Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#51 Dark Discussions at Cafe Infinity » Clock Quotes - III » 2025-09-06 17:41:33
- Jai Ganesh
- Replies: 0
Clock Quotes - III
1. I remember the 2015 World Cup looking up at the scoreboard and seeing that big Hublot sign and that clock and I just think it's a real compliment to the game of cricket. - Michael Clarke
2. If I am not excited about a project, then how do I expect my audience to watch my film? Give me good roles, and I will be working round the clock. -Dimple Kapadia
3. I marketed pens - on the phone. But the beauty of the gig was that you had to call these strangers and say, 'Hi, how ya doing?' You made up a name, like, 'Hey, it's Edward Quartermaine from California. You're eligible to receive this grandfather clock or a trip to Tahiti.' You promise them all these things if they buy a gross of pens. - Johnny Depp
4. I need to know how the clock is made after you tell me what time it is. I want to know all the details so I can understand how it works. - Sandra Bullock
5. I never really did years of movie-after-movie-after-movie but when you've got three toddlers in the house you're performing all day long, anyway, with puppet shows and stories - I act around the clock. - Julia Roberts
6. Food makes me happy. Make me work round the clock, but just feed me first! - Deepika Padukone
7. When it comes to the New Year, I make it a point to catch my mum and dad awake before the clock strikes 12. Then, I celebrate the night with friends. - Amy Jackson
8. Since my international debut in 2014 the miles on the clock have probably crept up and Test cricket is the level where the pressure and scrutiny are greatest. - Moeen Ali
9. You realize as an athlete that there is a bit of a clock, and you don't want to look back on a career and say, 'I wish I had done this a bit differently.' - Pam Shriver.
#52 Jokes » Lawyer Jokes - XIII » 2025-09-06 17:03:55
- Jai Ganesh
- Replies: 0
Q: What do you call a spider working at a law firm?
A: A Spin Doctor.
* * *
Q: What is the definition of a "crying shame"?
A: There was an empty seat.
* * *
Q. Where can you find a good lawyer?
A. In the cemetery.
* * *
Q: What do lawyers use for birth control?
A: Their personalities.
* * *
Q: What is the difference between a tick and a lawyer?
A: A tick falls off of you when you die.
* * *
#53 Science HQ » Rhenium » 2025-09-06 16:44:34
- Jai Ganesh
- Replies: 0
Rhenium
Gist
Rhenium (Re) is a rare, dense, silvery-white transition metal with atomic number 75, known for its extreme high-temperature stability and ductility. It is a critical component in high-temperature superalloys for jet engine parts, and also functions as a catalyst in petrochemical processes. Rhenium is primarily found in low concentrations in copper and molybdenum ores and is the last stable element to be discovered.
Rhenium is primarily used in high-temperature superalloys for aircraft engine and gas turbine components, as well as a component in platinum-rhenium catalysts for petroleum reforming. Other applications include tungsten-rhenium alloys for high-temperature thermocouples and filaments, radioactive isotopes in cancer treatment, and as an additive to enhance the properties of other metals in alloys for electronics and welding.
Summary
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. It has one of the highest melting and boiling points of any element. It resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. It shows in its compounds a wide variety of oxidation states ranging from −1 to +7.
Rhenium was originally discovered in 1908 by Masataka Ogawa, but he mistakenly assigned it as element 43 (now known as technetium) rather than element 75 and named it nipponium. It was rediscovered in 1925 by Walter Noddack, Ida Tacke and Otto Berg, who gave it its present name. It was named after the river Rhine in Europe, from which the earliest samples had been obtained and worked commercially.
Nickel-based superalloys of rhenium are used in combustion chambers, turbine blades, and exhaust nozzles of jet engines. These alloys contain up to 6% rhenium, making jet engine construction the largest single use for the element. The second-most important use is as a catalyst: it is an excellent catalyst for hydrogenation and isomerization, and is used for example in catalytic reforming of naphtha for use in gasoline (rheniforming process). Because of the low availability relative to demand, rhenium is expensive, with price reaching an all-time high in 2008–09 of US$10,600 per kilogram (US$4,800 per pound). As of 2018, its price had dropped to US$2,844 per kilogram (US$1,290 per pound) due to increased recycling and a drop in demand for rhenium catalysts.
Details
Rhenium (Re) is a chemical element, a very rare metal of Group 7 (VIIb) of the periodic table and one of the densest elements. Predicted by the Russian chemist Dmitry Ivanovich Mendeleyev (1869) as chemically related to manganese, rhenium was discovered (1925) by the German chemists Ida and Walter Noddack and Otto Carl Berg. The metal and its alloys have found limited application as turbine blades in fighter-jet engines, fountain pen points, high-temperature thermocouples (with platinum), catalysts, electrical contact points, and instrument-bearing points and in electrical components, such as in flashbulb filaments as an alloy with tungsten.
Rhenium does not occur free in nature or as a compound in any distinct mineral; instead it is widely distributed in small amounts in other minerals, usually in concentrations averaging about 0.001 parts per million. Chile is the world leader in rhenium recovery, followed by the United States, Poland, Uzbekistan, and Kazakhstan.
Rhenium occurs up to about 20 parts per million in molybdenite and to a lesser extent in sulfidic copper ores. The recovery of rhenium is aided by the concentration of its volatile heptoxide (Re2O7) in the flue dust and gases given off during the smelting of molybdenite ore or from its concentration with the platinum metals in the anode sludge during electrolytic copper refining. The black metal powder is extracted from the gases and dust by leaching or scrubbing them with water to dissolve the oxide, Re2O7, which in turn can be converted to ammonium perrhenate, NH4ReO4, and then reduced to the metal with hydrogen. The powder may be compressed and sintered into bars in hydrogen at elevated temperatures. Cold-working and annealing permit the fabrication of wire or foil.
Rhenium metal is silvery white and extremely hard; it resists wear and corrosion very well and has one of the highest melting points of the elements. (The melting point of rhenium, 3,180 °C [5,756 °F], is exceeded only by those of tungsten and carbon.) The metal powder slowly oxidizes in air above 150 °C (300 °F) and rapidly at higher temperatures to form the yellow heptoxide, Re2O7. The metal is not soluble in hydrochloric acid and dissolves only slowly in other acids. There is evidence for the existence of rhenium in each of the oxidation states from −1 to +7; the most common states are +3, +4, +5, and especially +7. Rhenium’s most characteristic and important compounds are formed in the oxidation states +4 and +7, although compounds are known in all formal oxidation states from −1 to +7. Perrhenic acid (HReO4) and its anhydride, the heptoxide, and the perrhenates are common stable compounds in which rhenium is in the +7 state. Natural rhenium is a mixture of the stable isotope rhenium-185 (37.4 percent) and the radioactive rhenium-187 (62.6 percent, 4.1 × {10}^{10}-year half-life).
Element Properties:
atomic number : 75
atomic weight : 186.2
melting point : 3,180 °C (5,756 °F)
boiling point : 5,627 °C (10,161 °F)
specific gravity : 20.5 at 20 °C (68 °F)
oxidation states : +1, +2, +3, +4, +5, +6, +7.
Additional Information:
Appearance
A metal with a very high melting point. Tungsten is the only metallic element with a higher melting point.
Uses
Rhenium is used as an additive to tungsten- and molybdenum-based alloys to give useful properties. These alloys are used for oven filaments and x-ray machines. It is also used as an electrical contact material as it resists wear and withstands arc corrosion.
Rhenium catalysts are extremely resistant to poisoning (deactivation) and are used for the hydrogenation of fine chemicals. Some rhenium is used in nickel alloys to make single-crystal turbine blades.
Biological role
Rhenium has no known biological role.
Natural abundance
Rhenium is among the rarest metals on Earth. It does not occur uncombined in nature or as a compound in a mineable mineral species. It is, however, widely spread throughout the Earth’s crust to the extent of about 0.001 parts per million. Commercial production of rhenium is by extraction from the flue dusts of molybdenum smelters.

#54 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-06 15:38:17
Hi,
#10549. What does the term in Biology Chloroplast mean?
#10550. What does the term in Biology Cholesterol mean?
#55 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-06 15:20:41
Hi,
#5739. What does the noun optimism mean?
#5740. What does the noun optometry mean?
#56 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-06 15:05:50
Hi,
#2461. What does the medical term Premolar mean?
#57 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-06 14:43:45
Hi,
#9728.
#58 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-06 14:10:11
Hi,
#6234.
#59 Re: Exercises » Compute the solution: » 2025-09-06 13:40:27
Hi,
Correct!
2576.
#60 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-09-05 23:18:32
2234) David Baker (biochemist)
Gist:
Work
Proteins control and drive all the chemical reactions that together are the basis of life. Proteins generally consist of 20 different amino acids, which can be described as life’s building blocks. In 2003, David Baker succeeded in using these blocks to design a new protein that was unlike any other protein. Baker used computer-based methods, which he had developed. This created opportunities to develop an enormous diversity of new proteins. New proteins can be used in, for example, drugs, vaccines and materials and sensors.
Summary
David Baker (born 1962, Seattle, Washington, U.S.) is an American biochemist and computational biologist who developed computerized methods for the de novo (from scratch) design of proteins with entirely new functions. Baker’s work on protein structure prediction and design fueled advances in synthetic biology and in the development of novel drugs and proteins for industrial applications. He was awarded the 2024 Nobel Prize in Chemistry (shared with English computer scientist Demis Hassabis and American researcher John M. Jumper) for his breakthroughs in computational protein design.
Education and early career
Baker grew up in Seattle. As an undergraduate, he attended Harvard University, where he studied philosophy and social science until his last year, when he transitioned to biology. After earning a bachelor’s degree from Harvard in 1984, he went to the University of California, Berkeley, to study biochemistry. There he carried out research on systems of protein transport in yeast. He earned a Ph.D. in 1989 and subsequently became a postdoctoral researcher at the University of California, San Francisco, where his focus shifted to structural biology. In 1993 he joined the faculty at the University of Washington and began to more deeply investigate protein folding and the ways in which sequences of amino acids form the wide variety of protein shapes observed in cells.
Rosetta
In the early 1990s Baker’s research team employed standard approaches available for protein analysis, including mass spectrometry and nuclear magnetic resonance spectroscopy. Eventually, however, they broadened their investigations to include computer modeling, which provided critical insight into the three-dimensional shapes that result from protein folding and the relevance of these shapes to protein function. This work led to his development of Rosetta, a revolutionary computational platform for predicting and designing protein structures that is based on a Monte Carlo sampling algorithm. Rosetta allows researchers to model protein folding and to design new proteins with desired functions.
In 1998 Baker used Rosetta in a competition known as Critical Assessment of protein Structure Prediction (CASP), a biennial scientific experiment in which participants attempt to predict the structures of proteins from known amino acids sequences. Rosetta performed well in the competition, successfully predicting how a given sequence would fold. After a series of refinements, Baker’s team began using Rosetta in reverse, obtaining information on amino acid sequences based on a given protein structure. In this way, researchers gained the ability to design proteins according to desired shapes.
Baker’s major breakthrough came in 2003, when Rosetta was fed an entirely new protein structure and computed a corresponding sequence consisting of 93 amino acids. The researchers verified Rosetta’s output by inserting the gene for the novel sequence into bacterial cells, which then produced the artificial protein, known as Top7. Top7 was the first protein to be designed and produced de novo. Baker subsequently released Rosetta’s code, making it available to other researchers. The technology has enabled Baker and others to create novel proteins with valuable functions, such as enzymes that specialize in breaking down toxic chemicals. Baker has also developed mini-proteins for therapeutic applications, including a mini-protein of about 56 amino acids capable of inhibiting SARS-CoV-2, the virus responsible for the COVID-19 pandemic.
Awards and honors
Baker has received various awards throughout his career, in addition to the Nobel Prize. In 2002 he was awarded the Overton Prize by the International Society for Computational Biology, and in 2004 he received the Feynman Prize in nanotechnology from the Foresight Institute (shared with Brian Kuhlman). In 2021 he was recognized with the prestigious Breakthrough Prize in Life Sciences and the following year received the Wiley Prize in Biomedical Sciences (shared with Hassabis and Jumper). He is a Howard Hughes Medical Institute investigator (2000) and a member of the National Academy of Sciences (2006).
Details
David Baker (born October 6, 1962) is an American biochemist and computational biologist who has pioneered methods to design proteins and predict their three-dimensional structures. He is the Henrietta and Aubrey Davis Endowed Professor in Biochemistry, an investigator with the Howard Hughes Medical Institute, and an adjunct professor of genome sciences, bioengineering, chemical engineering, computer science, and physics at the University of Washington. He was awarded the shared 2024 Nobel Prize in Chemistry for his work on computational protein design.
Baker is a member of the United States National Academy of Sciences and the director of the University of Washington's Institute for Protein Design. He has co-founded more than a dozen biotechnology companies and was included in Time magazine's inaugural list of the 100 Most Influential People in health in 2024.
Biography:
Early life and education
Baker was born into a Jewish family in Seattle, Washington on October 6, 1962, the son of physicist Marshall Baker and geophysicist Marcia (née Bourgin) Baker. He graduated from Seattle's Garfield High School.
Baker received a Bachelor of Arts degree with a major in biology from Harvard University in 1984. He then joined the laboratory of Randy Schekman, where he worked primarily on protein transport and trafficking in yeast, and obtained a Doctor of Philosophy in biochemistry from the University of California, Berkeley in 1989. In 1993, he completed his postdoctoral training in biophysics with David Agard at the University of California, San Francisco.
Career
Baker joined the Department of Biochemistry at the University of Washington School of Medicine as a faculty member in 1993. He became a Howard Hughes Medical Institute investigator in 2000. Baker was elected a Fellow of the American Academy of Arts and Sciences in 2009.
Personal life
Baker is married to Hannele Ruohola-Baker, another biochemist at the University of Washington. They have two children.
Research
Although primarily known for the development of computational methods for predicting and designing the structures and functions of proteins, Baker maintains an active experimental biochemistry group. He has authored over 600 scientific papers.
Baker's group developed the Rosetta algorithm for ab initio protein structure prediction, which has been extended into a tool for protein design, a distributed computing project called Rosetta@home, and the computer game Foldit. Baker served as the director of the Rosetta Commons, a consortium of labs and researchers that develop biomolecular structure prediction and design software. His group has regularly competed in the CASP structure prediction competition, specializing in ab initio methods, including both manually assisted and automated variants of the Rosetta protocol. Using artificial intelligence, his group has developed later a newer version of the program known as RoseTTAFold.
Baker's group is also active in the field of protein design; they are noted for designing Top7, the first artificial protein with a novel fold.
In 2017, Baker's Institute for Protein Design received over $11 million from Open Philanthropy, followed by an additional $3 million donation in 2021.
In April 2019, Baker gave a TED talk titled "5 challenges we could solve by designing new proteins" at TED2019 in Vancouver, Canada.
Baker has co-founded several biotechnology companies, including Prospect Genomics which was acquired by an Eli Lilly subsidiary in 2001, Icosavax which was acquired by AstraZeneca in 2023, Sana Biotechnology, Lyell Immunotherapeutics, and Xaira Therapeutics.
Awards
For his work on protein folding, Baker has received numerous awards, including the Overton Prize (2002), the Sackler International Prize in Biophysics (2008), the Wiley Prize (2022) and the BBVA Foundation Frontiers of Knowledge Award in the category "Biology and Biomedicine" (2022).
For his work on protein design, Baker has received the Newcomb Cleveland Prize (2004), the Feynman Prize in Nanotechnology (2004), and the Breakthrough Prize in Life Sciences (2021).
In 2024, Baker was awarded half of the Nobel Prize in Chemistry for his work on protein design; the other half went to John M. Jumper and Demis Hassabis for development of AlphaFold, a program for protein structure prediction.

#61 This is Cool » Sodium Carbonate » 2025-09-05 19:11:46
- Jai Ganesh
- Replies: 0
Sodium Carbonate
Gist
Sodium carbonate is a white, water-soluble, inorganic salt with the chemical formula Na₂CO₃. Also known as soda ash, washing soda, or soda crystals, it is a weak base used extensively in the manufacture of glass and soap, as a cleaning agent, and for softening water. Industrially, it is produced primarily by the Solvay process or mined from natural deposits like trona.
Sodium carbonate is used as a key ingredient in making glass, paper, soaps, and detergents. It's also a common water softener, a cleaning agent, a food additive for pH regulation, and an agent for adjusting pH in textiles. Additionally, it's used in mining for separating minerals, as a laboratory reagent for acid standardization, and as a constituent in some effervescent tablets.
Summary
Sodium carbonate is a chemical compound. It is composed of sodium and carbonate ions. Its chemical formula is Na2CO3. It is a base. It reacts with acids to produce carbon dioxide. It is produced industrially by the Solvay process. It is used to make glass. It is also used in pools and cooking. It is used as an electrolyte.
Preparation
It is made by the reaction of sodium hydroxide with carbon dioxide. Water is also a byproduct during this reaction. It is also made when sodium bicarbonate is heated.
Details
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odorless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.
Applications
Some common applications of sodium carbonate include:
* As a cleansing agent for domestic purposes like washing clothes. Sodium carbonate is a component of many dry soap powders. It has detergent properties through the process of saponification, which converts fats and grease to water-soluble salts (specifically, soaps).
* It is used for lowering the hardness of water.
* It is used in the manufacture of glass, soap, and paper.
* It is used in the manufacture of sodium compounds like borax (sodium borate).
Glass manufacture
Sodium carbonate serves as a flux for silica (SiO2, melting point 1,713 °C), lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass insoluble. Bottle and window glass ("soda–lime glass" with transition temperature ~570 °C) is made by melting such mixtures of sodium carbonate, calcium carbonate, and silica sand (silicon dioxide (SiO2)). When these materials are heated, the carbonates release carbon dioxide. In this way, sodium carbonate is a source of sodium oxide. Soda–lime glass has been the most common form of glass for centuries. It is also a key input for tableware glass manufacturing.
Water softening
Hard water usually contains calcium or magnesium ions. Sodium carbonate is used for removing these ions and replacing them with sodium ions.
Food additive and cooking
Sodium carbonate has several uses in cuisine, largely because it is a stronger base than baking soda (sodium bicarbonate) but weaker than lye (which may refer to sodium hydroxide or, less commonly, potassium hydroxide). Alkalinity affects gluten production in kneaded doughs, and also improves browning by reducing the temperature at which the Maillard reaction occurs. To take advantage of the former effect, sodium carbonate is therefore one of the components of kansui, a solution of alkaline salts used to give Japanese ramen noodles their characteristic flavour and chewy texture; a similar solution is used in Chinese cuisine to make lamian, for similar reasons. Cantonese bakers similarly use sodium carbonate as a substitute for lye-water to give moon cakes their characteristic texture and improve browning. In German cuisine (and Central European cuisine more broadly), breads such as pretzels and lye rolls traditionally treated with lye to improve browning can be treated instead with sodium carbonate; sodium carbonate does not produce quite as strong a browning as lye, but is much safer and easier to work with.
Sodium carbonate is used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citric acid, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva.
Sodium carbonate also finds use in the food industry as a food additive (European Food Safety Authority number E500) as an acidity regulator, anticaking agent, raising agent, and stabilizer. It is also used in the production of snus to stabilize the pH of the final product.
Other applications
Sodium carbonate is also used as a relatively strong base in various fields. As a common alkali, it is preferred in many chemical processes because it is cheaper than sodium hydroxide and far safer to handle. Its mildness especially recommends its use in domestic applications.
For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It is also a common additive in swimming pools and aquarium water to maintain a desired pH and carbonate hardness (KH). In dyeing with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used as mordant to ensure proper chemical bonding of the dye with cellulose (plant) fiber. It is also used in the froth flotation process to maintain a favourable pH as a float conditioner besides CaO and other mildly basic compounds.
Miscellaneous
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay. In casting, it is referred to as "bonding agent" and is used to allow wet alginate to adhere to gelled alginate. Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
Sodium carbonate is also used in the processing and tanning of animal hides.
Occurrence as natural mineral
Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (evaporites) formed when seasonal lakes evaporate. Deposits of the mineral natron have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of mummies and in the early manufacture of glass.
The anhydrous mineral form of sodium carbonate is quite rare and called natrite. Sodium carbonate also erupts from Ol Doinyo Lengai, Tanzania's unique volcano, and it is presumed to have erupted from other volcanoes in the past, but due to these minerals' instability at the Earth's surface, are likely to be eroded. All three mineralogical forms of sodium carbonate, as well as trona, trisodium hydrogendi carbonate dihydrate, are also known from ultra-alkaline pegmatitic rocks, that occur for example in the Kola Peninsula in Russia.
Extra terrestrially, known sodium carbonate is rare. Deposits have been identified as the source of bright spots on Ceres, interior material that has been brought to the surface. While there are carbonates on Mars, and these are expected to include sodium carbonate, deposits have yet to be confirmed, this absence is explained by some as being due to a global dominance of low pH in previously aqueous Martian soil.
Additional Information
Sodium carbonate also called washing soda or soda ash is an inorganic compound or salt with the chemical formula Na2CO3. In pure form, sodium carbonate is a white crystalline powder. Sodium carbonate produces an alkaline solution that contains carbonic acid and sodium hydroxide when dissolved in water. Sodium carbonate uses widely for making detergents and soaps, paper, glass, and brick industry, and for modifying pH and softening water. Washing soda or sodium carbonate is now exclusively manufactured by the Solvay process where sodium chloride, carbon dioxide, and ammonia react to form sodium bicarbonate or baking soda. Baking soda is converted to sodium carbonate and washing soda when heating and recrystallization.
One common source of washing soda or sodium carbonate is the ashes of burned plants. Therefore, it is sometimes called soda ash. It is a strong base with a pH of about 11. Therefore, it is used as an antacid because it is non-corrosive and safer to handle.
Sodium carbonate in washing soda softens water. Therefore, it helps other cleaning ingredients lift soil from fabrics and suspend the soil in the wash water. It is also used for making the sodium compound borax. In laboratory and analytical chemistry, it is used to standardize an acid and an analytical reagent.

#62 Re: This is Cool » Miscellany » 2025-09-05 18:12:25
2381) Niagra Falls
Gist
What makes Niagara Falls a natural wonder? Statistically speaking, Niagara Falls is recognized as having the greatest flow rate of any waterfall throughout the globe. The falls have more than six million cubic feet (168,000 cubic meters) flow over the top of the falls every minute during the peak season.
Niagara Falls is a group of three waterfalls—American, Bridal Veil, and Horseshoe Falls—located on the Niagara River at the border between Ontario, Canada, and New York, USA. Known for the immense flow of water and spectacular views, the falls have the highest flow rate of any waterfall globally. The site is a popular tourist attraction, a source of hydroelectric power, and home to Niagara Falls State Park, the oldest state park in the United States.
The falls consist of the American Falls, the Bridal Veil Falls, and the much larger Horseshoe Falls.
Summary
Niagara Falls[a] is a group of three waterfalls at the southern end of Niagara Gorge, spanning the border between the province of Ontario in Canada and the state of New York in the United States. The largest of the three is Horseshoe Falls, which straddles the international border of the two countries. It is also known as the Canadian Falls. The smaller American Falls and Bridal Veil Falls lie within the United States. Bridal Veil Falls is separated from Horseshoe Falls by Goat Island and from American Falls by Luna Island, with both islands situated in New York.
Formed by the Niagara River, which drains Lake Erie into Lake Ontario, the combined falls have the highest flow rate of any waterfall in North America that has a vertical drop of more than 50 m (164 ft). During peak daytime tourist hours, more than 168,000 {m}^{3} (5.9 million cu ft) of water goes over the crest of the falls every minute. Horseshoe Falls is the most powerful waterfall in North America, as measured by flow rate. Niagara Falls is famed for its beauty and is a valuable source of hydroelectric power. Balancing recreational, commercial, and industrial uses has been a challenge for the stewards of the falls since the 19th century.
Niagara Falls is 27 km (17 mi) northwest of Buffalo, New York, and 69 km (43 mi) southeast of Toronto, between the twin cities of Niagara Falls, Ontario, and Niagara Falls, New York. Niagara Falls was formed when glaciers receded at the end of the Wisconsin glaciation (the last ice age), and water from the newly formed Great Lakes carved a path over and through the Niagara Escarpment en route to the Atlantic Ocean.
Details
Niagara Falls, waterfall on the Niagara River in northeastern North America, one of the continent’s most famous spectacles. The falls lie on the border between Ontario, Canada, and New York state, U.S. For many decades the falls were an attraction for honeymooners and for such stunts as walking over the falls on a tightrope or going over them in a barrel. Increasingly, however, the appeal of the site has become its beauty and uniqueness as a physical phenomenon.
The falls are in two principal parts, separated by Goat Island. The larger division, adjoining the left, or Canadian, bank, is Horseshoe Falls; its height is 188 feet (57 metres), and the length of its curving crest line is about 2,200 feet (670 metres). The American Falls, adjoining the right bank, are 190 feet (58 metres) high and 1,060 feet (320 metres) across.
The formation of the Niagara gorge (downriver) and the maintenance of the falls as a cataract depend upon peculiar geologic conditions. The rock strata from the Silurian Period (about 444 to 419 million years ago) in the Niagara gorge are nearly horizontal, dipping southward only about 20 feet per mile (almost 4 metres per km). An upper layer of hard dolomite is underlain by softer layers of shale. Water exerts hydrostatic pressure and only slowly dissolves the dolomite after infiltrating its joints. Dolomite blocks fall away as water from above infiltrates and rapidly erodes the shale at the falls itself. The disposition of the rock strata provides the conditions for keeping the water constantly falling vertically from an overhanging ledge during a long period of recession (movement upstream) of the cataract. As blocks of dolomite are undercut, they fall off and are rapidly destroyed by the falling water, further facilitating the retreat of the falls and the maintenance of a vertical cataract.
The water flowing over the falls is free of sediment, and its clearness contributes to the beauty of the cataract. In recognition of the importance of the waterfall as a great natural spectacle, the province of Ontario and the state of New York retained or acquired title to the adjacent lands and converted them into public parks.
The very large diversion of water above the falls for hydroelectric power purposes has lessened the rate of erosion. Elaborate control works upstream from the falls have maintained an even distribution of flow across both the U.S. and Canadian cataracts, thereby preserving the curtains of the waterfalls. A large part of the great river above the falls is diverted and disappears into four great tunnels for use in the power plants downstream. Because of concern over the possibility of major rockfalls, water was diverted from the American Falls in 1969, and some cementing of the bedrock was done; an extensive boring and sampling program was also carried out. River flow was returned to the American Falls in November of that year, and it was decided that safety measures for the viewing public should be implemented and that measures to stem natural processes were both too expensive and undesirable.
Excellent views of the falls are obtained from Queen Victoria Park on the Canadian side; from Prospect Point on the U.S. side at the edge of the American Falls; and from Rainbow Bridge, which spans the Niagara gorge about 1,000 feet (300 metres) downstream from Prospect Point. Visitors may cross from the U.S. shore to Goat Island by footbridge and may take an elevator to the foot of the falls and visit the Cave of the Winds behind the curtain of falling water. The Horseshoe Falls, which carry about 90 percent of the river’s discharge, receded upstream at an average rate of about 5.5 feet (1.7 metres) per year in 1842–1905. Thereafter, control works and the diversion of water decreased the erosion rate, which is presently so slow at the American Falls that large blocks of dolomite accumulate at the base of the falls, threatening to turn it into rapids.
Additional Information
Situated between the state of New York and the province of Ontario, Niagara Falls is one of the most spectacular natural wonders in North America. This series of waterfallsis on the Niagara River, which flows between the United States and Canada from Lake Erie to Lake Ontario. The falls are about 17 miles (27 kilometers) northwest of Buffalo, New York. The industrial city and tourist center of Niagara Falls, New York, is adjacent to the American side of the falls. Niagara Falls, Ontario, Canada, is across the river.
The falls are divided into two parts by Goat Island. The larger portion, on the southwest side, is the Canadian falls, known as the Horseshoe Falls. It measures 2,600 feet (790 meters) along its curve and drops 162 feet (49 meters). The smaller American Falls is northeast of Goat Island. It is 1,000 feet (305 meters) across and drops about 167 feet (51 meters). Between the American Falls and Goat Island are the small Luna Island and the small Luna, or Bridal Veil, Falls.
Just before flowing over the ledge, the American stream is only about 3 1/2 feet (1 meter) deep. The Canadian stream is about 20 feet (6 meters) deep and carries some 95 percent of the Niagara River’s water.
Every minute about 12,000,000 cubic feet (340,000 cubic meters), or 379,000 tons, of water pours in torrents over the cliffs of the falls of Niagara. As the water plunges from the brink of the falls, it fills the air with a silvery mist, which under the sunlight displays many rainbows. The plunging water also sends out a never-ending roar as it strikes the bottom. For this reason the Haudenosaunee (Iroquois) people called the falls Niagara, meaning “thunder of waters.”
The plunging water has worn the lower rocks away so that there are caves behind the sheets of water of both falls. Sightseers may enter the Cave of the Winds at the foot of the American Falls and get an unusual view. The Horseshoe Falls have carved a plunge basin 192 feet (59 meters) deep.
Both the United States and Canadian governments have built parks, viewing platforms, paths, and highways. The Niagara Reservation State Park was established in 1885 and is New York’s oldest state park. It includes an observation tower, elevators that descend into the gorge at the base of the American Falls, and boat trips into the waters at the base of the Horseshoe Falls.
The park area has long been a tourist site and a favorite spot for couples to spend their honeymoons. At night colored lights illuminate the falls.
Almost all the drainage from four of the Great Lakes pours over the crest of Niagara Falls. This tremendous volume of water is used to generate power in six hydroelectric plants. They develop a maximum of about 5 3/4 million horsepower, some 55 percent on the American side and about 45 percent on the Canadian. The plants draw water from the river above the falls through canals. Near each plant the water drops through penstocks to powerhouses on the Niagara River below the falls. There it turns turbine generators.
The control of Niagara Falls between the United States and Canada has long offered the world an example of international cooperation. A treaty in 1910 and later agreements fixed the amounts of water that could be diverted. An international Niagara Control Board was established in 1923.
In 1950 the two countries signed a new treaty that specified the minimum flow to be maintained over the falls. This treaty made possible greater hydroelectric development. It provides that 100,000 cubic feet (2,830 cubic meters) per second of water must flow over the falls during the tourist season in the daytime and 50,000 (1,415) at night and during the off-tourist season in the daytime. The remainder is equally divided between Canada and the United States. An average of 202,300 cubic feet (5,729 cubic meters) per second flows over the falls.
Between 1954 and 1958 the United States and Canada completed the Niagara Remedial Works Project. This enormous operation checked erosion with a gated control structure, excavations, and fills.
The Hydro-Electric Power Commission of Ontario completed the Sir Adam Beck-Niagara Generating Station No. 1 in 1925 and No. 2 in 1958. The combined capacity of the plants is 1,443,000 kilowatts.
In 1957 the United States Congress approved the construction of the Niagara Power Project by the Power Authority of the State of New York. It has a capacity of 2,190,000 kilowatts. The first electric current from the project was delivered in 1961.
The falls of Niagara are about 25,000 years old. The hard rock (Lockport dolomite) at the brink of the falls is much older. It was made on the bed of an inland sea in the Silurian period (about 443 million to 419 million years ago). Gradually the limy sediment hardened to stone—either limestone or dolomite, a limestone with magnesium.
Later the Niagara region was raised in a widespread uplift centered in Michigan. Streams wore down the land. The layer of tough rock, however, resisted erosion. The edge of the deposit formed a great cliff—the Niagara escarpment. It runs west from Rochester, New York, between Lakes Erie and Ontario, then swings northward through the province of Ontario. It is capped by hard Niagara limestone or Lockport dolomite.
Glaciers covered the Niagara region during the Ice Age that took place during the Pleistocene Epoch (about 2.6 million to 11,700 years ago). As the last glacier retreated, it left Lake Erie at its southern edge. Water from the lake began to spill over the Niagara escarpment into the Ontario basin below, just south of where Queenston and Lewiston now stand.
The new falls did not wear away the dolomite caprock as fast as it churned away the softer rock below. From time to time blocks of the undermined caprock broke off. The falls worked back toward Lake Erie, forming a steep-walled gorge.
Niagara’s rate of cutting has changed many times. It started slowly, for at first the river drained Lake Erie only. Lakes Superior, Michigan, and Huron had a northerly outlet. The drainage changed as glaciers retreated. Water from all four lakes then poured over the falls. When the river spread to the point where the famous whirlpool now is, it reached an ancient valley that had cut into the dolomite from the west. Later the valley filled with glacial debris. The river wore away the soft material, forming the 60-acre (24- hectare) basin.
Louis Hennepin, a priest who accompanied the French explorer René-Robert Cavelier, sieur de La Salle, was the first European to view Niagara Falls, in 1678. The site was of strategic use to the British and French in the struggle to control the Great Lakes. The British built Fort Schlosser there in 1761.

#63 Dark Discussions at Cafe Infinity » Clock Quotes - II » 2025-09-05 17:18:52
- Jai Ganesh
- Replies: 0
Clock Quotes - II
1. I don't have an alarm clock. If someone needs to wake me up, then I have my BlackBerry next to me. - Mark Zuckerberg
2. Work is a necessity for man. Man invented the alarm clock. - Pablo Picasso
3. Like the United Nations, there is something inspirational about New York as a great melting pot of different cultures and traditions. And if this is the city that never sleeps, the United Nations works tirelessly, around the clock around the world. - Ban Ki-moon
4. By putting forward the hands of the clock you shall not advance the hour. - Victor Hugo
5. Switzerland is a small, steep country, much more up and down than sideways, and is all stuck over with large brown hotels built on the cuckoo clock style of architecture. - Ernest Hemingway
6. When I was 5, my parents split and my mother raised us mostly on her own. Like so many mothers, she worked around the clock to make it work - packing lunches before we woke up - and paying bills after we went to bed. Helping us with homework at the kitchen table - and shuttling us to church for choir practice. - Kamala Harris
7. I think when you're off the clock, you should be off the clock. - Jennifer Aniston
8. To me, Dan Evans is an example of somebody that puts the clock back a little bit and tells everybody: 'Listen, tennis is not a freak sport where you need to have rich parents, who sit in your players' box for every single week of the whole year, and you need to talk to your coaches' box between every shot.' - Mats Wilander.
#64 Science HQ » Tungsten » 2025-09-05 16:54:03
- Jai Ganesh
- Replies: 0
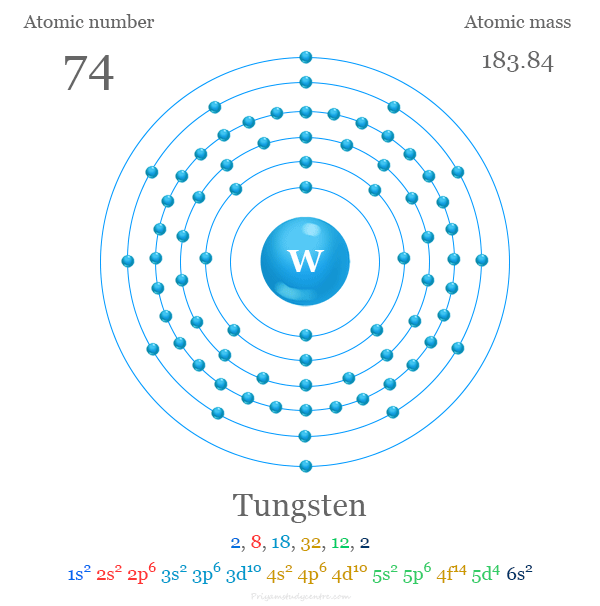
Tungsten
Gist
Tungsten (symbol W) is a rare, hard, refractory metal with the highest melting point of all elements, making it useful for high-temperature applications like light bulb filaments, heating elements, and tool bits. Also known as wolfram, it is a Group 6 element found naturally in minerals such as wolframite and scheelite. The metal is used in alloys like tungsten carbide, a very hard and wear-resistant material found in industrial cutting tools, drill tips, and even some jewelry.
Tungsten is used in products where extreme heat resistance, hardness, and density are needed. Key applications include light bulb filaments, cutting tools and drill bits made of tungsten carbide, X-ray tubes, electronic components, high-temperature furnace parts, radiation shielding, and alloys for aerospace and automotive components. Its high density and ability to strengthen other metals also make it useful for ballast weights, darts, and jewelry.
Summary
Tungsten (also called wolfram) is a chemical element; it has symbol W (from Latin: Wolframium). Its atomic number is 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternative name.
The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements, melting at 3,422 °C (6,192 °F; 3,695 K). It also has the highest boiling point, at 5,930 °C (10,706 °F; 6,203 K). Its density is 19.254 g/{cm}^{3}, comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work into metal. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw.
Tungsten occurs in many alloys, which have numerous applications, including incandescent light bulb filaments, X-ray tubes, electrodes in gas tungsten arc welding, superalloys, and radiation shielding. Tungsten's hardness and high density make it suitable for military applications in penetrating projectiles. Tungsten compounds are often used as industrial catalysts. Its largest use is in tungsten carbide, a wear-resistant material used in metalworking, mining, and construction. About 50% of tungsten is used in tungsten carbide, with the remaining major use being alloys and steels: less than 10% is used in other compounds.
Tungsten is the only metal in the third transition series that is known to occur in biomolecules, being found in a few species of bacteria and archaea. However, tungsten interferes with molybdenum and copper metabolism and is somewhat toxic to most forms of animal life.
Details
Tungsten (W), chemical element, is an exceptionally strong refractory metal of Group 6 (VIb) of the periodic table, used in steels to increase hardness and strength and in lamp filaments.
Tungsten metal was first isolated (1783) by the Spanish chemists and mineralogists Juan José and Fausto Elhuyar by charcoal reduction of the oxide (WO3) derived from the mineral wolframite. Earlier (1781) the Swedish chemist Carl Wilhelm Scheele had discovered tungstic acid in a mineral now known as scheelite, and his countryman Torbern Bergman concluded that a new metal could be prepared from the acid. The names tungsten and wolfram have been used for the metal since its discovery, though everywhere Jön Jacob Berzelius’s symbol W prevails. In British and American usage, tungsten is preferred; in Germany and a number of other European countries, wolfram is accepted.
Element Properties
atomic number : 74
atomic weight : 183.85
melting point : 3,410 °C (6,152 °F)
boiling point : 5,660 °C (10,220 °F)
density : 19.3 grams/{cm}^{3} at 20 °C (68 °F)
oxidation states : +2, +3, +4, +5, +6
Occurrence, properties, and uses
The amount of tungsten in Earth’s crust is estimated to be 1.5 parts per million, or about 1.5 grams per ton of rock. China is the dominant producer of tungsten; in 2016 it produced over 80 percent of total tungsten mined, and it contained nearly two-thirds of the world’s reserves. Vietnam, Russia, Canada, and Bolivia produce most of the remainder. Tungsten does not occur as a free metal. It is about as abundant as tin or as molybdenum, which it resembles, and half as plentiful as uranium. Although tungsten occurs as tungstenite—tungsten disulfide, WS2—the most important ores in this case are the tungstates such as scheelite (calcium tungstate, CaWO4), stolzite (lead tungstate, PbWO4), and wolframite—a solid solution or a mixture or both of the isomorphous substances ferrous tungstate (FeWO4) and manganous tungstate (MnWO4).
For tungsten the ores are concentrated by magnetic and mechanical processes, and the concentrate is then fused with alkali. The crude melts are leached with water to give solutions of sodium tungstate, from which hydrous tungsten trioxide is precipitated upon acidification, and the oxide is then dried and reduced to metal with hydrogen.
Tungsten is rather resistant to attack by acids, except for mixtures of concentrated nitric and hydrofluoric acids, and it can be attacked rapidly by alkaline oxidizing melts, such as fused mixtures of potassium nitrate and sodium hydroxide or sodium peroxide; aqueous alkalies, however, are without effect. It is inert to oxygen at normal temperature but combines with it readily at red heat, to give the trioxides, and is attacked by fluorine at room temperature, to give the hexafluorides.
Tungsten metal has a nickel-white to grayish lustre. Among metals it has the highest melting point, at 3,410 °C (6,170 °F), the highest tensile strength at temperatures of more than 1,650 °C (3,002 °F), and the lowest coefficient of linear thermal expansion (4.43 × {10}^{-6} per °C at 20 °C [68 °F]). Tungsten is ordinarily brittle at room temperature. Pure tungsten can, however, be made ductile by mechanical working at high temperatures and can then be drawn into very fine wire. Tungsten was first commercially employed as a lamp filament material and thereafter used in many electrical and electronic applications. It is used in the form of tungsten carbide for very hard and tough dies, tools, gauges, and bits. Much tungsten goes into the production of tungsten steels, and some has been used in the aerospace industry to fabricate rocket-engine nozzle throats and leading-edge reentry surfaces. (For information on the mining, recovery, and applications of tungsten, see tungsten processing.)
Natural tungsten is a mixture of five stable isotopes: tungsten-180 (0.12 percent), tungsten-182 (26.50 percent), tungsten-183 (14.31 percent), tungsten-184 (30.64 percent), and tungsten-186 (28.43 percent). Tungsten crystals are isometric and, by X-ray analysis, are seen to be body-centred cubic.
Compounds
Chemically, tungsten is relatively inert. Compounds have been prepared, however, in which the element has oxidation states from 0 to +6. The states above +2, especially +6, are most common. In the +4, +5, and +6 states, tungsten forms a variety of complexes.
The most important tungsten compound is tungsten carbide (WC), which is noted for its hardness (9.5 on the Mohs scale, where the maximum, diamond, is 10). It is used alone or in combination with other metals to impart wear-resistance to cast iron and the cutting edges of saws and drills. Tungsten also forms hard, refractory, and chemically inert interstitial compounds with boron, nitrogen, and silicon upon direct reaction with those elements at high temperatures.
Additional Information:
Appearance
A shiny, silvery-white metal.
Uses
Tungsten was used extensively for the filaments of old-style incandescent light bulbs, but these have been phased out in many countries. This is because they are not very energy efficient; they produce much more heat than light.
Tungsten has the highest melting point of all metals and is alloyed with other metals to strengthen them. Tungsten and its alloys are used in many high-temperature applications, such as arc-welding electrodes and heating elements in high-temperature furnaces.
Tungsten carbide is immensely hard and is very important to the metal-working, mining and petroleum industries. It is made by mixing tungsten powder and carbon powder and heating to 2200°C. It makes excellent cutting and drilling tools, including a new ‘painless’ dental drill which spins at ultra-high speeds.
Calcium and magnesium tungstates are widely used in fluorescent lighting.
Biological role
Tungsten is the heaviest metal to have a known biological role. Some bacteria use tungsten in an enzyme to reduce carboxylic acids to aldehydes.
Natural abundance
The principal tungsten-containing ores are scheelite and wolframite. The metal is obtained commercially by reducing tungsten oxide with hydrogen or carbon.
#65 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-05 15:55:51
Hi,
#10547. What does the term in Biology Centrosome mean?
#10548. What does the term in Biology Chlorophyll mean?
#66 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-05 15:29:40
Hi,
#5737. What does the verb (used with object) exert mean?
#5738. What does the verb (used with without object) exhale mean?
#67 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-05 15:02:34
Hi,
#2460. What does the medical term Laryngeal mask airway signify?
#68 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-05 14:31:36
Hi,
#9727.
#69 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-05 14:10:04
Hi,
#6233.
#70 Jokes » Lawyer Jokes - XII » 2025-09-05 13:48:32
- Jai Ganesh
- Replies: 0
Q: How do you get a lawyer out of a tree?
A: Cut the rope.
* * *
Q: Do you know how to save a drowning lawyer?
A: Take your foot off his head.
* * *
Q: Do you know how to save a drowning lawyer?
A: No? Good!
* * *
Q: What's the difference between a lawyer and a bucket of pond scum?
A: The bucket.
* * *
Q: What is the definition of a shame (as in "that's a shame")?
A: When a busload of lawyers goes off a cliff.
* * *
#71 Re: Exercises » Compute the solution: » 2025-09-05 13:35:16
Hi,
Well done!
2575.
#72 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-09-04 23:07:28
2233) Geoffrey Hinton
Gist:
Work
When we talk about artificial intelligence, we often mean machine learning using artificial neural networks. This technology was originally inspired by the structure of the brain. In an artificial neural network, the brain’s neurons are represented by nodes that have different values. In 1983–1985, Geoffrey Hinton used tools from statistical physics to create the Boltzmann machine, which can learn to recognise characteristic elements in a set of data. The invention became significant, for example, for classifying and creating images.
Summary
Geoffrey Hinton (born December 6, 1947, London, England) is a British-Canadian cognitive psychologist and computer scientist known as the “godfather of AI.” He revolutionized the field of artificial intelligence with his work on neural network models. He contributed significantly to AI research with novel insights and key discoveries in the areas of backpropagation, Boltzmann machines, distributed representations, and time-delay neural networks. Although he spent the majority of his career advancing AI, Hinton became an outspoken critic of the technology in 2023 and highlighted its potential harms. He was awarded the 2024 Nobel Prize in Physics and shared the prize with American physicist John J. Hopfield.
Hinton was born into a family with a rich intellectual history. His father, Howard Everest Hinton, was a distinguished entomologist, and all three of his siblings conducted scholarly work. His family includes multiple mathematicians, among them Mary Everest Boole and her husband, George Boole, whose algebra of logic (known as Boolean logic) became the basis for modern computing. Other notable relatives include Joan Hinton, one of the few women to work on the Manhattan Project; Charles Howard Hinton, the mathematician famous for visualizing higher dimensions; and George Everest, the surveyor Mount Everest is named for.
Hinton attended the University of Cambridge, where he switched his studies between physiology, philosophy, and physics before earning a degree in experimental psychology in 1970. He then attended the University of Edinburgh, where he received a Ph.D. in AI in 1978. Although discouraged by his professors, Hinton embraced unconventional computer networks modeled after neural nodes and the structure of the human brain. He began researching systems known as neural networks and completed postdoctoral research at the University of California, San Diego.
In 1982 Hinton joined the faculty of Carnegie Mellon University, where he worked with psychologist David Rumelhart and computer scientist Ronald J. Williams to develop an algorithm to work backward from output to input when measuring error. The process, called “backpropagation,” was discussed by the trio in 1986 in an influential paper that laid the groundwork for neural network development.
Hinton left the United States for Canada in 1987, a decision fueled by disdain for the U.S. military and the Reagan administration. The majority of American AI research at the time was funded by the U.S. Department of Defense, and Hinton opposed using AI for combat. He continued his research, this time as a professor at the University of Toronto, for the next 11 years. In 1998 Hinton left Toronto to found and direct the Gatsby Computational Neuroscience Unit at University College London. While a researcher there, he studied neural networks and their applications.
Hinton returned to the University of Toronto in 2001 and continued to make advances in neural network models. His research group developed and began to apply practical means for deep-learning technology in the 2000s. In 2012 Hinton and two of his graduate students, Alex Krizhevsky and Ilya Sutskever, developed an eight-layer neural network program, which they named AlexNet, to identify images on ImageNet, a massive online dataset of images. AlexNet outperformed the next most accurate program by more than 40 percent. The trio created a company, DDNresearch, for AlexNet. In 2013 Google acquired the company for $44 million. That same year Hinton joined Google Brain, the company’s AI research team, and he was eventually named a vice president and engineering fellow.
In May 2023 Hinton quit his job at Google, because he wanted to be able to speak freely about the risks of commercial AI use. He expressed concerns particularly about its power to create fake content and its potential to upend the job market. Hinton has stated that he does not fully regret his life’s work but fears that AI will become uncontrollable in the long run.
Hinton has received extensive recognition for his role in revolutionizing AI. Among his numerous awards are the Cognitive Science Society’s first-ever David E. Rumelhart Prize (2001) and the Gerhard Herzberg Canada Gold Medal (2010), the country’s highest award for science and engineering. In 2018 Hinton was named a joint recipient of the Turing Award, often described as the “Nobel Prize of Computing,” for his breakthrough research on neural networks, and four years later he received the Royal Society’s Royal Medal for his pioneering work on deep learning.
Details
Geoffrey Everest Hinton (born 6 December 1947) is a British-Canadian computer scientist, cognitive scientist, and cognitive psychologist known for his work on artificial neural networks, which earned him the title "the Godfather of AI".
Hinton is University Professor Emeritus at the University of Toronto. From 2013 to 2023, he divided his time working for Google (Google Brain) and the University of Toronto before publicly announcing his departure from Google in May 2023, citing concerns about the many risks of artificial intelligence (AI) technology. In 2017, he co-founded and became the chief scientific advisor of the Vector Institute in Toronto.
With David Rumelhart and Ronald J. Williams, Hinton was co-author of a highly cited paper published in 1986 that popularised the backpropagation algorithm for training multi-layer neural networks, although they were not the first to propose the approach. Hinton is viewed as a leading figure in the deep learning community. The image-recognition milestone of the AlexNet designed in collaboration with his students Alex Krizhevsky and Ilya Sutskever for the ImageNet challenge 2012 was a breakthrough in the field of computer vision.
Hinton received the 2018 Turing Award, together with Yoshua Bengio and Yann LeCun for their work on deep learning. They are sometimes referred to as the "Godfathers of Deep Learning" and have continued to give public talks together. He was also awarded, along with John Hopfield, the 2024 Nobel Prize in Physics for foundational discoveries and inventions that enable machine learning with artificial neural networks.
In May 2023, Hinton announced his resignation from Google to be able to "freely speak out about the risks of A.I." He has voiced concerns about deliberate misuse by malicious actors, technological unemployment, and existential risk from artificial general intelligence. He noted that establishing safety guidelines will require cooperation among those competing in use of AI in order to avoid the worst outcomes. After receiving the Nobel Prize, he called for urgent research into AI safety to figure out how to control AI systems smarter than humans.
Education
Hinton was born on 6 December 1947 in Wimbledon, England, and was educated at Clifton College in Bristol. In 1967, he enrolled as an undergraduate student at King's College, Cambridge, and after repeatedly switching between different fields, like natural sciences, history of art, and philosophy, he eventually graduated with a Bachelor of Arts degree in experimental psychology at the University of Cambridge in 1970. He spent a year apprenticing carpentry before returning to academic studies. From 1972 to 1975, he continued his study at the University of Edinburgh, where he was awarded a PhD in artificial intelligence in 1978 for research supervised by Christopher Longuet-Higgins, who favored the symbolic AI approach over the neural network approach.
Career and research
After his PhD, Hinton initially worked at the University of Sussex and at the MRC Applied Psychology Unit. After having difficulty getting funding in Britain, he worked in the US at the University of California, San Diego and Carnegie Mellon University.[38] He was the founding director of the Gatsby Charitable Foundation Computational Neuroscience Unit at University College London. He is currently University Professor Emeritus in the Department of Computer Science at the University of Toronto, where he has been affiliated since 1987.
Upon arrival in Canada, Geoffrey Hinton was appointed at the Canadian Institute for Advanced Research (CIFAR) in 1987 as a Fellow in CIFAR's first research program, Artificial Intelligence, Robotics & Society. In 2004, Hinton and collaborators successfully proposed the launch of a new program at CIFAR, "Neural Computation and Adaptive Perception" (NCAP), which today is named "Learning in Machines & Brains". Hinton would go on to lead NCAP for ten years. Among the members of the program are Yoshua Bengio and Yann LeCun, with whom Hinton would go on to win the ACM A.M. Turing Award in 2018. All three Turing winners continue to be members of the CIFAR Learning in Machines & Brains program.
Hinton taught a free online course on Neural Networks on the education platform Coursera in 2012. He co-founded DNNresearch Inc. in 2012 with his two graduate students Alex Krizhevsky and Ilya Sutskever at the University of Toronto’s department of computer science. In March 2013, Google acquired DNNresearch Inc. for $44 million, and Hinton planned to "divide his time between his university research and his work at Google".
Hinton's research concerns ways of using neural networks for machine learning, memory, perception, and symbol processing. He has written or co-written more than 200 peer-reviewed publications.
While Hinton was a postdoc at UC San Diego, David E. Rumelhart and Hinton and Ronald J. Williams applied the backpropagation algorithm to multi-layer neural networks. Their experiments showed that such networks can learn useful internal representations of data. In a 2018 interview, Hinton said that "David E. Rumelhart came up with the basic idea of backpropagation, so it's his invention". Although this work was important in popularising backpropagation, it was not the first to suggest the approach. Reverse-mode automatic differentiation, of which backpropagation is a special case, was proposed by Seppo Linnainmaa in 1970, and Paul Werbos proposed to use it to train neural networks in 1974.
In 1985, Hinton co-invented Boltzmann machines with David Ackley and Terry Sejnowski. His other contributions to neural network research include distributed representations, time delay neural network, mixtures of experts, Helmholtz machines and product of experts. An accessible introduction to Geoffrey Hinton's research can be found in his articles in Scientific American in September 1992 and October 1993. In 2007, Hinton coauthored an unsupervised learning paper titled Unsupervised learning of image transformations. In 2008, he developed the visualization method t-SNE with Laurens van der Maaten.
In October and November 2017, Hinton published two open access research papers on the theme of capsule neural networks, which, according to Hinton, are "finally something that works well".
At the 2022 Conference on Neural Information Processing Systems (NeurIPS), Hinton introduced a new learning algorithm for neural networks that he calls the "Forward-Forward" algorithm. The idea of the new algorithm is to replace the traditional forward-backward passes of backpropagation with two forward passes, one with positive (i.e. real) data and the other with negative data that could be generated solely by the network.
In May 2023, Hinton publicly announced his resignation from Google. He explained his decision by saying that he wanted to "freely speak out about the risks of A.I." and added that a part of him now regrets his life's work.
Notable former PhD students and postdoctoral researchers from his group include Peter Dayan, Sam Roweis, Max Welling, Richard Zemel, Brendan Frey, Radford M. Neal, Yee Whye Teh, Ruslan Salakhutdinov, Ilya Sutskever, Yann LeCun, Alex Graves, Zoubin Ghahramani, and Peter Fitzhugh Brown.

#73 This is Cool » Sodium Chloride » 2025-09-04 20:59:00
- Jai Ganesh
- Replies: 0
Sodium Chloride
Gist
Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the primary salt in seawater and in the extracellular fluid of many multicellular organisms.
Sodium chloride is used as an electrolyte replenisher to help prevent heat cramps caused by too much sweating. This medicine is also used for the preparation of normal isotonic solution of sodium chloride. This medicine is available without prescription.
Summary
Sodium chloride is an essential nutrient and is used in healthcare to help prevent patients from becoming dehydrated.
It is used as a food preservative and as a seasoning to enhance flavor.
Sodium chloride is also used in manufacturing to make plastics and other products, and it is used to de-ice roads and sidewalks.
Salt is regulated by the FDA as a “generally recognized as safe” (GRAS) ingredient. A GRAS substance is one that has a long history of safe, common use in foods, or that is determined to be safe, for the intended use, based on proven science.
Uses & Benefits
Sodium chloride is an essential nutrient and is used in healthcare to help prevent patients from becoming dehydrated. It is used as a food preservative and as a seasoning to enhance flavor. Sodium chloride is also used in manufacturing to make plastics and other products. It is also used to de-ice roads and sidewalks.
Details
Salt (NaCl), is a mineral substance of great importance to human and animal health, as well as to industry. The mineral form halite, or rock salt, is sometimes called common salt to distinguish it from a class of chemical compounds called salts.
Salt is essential to the health of both people and animals. Table salt, used universally as a seasoning, is fine-grained and of high purity. To ensure that this hygroscopic (i.e., water-attracting) substance will remain free-flowing when exposed to the atmosphere, small quantities of sodium aluminosilicate, tricalcium phosphate, or magnesium silicate are added. Iodized salt—that is, salt to which small quantities of potassium iodide have been added—is widely used in areas where iodine is lacking from the diet, a deficiency that can cause swelling of the thyroid gland, commonly called goitre. Livestock also require salt; it is often made available in solid blocks.
The meat-packing, sausage-making, fish-curing, and food-processing industries use salt as a preservative or seasoning or both. It is employed for curing and preserving hides and as a brine for refrigeration.
In the chemical industry, salt is required in the manufacture of sodium bicarbonate (baking soda), sodium hydroxide (caustic soda), hydrochloric acid, chlorine, and many other chemicals. Salt is also employed in soap, glaze, and porcelain enamel manufacture and enters into metallurgical processes as a flux (a substance promoting fusing of metals).
When applied to snow or ice, salt lowers the melting point of the mixture. Thus, large amounts are used in northern climates to help rid thoroughfares of accumulated snow and ice. Salt is used in water-softening equipment that removes calcium and magnesium compounds from water.
History of use
In some parts of the Western Hemisphere and in India, the use of salt was introduced by Europeans, but in parts of central Africa it is still a luxury available only to the rich. Where people live mainly on milk and raw or roasted meat (so that its natural salts are not lost), sodium chloride supplements are unnecessary; nomads with their flocks of sheep or herds of cattle, for example, never eat salt with their food. On the other hand, people who live mostly on cereal, vegetable, or boiled meat diets require supplements of salt.
The habitual use of salt is intimately connected with the advance from nomadic to agricultural life, a step in civilization that profoundly influenced the rituals and cults of almost all ancient nations. The gods were worshipped as the givers of the kindly fruits of the earth, and salt was usually included in sacrificial offerings consisting wholly or partly of cereal elements. Such offerings were prevalent among the Greeks and Romans and among a number of the Semitic peoples.
Covenants were ordinarily made over a sacrificial meal, in which salt was a necessary element. The preservative qualities of salt made it a peculiarly fitting symbol of an enduring compact, sealing it with an obligation to fidelity. The word salt thus acquired connotations of high esteem and honour in ancient and modern languages. Examples include the Arab avowal “There is salt between us,” the Hebrew expression “to eat the salt of the palace,” and the modern Persian phrase namak ḥarām, “untrue to salt” (i.e., disloyal or ungrateful). In English the term “salt of the earth” describes a person held in high esteem.
Salt contributes greatly to our knowledge of the ancient highways of commerce. One of the oldest roads in Italy is the Via Salaria (Salt Route) over which Roman salt from Ostia was carried into other parts of Italy. Herodotus tells of a caravan route that united the salt oases of the Libyan Desert. The ancient trade between the Aegean and the Black Sea coast of southern Russia was largely dependent on the salt pans (ponds for evaporating seawater to obtain salt) at the mouth of the Dnieper River and on the salt fish brought from this district.
China, the United States, India, Germany, Canada, and Australia are the world’s largest salt producers in the early 21st century.
Additional Information
Sodium chloride, commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite. In its edible form, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.
Uses
In addition to the many familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 data) include chemicals and de-icing.
Chemical functions
Salt is used, directly or indirectly, in the production of many chemicals, which consumes most of the world's production.
Chlor-alkali industry
This electrolysis is conducted in either a mercury cell, a diaphragm cell, or a membrane cell. Each of those uses a different method to separate the chlorine from the sodium hydroxide. Other technologies are under development due to the high energy consumption of the electrolysis, whereby small improvements in the efficiency can have large economic paybacks. Some applications of chlorine include PVC thermoplastics production, disinfectants, and solvents.
Sodium hydroxide is extensively used in many different industries enabling production of paper, soap, aluminum, and more.
Soda-ash industry
Sodium chloride is used in the Solvay process to produce sodium carbonate and calcium chloride. Sodium carbonate, in turn, is used to produce glass, sodium bicarbonate, and dyes, as well as a myriad of other chemicals. In the Mannheim process, sodium chloride is used for the production of sodium sulfate and hydrochloric acid.
Miscellaneous industrial uses
Sodium chloride is heavily used, so even relatively minor applications can consume massive quantities. In oil and gas exploration, salt is an important component of drilling fluids in well drilling. It is used to flocculate and increase the density of the drilling fluid to overcome high downwell gas pressures. Whenever a drill hits a salt formation, salt is added to the drilling fluid to saturate the solution in order to minimize the dissolution within the salt stratum. Salt is also used to increase the curing of concrete in cemented casings.
In textiles and dyeing, salt is used as a brine rinse to separate organic contaminants, to promote "salting out" of dyestuff precipitates, and to blend with concentrated dyes to increase yield in dyebaths and make the colors look sharper. One of its main roles is to provide the positive ion charge to promote the absorption of negatively charged ions of dyes.
For use in the pulp and paper industry, it is used to manufacture sodium chlorate, which is then reacted with sulfuric acid and a reducing agent such as methanol to manufacture chlorine dioxide, a bleaching chemical that is widely used to bleach wood pulp.
In tanning and leather treatment, salt is added to animal hides to inhibit microbial activity on the underside of the hides and to attract moisture back into the hides.
In rubber manufacture, salt is used to make buna, neoprene, and white rubber types. Salt brine and sulfuric acid are used to coagulate an emulsified latex made from chlorinated butadiene.
Salt also is added to secure the soil and to provide firmness to the foundation on which highways are built. The salt acts to minimize the effects of shifting caused in the subsurface by changes in humidity and traffic load.

#74 Dark Discussions at Cafe Infinity » Clock Quotes - I » 2025-09-04 18:17:40
- Jai Ganesh
- Replies: 1
Clock Quotes - I
1. Quiet minds cannot be perplexed or frightened but go on in fortune or misfortune at their own private pace, like a clock during a thunderstorm. - Robert Louis Stevenson
2. A person who has not done one half his day's work by ten o clock, runs a chance of leaving the other half undone. - Emily Bronte
3. When times are tough, constant conflict may be good politics but in the real world, cooperation works better. After all, nobody's right all the time, and a broken clock is right twice a day. - William J. Clinton
4. It's a wise man who understands that every day is a new beginning, because boy, how many mistakes do you make in a day? I don't know about you, but I make plenty. You can't turn the clock back, so you have to look ahead. - Mel Gibson
5. I must govern the clock, not be governed by it. - Golda Meir
6. The hours of folly are measured by the clock; but of wisdom, no clock can measure. - William Blake
7. Consider a clock thermostat, and set it so that you're not using energy when you don't need it, when you're out of your house. - Al Gore
8. I don't have an alarm clock. If someone needs to wake me up, then I have my BlackBerry next to me. - Mark Zuckerberg.
#75 Jokes » Lawyer Jokes - XI » 2025-09-04 17:35:45
- Jai Ganesh
- Replies: 0
Q: How many lawyers does it take to screw in a light bulb?
A: One; the lawyer holds it while the rest of the world revolves around him.
* * *
Q: What happened to the banker who went to law school?
A: Now she’s a loan shark.
* * *
Q: Where do vampires learn to drag blood?
A: Law school.
* * *
Q: How do you prevent a Lawyer from drowning?
A: Shoot him before he hits the water!
* * *
Q: How was wire invented?
A: Two lawyers pulling on a penny.
* * *