperatures in the lowlands reach as high as 90 °F (32 °C) in the summer and plunge to 20 °F (−7 °C) in the winter. In the highlands the temperature range is much wider, and readings below 0 °F (−18 °C) are not unusual. Frost occurs widely in the winter, when the Maloti Mountains are usually snowcapped.
Plant and animal life
Lesotho is largely covered in grasses, although trees also appear on the landscape. Indigenous trees include Cape willows, cheche bush (used for fuel), and wild olives. Other willows and white poplars have been introduced into the country. There are numerous indigenous species of aloes, which are commonly found in the cooler, wet areas. Overgrazing, overutilization, and soil erosion have drastically depleted and altered the grasslands, reedbeds, and woody bush on the slopes. Reforestation schemes have been attempted but have met with limited success.
In the mid-19th century, zebras, wildebeests, ostriches, and lions could be found in the country. However, hunting and deforestation have mostly eliminated the populations of large mammals; the last lion was killed in the 1870s. Smaller antelope and hares can still be found, and the hyrax, or dassie, is common. Sehlabathebe National Park in the southeastern highlands near Qacha’s Nek protects such birds as raptors and such mammals as mountain reedbuck and leopards. Lesotho is the last stronghold in Southern Africa of the magnificent bearded vulture, or lammergeier. Some rivers contain yellowfish and the rare Maloti minnow; trout and the North African catfish have also been introduced.
People:
Ethnic Groups
The Sotho (also known as Basotho) form the overwhelming majority of the country’s population. They were originally united by a common loyalty to the royal house of Moshoeshoe I, who founded the Sotho nation in the 19th century. Internally, divisions between different chiefdoms—and within the royal lineage itself—have had political significance, but externally a sense of Sotho nationhood and cultural unity remains strong. Lesotho is also home to a Zulu minority, a small population of Asian or mixed ancestry, and a European community that is dominated by expatriate teachers, missionaries, aid workers, technicians, and development advisers.
Languages
Except for English, all the main languages spoken in Lesotho are members of the Niger-Congo language family. Sotho (Sesotho), a Bantu language, is spoken by the majority of the population, though both Sotho and English are official languages in the country. Zulu is spoken by a small but significant minority. Phuthi, a dialect of Swati, and Xhosa are also spoken in parts of Lesotho.
Religion of Lesotho
Some four-fifths of the population profess Christianity, of which the largest denomination is Roman Catholic; other denominations include Lesotho Evangelical, Presbyterian, and Anglican. Independent churches are also present, together with Zionist sects (small African sects that blend Pentecostal Christianity and indigenous ritual belief). Other religions—including Islam, Hinduism, and Buddhism—are practiced by small percentages of the population, as are traditional religions. Some adherents of Christianity also embrace traditional religious beliefs.
Additional Information
Lesotho is a small country in southern Africa, it is an enclave of South Africa. Its population is about 1,800,000. The capital of Lesotho is called Maseru.
Geography
Lesotho has no coast on the sea nor on a lake. This type of country is called landlocked, meaning it is surrounded by land. All of Lesotho's trade must either be flown in by aeroplane, or brought in by land. Lesotho's position is unusual: it is completely surrounded by South Africa and has no borders with any other country. This type of country is called an enclave. Lesotho is one of the only three enclaved countries in the world (the other two are San Marino and the Vatican City). This makes Lesotho's relationship with South Africa very important to them both.
Lesotho has many mountains and is one of the most mountainous countries in the world. All of Lesotho is at least 1,400 m above sea level. People sometimes travel there to ski in winter. The many mountains in Lesotho mean that it rains there a lot. Lesotho uses some of its water to generate electricity and sells some of the water to South Africa.
History
Lesotho became a country in 1818, but it was then called Basutoland. A man called Moshoeshoe brought several of the groups of people in the area together and formed a new country with him as its king. This new country came under attack from its enemies and in 1868 Moshoeshoe asked Queen Victoria of Great Britain for help. Great Britain said it would help if Lesotho became part of the British Empire.
Lesotho eventually gained its independence on 4 October 1966. Now it is a member of the British Commonwealth. King Letsie III is the ruler of Lesotho.

#217 Re: This is Cool » Miscellany » 2025-08-23 18:16:02
2372) Dead Sea
Gist
The Dead Sea is one of the saltiest natural bodies of water on Earth. The special salt has been used in beauty products for thousands of years, but there's little chance of it running out!
The Dead Sea is called the "dead sea" because its extreme salinity, nearly ten times saltier than ocean water, prevents most aquatic life, such as fish and plants, from surviving in it. While most organisms cannot live there, some microorganisms like bacteria and algae have adapted to the harsh, mineral-rich waters, which are concentrated by intense evaporation in a hot desert climate.
Summary
The Dead Sea, also known by other names, is a landlocked salt lake bordered by Jordan to the east, the Israeli-occupied West Bank to the west and Israel to the southwest. It lies in the endorheic basin of the Jordan Rift Valley, and its main tributary is the Jordan River.
As of 2025, the lake's surface is 439.78 metres (1,443 ft) below sea level, making its shores the lowest land-based elevation on Earth. It is 304 m (997 ft) deep, the deepest hypersaline lake in the world. With a salinity of 342 g/kg, or 34.2% (in 2011), it is one of the world's saltiest bodies of water,[9] 9.6 times as salty as the ocean—and has a density of 1.24 kg/litre, which makes swimming similar to floating. This salinity makes for a harsh environment in which plants and animals cannot flourish, hence its name. The Dead Sea's main, northern basin is 50 kilometres (31 mi) long and 15 kilometres (9 mi) wide at its widest point.
The Dead Sea has attracted visitors from around the Mediterranean basin for thousands of years. It was one of the world's first health resorts, and it has been the supplier of a wide variety of products, from asphalt for Egyptian mummification to potash for fertilisers. Today, tourists visit the sea on its Israeli, Jordanian and West Bank coastlines.
The Dead Sea is receding at a swift rate; its surface area today is 605 sq km (234 sq mi), having been 1,050 sq km (410 sq mi) in 1930. Multiple canal and pipeline proposals, such as the scrapped Red Sea–Dead Sea Water Conveyance project, have been made to reduce its recession.
Details
Dead Sea, landlocked salt lake between Israel and Jordan in southwestern Asia. Its eastern shore belongs to Jordan, and the southern half of its western shore belongs to Israel. The northern half of the western shore lies within the Palestinian West Bank and has been under Israeli occupation since the 1967 Arab-Israeli war. The Jordan River, from which the Dead Sea receives nearly all its water, flows from the north into the lake.
The Dead Sea has the lowest elevation and is the lowest body of water on the surface of Earth. For several decades in the mid-20th century, the standard value given for the surface level of the lake was some 1,300 feet (400 metres) below sea level. Beginning in the 1960s, however, Israel and Jordan began diverting much of the Jordan River’s flow and increased the use of the lake’s water itself for commercial purposes. The result of those activities was a precipitous drop in the Dead Sea’s water level. By the mid-2010s, measurement of the lake level was more than 100 feet (some 30 metres) below the mid-20th-century figure—i.e., about 1,410 feet (430 metres) below sea level—but the lake continued to drop by about 3 feet (1 metre) annually.
Physical features:
Physiography and geology
The Dead Sea is situated between the hills of Judaea to the west and the Transjordanian plateaus to the east. Before the water level began dropping, the lake was some 50 miles (80 km) long, attained a maximum width of 11 miles (18 km), and had a surface area of about 394 square miles (1,020 square km). The peninsula of Al-Lisān (Arabic: “The Tongue”) divided the lake on its eastern side into two unequal basins: the northern basin encompassed about three-fourths of the lake’s total surface area and reached a depth of 1,300 feet (400 metres), and the southern basin was smaller and considerably shallower, less than 10 feet (3 metres) deep on average. During biblical times and until the 8th century ce, only the area around the northern basin was inhabited, and the lake was slightly lower than its present-day level. It rose to its highest level, 1,275 feet (389 metres) below sea level, in 1896 but receded again after 1935, stabilizing at about 1,300 feet (400 metres) below sea level for several decades.
The drop in the lake level in the late 20th and early 21st centuries changed the physical appearance of the Dead Sea. Most noticeably, the peninsula of Al-Lisān gradually extended eastward, until the lake’s northern and southern basins became separated by a strip of dry land. In addition, the southern basin was eventually subdivided into dozens of large evaporation pools (for the extraction of salt), so by the 21st century it had essentially ceased to be a natural body of water. The northern basin—effectively now the actual Dead Sea—largely retained its overall dimensions despite its great loss of water, mainly because its shoreline plunged downward so steeply from the surrounding landscape.
The Dead Sea region occupies part of a graben (a downfaulted block of Earth’s crust) between transform faults along a tectonic plate boundary that runs northward from the Red Sea–Gulf of Suez spreading centre to a convergent plate boundary in the Taurus Mountains of southern Turkey. The eastern fault, along the edge of the Moab Plateau, is more readily visible from the lake than is the western fault, which marks the gentler Judaean upfold.
In the Jurassic and Cretaceous periods (about 201 million to 66 million years ago), before the creation of the graben, an extended Mediterranean Sea covered Syria and Palestine. During the Miocene Epoch (23 million to 5.3 million years ago), as the Arabian Plate collided with the Eurasian Plate to the north, upheaval of the seabed produced the upfolded structures of the Transjordanian highlands and the central range of Palestine, causing the fractures that allowed the Dead Sea graben to drop. At that time the Dead Sea was probably about the size that it is today. During the Pleistocene Epoch (2,588,000 to 11,700 years ago), it rose to an elevation of about 700 feet (200 metres) above its modern level, forming a vast inland sea that stretched some 200 miles (320 km) from the H̱ula Valley area in the north to 40 miles (64 km) beyond its present southern limits. The Dead Sea did not spill over into the Gulf of Aqaba because it was blocked by a 100-foot (30-metre) rise in the highest part of Wadi Al-ʿArabah, a seasonal watercourse that flows in a valley to the east of the central Negev highlands.
Beginning about 2.5 million years ago, heavy streamflow into the lake deposited thick sediments of shale, clay, sandstone, rock salt, and gypsum. Later, strata of clay, marl, soft chalk, and gypsum were dropped onto layers of sand and gravel. Because the water in the lake evaporated faster than it was replenished by precipitation during the past 10,000 years, the lake gradually shrank to its present form. In so doing, it exposed deposits that now cover the Dead Sea valley to thicknesses of between about 1 and 4 miles (1.6 and 6.4 km).
The Al-Lisān region and Mount Sedom (historically Mount Sodom) resulted from movements of Earth’s crust. Mount Sedom’s steep cliffs rise up from the southwestern shore. Al-Lisān is formed of strata of clay, marl, soft chalk, and gypsum interbedded with sand and gravel. Both Al-Lisān and beds made of similar material on the western side of the Dead Sea valley dip to the east. It is assumed that the uplifting of Mount Sedom and Al-Lisān formed a southern escarpment for the Dead Sea. Later the sea broke through the western half of that escarpment to flood what is now the shallow southern remnant of the Dead Sea.
Another consequence resulting from the Dead Sea’s lower water level has been the appearance of sinkholes, especially in the southwestern part of the region. As the water in the lake dropped, it became possible for groundwater to rise up and dissolve large subterranean caverns in the overlying salt layer until the surface finally collapses. Several hundred sinkholes have formed, some of them in areas popular with tourists.
Climate and hydrology
The Dead Sea lies in a desert. Rainfall is scanty and irregular. Al-Lisān averages about 2.5 inches (65 mm) of rain a year, the industrial site of Sedom (near historical Sodom) only about 2 inches (50 mm). Because of the lake’s extremely low elevation and sheltered location, winter temperatures are mild, averaging 63 °F (17 °C) in January at the southern end at Sedom and 58 °F (14 °C) at the northern end; freezing temperatures do not occur. Summer is oppressively hot, averaging 93 °F (34 °C) in August at Sedom, with a recorded maximum of 124 °F (51 °C). Evaporation of the lake’s waters—estimated at about 55 inches (1,400 mm) per year—often creates a thick mist above the lake. On the rivers the atmospheric humidity varies from 45 percent in May to 62 percent in October. Lake and land breezes, which are relatively common, blow off the lake in all directions in the daytime and then reverse direction to blow toward the centre of the lake at night.
The inflow from the Jordan River, whose high waters occur in winter and spring, once averaged some 45.5 billion cubic feet (1.3 billion cubic metres) per year. However, the subsequent diversions of the Jordan’s waters reduced the river’s flow to a small fraction of the previous amount and became the principal cause for the drop in the Dead Sea’s water level. Four modest streams descend to the lake from Jordan to the east through deep gorges: the wadis (intermittent streams) Al-ʿUẓaymī, Zarqāʾ Māʿīn, Al-Mawjib, and Al-Ḥasā. Down numerous other wadis, streams flow spasmodically and briefly from the neighbouring heights as well as from the depression of Wadi Al-ʿArabah. Thermal sulfur springs also feed the rivers. Evaporation in summer and the inflow of water, especially in winter and spring, once caused noticeable seasonal variations of 12 to 24 inches (30 to 60 cm) in the level of the lake, but those fluctuations have been overshadowed by the more-dramatic annual drops in the Dead Sea’s surface level.
Salinity
The waters of the Dead Sea are extremely saline, and, generally, the concentration of salt increases toward the lake’s bottom. That phenomenon can create two different masses of water in the lake for extended periods of time. Such a situation existed for some three centuries, lasting until the late 1970s. Down to a depth of about 130 feet (40 metres), the temperature varied from 66 to 98 °F (19 to 37 °C), the salinity was slightly less than 300 parts per thousand, and the water was especially rich in sulfates and bicarbonates. Beneath a zone of transition located at depths between 130 and 330 feet (40 and 100 metres), the water had a uniform temperature of about 72 °F (22 °C) and a higher degree of salinity (approximately 332 parts per thousand); it contained hydrogen sulfide and strong concentrations of magnesium, potassium, chlorine, and bromine. The deep water was saturated with sodium chloride, which precipitated to the bottom. The deep water thus became fossilized (i.e., because it was highly salty and dense, it remained permanently on the bottom).
The dramatic reduction in inflow from the Jordan River that began in the 1960s gradually increased the salinity of the upper-layer waters of the Dead Sea. By the late 1970s that water mass had become more saline (and denser) than the lower layers, but, because it remained warmer than the layers beneath it, it did not sink. By the winter of 1978–79, however, the upper-level layer had become cool and saturated enough to sink, setting off an event known as an overturn (a mixing of the water layers). Since then the trend has been toward restoring the formerly stratified water layers, but with more instances of overturning.
The saline water has a high density that keeps bathers buoyant. The fresh water of the Jordan stays on the surface, and in the spring its muddy colour can be traced as it spreads southward from the point where the river empties into the Dead Sea. The lake’s extreme salinity excludes all forms of life except bacteria. Fish carried in by the Jordan or by smaller streams when in flood die quickly. Apart from the vegetation along the rivers, plant life along the shores is discontinuous and consists mainly of halophytes (plants that grow in salty or alkaline soil).
Human imprint
The name Dead Sea can be traced at least to the Hellenistic Age (323 to 30 bce). The Dead Sea figures in biblical accounts dating to the time of Abraham (first of the Hebrew patriarchs) and the destruction of Sodom and Gomorrah (the two cities along the lake, according to the Hebrew Bible, that were destroyed by fire from heaven because of their wickedness). The desolate wilderness beside the lake offered refuge to David (king of ancient Israel) and later to Herod I (the Great; king of Judaea), who at the time of the siege of Jerusalem by the Parthians in 40 bce barricaded himself in a fortress at Masada, Israel, just west of Al-Lisān. Masada was the scene of a two-year siege that culminated in the mass suicide of its Jewish Zealot defenders and the occupation of the fortress by the Romans in 73 ce. The Jewish sect that left the biblical manuscripts known as the Dead Sea Scrolls took shelter in caves at Qumrān, just northwest of the lake.
The Dead Sea constitutes an enormous salt reserve. Rock salt deposits also occur in Mount Sedom along the southwestern shore. The salt has been exploited on a small scale since antiquity. In 1929 a potash factory was opened near the mouth of the Jordan. Subsidiary installations were later built in the south at Sedom, but the original factory was destroyed during the 1948–49 Arab-Israeli war. A factory producing potash, magnesium, and calcium chloride was opened in Sedom in 1955. Another plant produces bromine and other chemical products. There are also chemical-processing facilities on the Jordanian side of the southern basin. Water for the extensive array of evaporation pools in the south, from which those minerals are extracted, is supplied by artificial canals from the northern basin.
Because of its location on the contested Jordanian-Israeli frontier, navigation on the Dead Sea is negligible. Its shores are nearly deserted, and permanent establishments are rare. Exceptions are the factory at Sedom, a few hotels and spas in the north, and, in the west, a kibbutz (an Israeli agricultural community) in the region of the ʿEn Gedi oasis. Small cultivated plots are also occasionally found on the lakeshore.
Concern mounted quickly over the continued drop in the Dead Sea’s water level, prompting studies and calls for greater conservation of the Jordan River’s water resources. In addition to proposals for reducing the amount of river water diverted by Israel and Jordan, those two countries discussed proposals for canals that would bring additional water to the Dead Sea. One such project, which received approval from both sides in 2015, would involve constructing a canal northward from the Red Sea. The plan, which would include desalinization and hydroelectric plants along the course of the canal, would deliver large quantities of brine (a by-product of the desalinization process) to the lake. However, the project met with skepticism and opposition from environmentalists and others who questioned the potentially harmful effects of mixing water from the two sources.
Additional Information
The Dead Sea is a lake located in the southwestern of Asia. It is the Earth's lowest surface, located 420 meters (1,380 feet) below sea level. The sea is drying up as basin countries use water from its tributaries for drinking water and processes like irrigation.
The Dead Sea is almost nine times as salty as the ocean because a lot of water evaporates out of it, leaving salt behind. Most life cannot survive its high salinity, which is why it is called the "Dead Sea". However, some microbes have adapted to the high salinity and are able to survive in the Dead Sea's harsh environment.
Because the water is so salty, it weighs more than fresh water. This allows people to float in the Dead Sea without any effort. Tourists come from around the world to float in the water.

#218 Dark Discussions at Cafe Infinity » Climate Quotes - VII » 2025-08-23 17:06:29
- Jai Ganesh
- Replies: 0
Climate Quotes - VII
1. Nuclear tests poison the environment - and they also poison the political climate. They breed mistrust, isolation and fear. - Ban Ki-moon
2. Climate change, in some regions, has aggravated conflict over scarce land, and could well trigger large-scale migration in the decades ahead. And rising sea levels put at risk the very survival of all small island states. These and other implications for peace and security have implications for the United Nations itself. - Ban Ki-moon
3. I have been very encouraged by President Obama's call to action on climate change both at his Inauguration and in the State of the Union Address. This is a global imperative. I also welcome President Obama's intention to pursue reductions in nuclear math. - Ban Ki-moon
4. Climate change, demographics, water, food, energy, global health, women's empowerment - these issues are all intertwined. We cannot look at one strand in isolation. Instead, we must examine how these strands are woven together. - Ban Ki-moon
5. Look at climate change; don't put your head in the sand. Understand that it is going to have profound effects on our resources and so much else. - Hillary Clinton
6. America's experience, like many others, teaches us that fostering entrepreneurship is not just about crafting the right economic policy or developing the best educated curricula. It's about creating an entire climate in which innovation and ideas flourish. - Joe Biden
7. I'm not in favor of just taking short-term isolated situations and depleting our resources to keep our climate just the way it is today. - Buzz Aldrin
8. The effects of climate change are real and must be acted on. - Joe Biden.
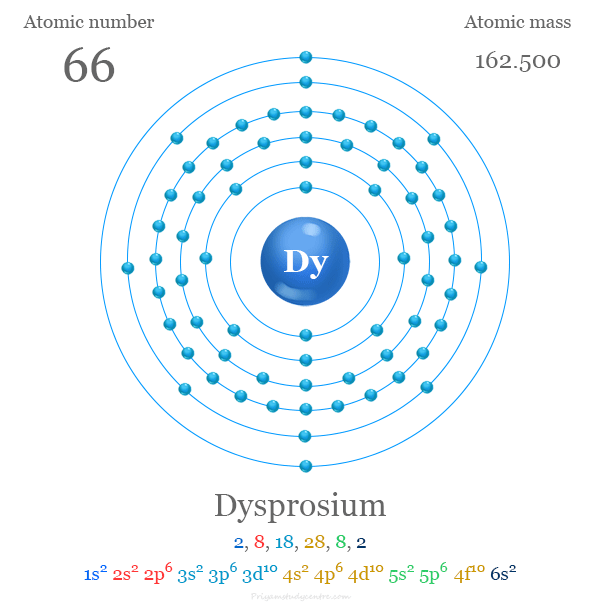
#219 Science HQ » Dysprosium » 2025-08-23 16:14:58
- Jai Ganesh
- Replies: 0
Dysprosium
Gist
Dysprosium (Dy) is a silvery, metallic rare-earth element with atomic number 66. Named for its "hard to obtain" nature, it's found in minerals like xenotime and is used in lasers, nuclear reactor control rods, and magnets due to its unique magnetic and neutron-absorbing properties.
Dysprosium's main use is in alloys for neodymium-based magnets. This is because it is resistant to demagnetisation at high temperatures. This property is important for magnets used in motors or generators. These magnets are used in wind turbines and electrical vehicles, so demand for dysprosium is growing rapidly.
Summary
Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it is found in various minerals, such as xenotime. Naturally occurring dysprosium is composed of seven isotopes, the most abundant of which is 164Dy.
Dysprosium was first identified in 1886 by Paul Émile Lecoq de Boisbaudran, but it was not isolated in pure form until the development of ion-exchange techniques in the 1950s. Dysprosium is used to produce neodymium-iron-boron (NdFeB) magnets, which are crucial for electric vehicle motors and the efficient operation of wind turbines.[9] It is used for its high thermal neutron absorption cross-section in making control rods in nuclear reactors, for its high magnetic susceptibility in data-storage applications, and as a component of Terfenol-D (a magnetostrictive material). Soluble dysprosium salts are mildly toxic, while the insoluble salts are considered non-toxic.
Details
Dysprosium (Dy) is a chemical element, a rare-earth metal of the lanthanide series of the periodic table.
Dysprosium is a relatively hard metal and is silvery white in its pure form. It is quite stable in air, remaining shiny at room temperature. Dysprosium turnings ignite easily and burn white-hot. The metal slowly reacts with water and quickly dissolves in diluted acids—except hydrofluoric acid (HF), in which it forms a protective layer of insoluble DyF3. The metal is a very strong paramagnet above approximately 180 K (−93 °C, or −136 °F); it is antiferromagnetic between about 90 (−183 °C, or −298 °F) and 180 K and ferromagnetic below 90 K.
French chemist Paul-Émile Lecoq de Boisbaudran first found this element (1886) associated with holmium and other heavy lanthanides; French chemist Georges Urbain later (1906) was able to prepare a reasonably pure fraction. Some important mineral sources of dysprosium are laterite ionic clays, xenotime, fergusonite, gadolinite, euxenite, polycrase, and blomstrandine. It also occurs in the products of nuclear fission.
The naturally occurring isotopes are all stable and have mass numbers 164 (natural abundance 28.3 percent), 162 (25.5 percent), 163 (24.9 percent), 161 (18.9 percent), 160 (2.33 percent), 158 (0.10 percent), and 156 (0.06 percent). Excluding nuclear isomers, a total of 29 radioactive isotopes of dysprosium are known. They range in mass from 138 to 173. The least stable is dysprosium-139 (half-life 0.6 second), and the most stable is dysprosium-154 (half-life 3.0 × {10}^{6} years).
Commercial separation is performed by liquid-liquid extraction or ion-exchange methods. The metal has been prepared by metallothermic reduction of the anhydrous halides with alkali or alkaline-earth metals. The metal is further purified by vacuum distillation. Dysprosium exists in three allotropic (structural) forms. The α-phase is close-packed hexagonal with a = 3.5915 Å and c = 5.6501 Å at room temperature. When cooled below ~90 K, the ferromagnetic ordering is accompanied by an orthorhombic distortion, β-Dy, of the hexagonal close-packed lattice. The β-phase has a = 3.595 Å, b = 6.184 Å, and c = 5.678 Å at 86 K (−187 °C, or −305 °F). The γ-phase is body-centred cubic with a = 4.03 Å at 1,381 °C (2,518 °F).
The major use of dysprosium is as an alloying addition to Nd2Fe14B permanent magnet materials (in which some of the neodymium is substituted with dysprosium) to increase both the Curie point and especially the coercivity and, therefore, improve the high-temperature performance of the alloy. The metal is also a component of magnetostrictive Terfenol D (Tb0.3Dy0.7Fe2). Dysprosium is used in control rods for nuclear reactors because of its relatively high neutron-absorption cross section; its compounds have been used for making laser materials and phosphor activators, and in metal halide lamps.
Chemically, dysprosium behaves as a typical trivalent rare earth and forms a series of pale yellow compounds in which its oxidation state is +3.
Element Properties
atomic number : 66
atomic weight : 162.5
melting point : 1,412 °C (2,574 °F)
boiling point : 2,567 °C (4,653 °F)
density : 8.551 gram/{cm}^{3} (24 °C, or 75 °F)
oxidation state : +3.
Additional Information:
Appearance
A bright, silvery metallic element.
Uses
As a pure metal it is little used, because it reacts readily with water and air. Dysprosium’s main use is in alloys for neodymium-based magnets. This is because it is resistant to demagnetisation at high temperatures. This property is important for magnets used in motors or generators. These magnets are used in wind turbines and electrical vehicles, so demand for dysprosium is growing rapidly.
Dysprosium iodide is used in halide discharge lamps. The salt enables the lamps to give out a very intense white light.
A dysprosium oxide-nickel cermet (a composite material of ceramic and metal) is used in nuclear reactor control rods. It readily absorbs neutrons, and does not swell or contract when bombarded with neutrons for long periods.
Biological role
Dysprosium has no known biological role. It has low toxicity.
Natural abundance
In common with many other lanthanides, dysprosium is found in the minerals monazite and bastnaesite. It is also found in smaller quantities in several other minerals such as xenotime and fergusonite.
It can be extracted from these minerals by ion exchange and solvent extraction. It can also be prepared by the reduction of dysprosium trifluoride with calcium metal.
#220 Re: Jai Ganesh's Puzzles » General Quiz » 2025-08-23 15:47:45
Hi,
#10529. What does the term in Geography Billabong mean?
#10530. What does the term in Geography Biogeography mean?
#221 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-08-23 15:21:31
Hi,
#5719. What does the noun ministration mean?
#5720. What does the noun mink mean?
#222 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-08-23 15:10:57
Hi,
#2451. What does the medical term Insulin receptor mean?
#223 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-08-23 15:02:02
Hi,
#9719.
#224 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-08-23 14:39:29
Hi,
#6224.
#225 Re: Exercises » Compute the solution: » 2025-08-23 14:22:18
Hi,
2565.