Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#276 Re: Exercises » Compute the solution: » 2025-10-28 13:30:54
Hi,
2630.
#277 This is Cool » Unicellular Organisms » 2025-10-27 22:13:36
- Jai Ganesh
- Replies: 0
Unicellular Organisms
Gist
In biology, the adjective unicellular describes an organism that has only one single cell, like most kinds of bacteria. You're most likely to see the word unicellular in a biology textbook, where it is used to talk about microscopic, single-celled organisms.
Many organisms are unicellular, including bacteria, archaea, protozoa like amoeba and paramecium, and some fungi like yeast. These single-celled organisms perform all necessary life functions within that one cell and are often microscopic, though some can be large enough to see with the naked eye, such as Valonia ventricosa.
Summary
Unicellular organisms are organisms consisting of one cell only that performs all vital functions including metabolism, excretion, and reproduction. Unicellular organisms can either be prokaryotes or eukaryotes. Examples of unicellular organisms are bacteria, archaea, unicellular fungi, and unicellular protists. Even though unicellular organisms are not seen by the naked eye, they have an indispensable role in the environment, industry, and medicine. Some of them may also be infectious or pathogenic to humans, animals, and plants.
Unicellular Definition
What is a unicellular organism? In contrast to multicellular organisms, single-celled organisms — or unicellular organisms — are groups of different living organisms consisting of one cell only. And that cell performs all vital functions, such as homeostasis, metabolism, and reproduction. Moreover, a single cell must be able to obtain and use energy, get rid of wastes, and transport materials. In contrast, multicellular organisms are made up of multiple cells and these cells have specific roles and may function together as a unit (tissue).
The cell of a unicellular organism has a protoplasm that contains various proteins, lipids, carbohydrates, and nucleic acids. The protoplasm is surrounded by a cell membrane that separates the internal components of the cell from the external environment. However, any cell should be able to interact with its external environment to obtain molecules from the outside and expel wastes to the outside.
Are bacteria unicellular? Yes! In fact, not only bacteria are unicellular but also archaea. Both bacteria and archaea are prokaryotic organisms. Unicellularity, though, is not exclusive to prokaryotes. Some eukaryotes live singly as well. Examples of single-celled eukaryotes are the unicellular algae, unicellular fungi, and protozoa.
Most living things composed of only one cell are microscopic and cannot be seen by the naked eyes. Unicellular organisms abound in nature. Even extreme habitats contain unicellular organisms. Some archaea, for instance, can survive in extreme environments, and so they are called extremophiles. They are typically resistant to extreme conditions such as temperature or pH.
Details
A unicellular organism, also known as a single-celled organism, is an organism that consists of a single cell, unlike a multicellular organism that consists of multiple cells. Organisms fall into two general categories: prokaryotic organisms and eukaryotic organisms. Most prokaryotes are unicellular and are classified into bacteria and archaea. Many eukaryotes are multicellular, but some are unicellular such as protozoa, unicellular algae, and unicellular fungi. Unicellular organisms are thought to be the oldest form of life, with early organisms emerging 3.5–3.8 billion years ago.
Although some prokaryotes live in colonies, they are not specialised cells with differing functions. These organisms live together, and each cell must carry out all life processes to survive. In contrast, even the simplest multicellular organisms have cells that depend on each other to survive.
Most multicellular organisms have a unicellular life-cycle stage. Gametes, for example, are reproductive unicells for multicellular organisms.
Some organisms are partially unicellular, like Dictyostelium discoideum. Additionally, unicellular organisms can be multinucleate, like Caulerpa, Plasmodium, and Myxogastria.
Evolutionary hypothesis
The origin of life is largely still a mystery. Primitive protocells are thought to be the precursors to today's unicellular organisms.
In one theory, known as the RNA world hypothesis, early RNA molecules would have been the basis for catalyzing organic chemical reactions and self-replication.
Compartmentalization was necessary for chemical reactions to be more likely as well as to differentiate reactions with the external environment. For example, an early RNA replicator ribozyme may have replicated other replicator ribozymes of different RNA sequences if not kept separate. Such hypothetic cells with an RNA genome instead of the usual DNA genome are called 'ribocells' or 'ribocytes'.
When amphiphiles like lipids are placed in water, the hydrophobic tails aggregate to form micelles and vesicles, with the hydrophilic ends facing outwards. Primitive cells likely used self-assembling fatty-acid vesicles to separate chemical reactions and the environment. Because of their simplicity and ability to self-assemble in water, it is likely that these simple membranes predated other forms of early biological molecules.
Additional Information
A unicellular organism is a living thing that is just one cell. There are different types of unicellular organism, including:
* Unicellular fungi
* Protozoa
* Bacteria
These organisms have adaptations that make them well suited for life in their environment.
Unicellular fungi
Yeast are unicellular fungi. They are used by brewers and wine-makers because they convert sugar into alcohol, and by bakers because they can produce carbon dioxide to make bread to rise. Fungi can also form into mushrooms and toadstools.
Protozoa
Protozoa are unicellular organisms that live in water or in damp places, for example, the amoeba.
Bacteria
Even though a bacterium is just one cell, it can carry out all seven life processes - movement, respiration, sensitivity, growth, reproduction, excretion and nutrition.
#278 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-27 17:25:15
2376) Luis Walter Alvarez
Gist:
Work
Opportunities to investigate our world’s smallest components were revolutionized by C.T.R. Wilson’s invention of the cloud chamber and Donald Glaser’s invention of the bubble chamber. In these devices electrically charged particles leave trails behind them. In the latter part of the 1950s, Luis Alvarez further developed the bubble chamber by using liquid hydrogen. He also developed new measurement systems and computer-based methods for analyzing large quantities of data. This has led to the discovery of a number of previously unknown particles.
Summary
Luis Walter Alvarez (June 13, 1911 – September 1, 1988) was an American experimental physicist, inventor, and professor of Spanish descent who was awarded the Nobel Prize in Physics in 1968 for his discovery of resonance states in particle physics using the hydrogen bubble chamber. In 2007 the American Journal of Physics commented, "Luis Alvarez was one of the most brilliant and productive experimental physicists of the twentieth century."
After receiving his PhD from the University of Chicago in 1936, Alvarez went to work for Ernest Lawrence at the Radiation Laboratory at the University of California, Berkeley. Alvarez devised a set of experiments to observe K-electron capture in radioactive nuclei, predicted by the beta decay theory but never before observed. He produced tritium using the cyclotron and measured its lifetime. In collaboration with Felix Bloch, he measured the magnetic moment of the neutron.
In 1940, Alvarez joined the MIT Radiation Laboratory, where he contributed to a number of World War II radar projects, from early improvements to Identification friend or foe (IFF) radar beacons, now called transponders, to a system known as VIXEN for preventing enemy submarines from realizing that they had been found by the new airborne microwave radars. The radar system for which Alvarez is best known and which has played a major role in aviation, most particularly in the post war Berlin airlift, was Ground Controlled Approach (GCA). Alvarez spent a few months at the University of Chicago working on nuclear reactors for Enrico Fermi before coming to Los Alamos to work for Robert Oppenheimer on the Manhattan Project. Alvarez worked on the design of explosive lenses, and the development of exploding-bridgewire detonators. As a member of Project Alberta, he observed the Trinity nuclear test from a B-29 Superfortress, and later the bombing of Hiroshima from the B-29 The Great Artiste.
After the war Alvarez was involved in the design of a liquid hydrogen bubble chamber that allowed his team to take millions of photographs of particle interactions, develop complex computer systems to measure and analyze these interactions, and discover entire families of new particles and resonance states. This work resulted in his being awarded the Nobel Prize in 1968. He was involved in a project to x-ray the Egyptian pyramids to search for unknown chambers. With his son, geologist Walter Alvarez, he developed the Alvarez hypothesis which proposes that the extinction event that wiped out the non-avian dinosaurs was the result of an asteroid impact.
Details
Luis Alvarez (born June 13, 1911, San Francisco, California, U.S.—died September 1, 1988, Berkeley, California) was an American experimental physicist who was awarded the Nobel Prize for Physics in 1968 for work that included the discovery of many resonance particles (subatomic particles having extremely short lifetimes and occurring only in high-energy nuclear collisions).
Alvarez studied physics at the University of Chicago (B.S., 1932; M.S., 1934; Ph.D., 1936). He joined the faculty of the University of California, Berkeley, in 1936, becoming professor of physics in 1945 and professor emeritus in 1978. In 1938 Alvarez discovered that some radioactive elements decay by orbital-electron capture; i.e., an orbital electron merges with its nucleus, producing an element with an atomic number smaller by one. In 1939 he and Felix Bloch made the first measurement of the magnetic moment of the neutron, a characteristic of the strength and direction of its magnetic field.
Alvarez, Luis
Alvarez, LuisLuis Alvarez (far left) and visiting scientists examining the bubble chamber at the Lawrence Berkeley National Laboratory, California, 1959.
Alvarez worked on microwave radar research at the Massachusetts Institute of Technology, Cambridge (1940–43), and participated in the development of the atomic bomb at the Los Alamos Scientific Laboratory, Los Alamos, New Mexico, in 1944–45. He suggested the technique for detonating the implosion type of atomic bomb. He also participated in the development of microwave beacons, linear radar antennas, the ground-controlled landing approach system, and a method for aerial bombing using radar to locate targets. After World War II Alvarez helped construct the first proton linear accelerator. In this accelerator, electric fields are set up as standing waves within a cylindrical metal “resonant cavity,” with drift tubes suspended along the central axis. The electric field is zero inside the drift tubes, and, if their lengths are properly chosen, the protons cross the gap between adjacent drift tubes when the direction of the field produces acceleration and are shielded by the drift tubes when the field in the tank would decelerate them. The lengths of the drift tubes are proportional to the speeds of the particles that pass through them. In addition to this work, Alvarez also developed the liquid hydrogen bubble chamber in which subatomic particles and their reactions are detected.
In about 1980 Alvarez helped his son, the geologist Walter Alvarez, publicize Walter’s discovery of a worldwide layer of clay that has a high iridium content and which occupies rock strata at the geochronological boundary between the Mesozoic and Cenozoic eras (i.e., about 65.5 million years ago). They postulated that the iridium had been deposited following the impact on Earth of an asteroid or comet and that the catastrophic climatic effects of this massive impact triggered a mass extinction event, killing the dinosaurs. Though initially controversial, this widely publicized theory gradually gained support as the most plausible explanation for the abrupt demise of dinosaurs.
Alvarez’s autobiography, Alvarez: Adventures of a Physicist, was published in 1987.

#279 Re: This is Cool » Miscellany » 2025-10-27 17:08:41
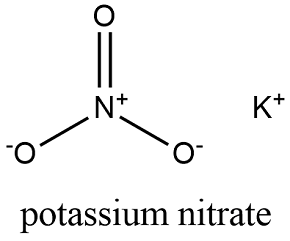
2428) Potassium Nitrate
Gist
Potassium nitrate (KNO3) is a chemical compound, also known as saltpeter or nitre, with applications in agriculture, industry, and medicine. It is an ionic salt of potassium and nitrate ions, appearing as a white crystalline powder that is soluble in water. Key properties include a density of approximately 2.11 g/{cm}^{3}, a melting point of 334 degrees Centigrade, and a boiling point of 400 degrees Centigrade before it decomposes.
Potassium nitrate (KNO3) has a wide range of uses, including as a component in fertilizers for plant growth, a food preservative, and in the production of fireworks and explosives. It is also used in specialty toothpastes to treat sensitive teeth, to remove tree stumps by accelerating decomposition, and in some industrial processes like solar power storage and glass manufacturing.
Summary
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula KNO3. It is a potassium salt of nitric acid. This salt consists of potassium cations K+ and nitrate anions NO(−3), and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or nitre outside the United States). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the United States).
Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color.
Details
Potassium nitrate (KNO3) is an ionic white crystalline salt made up of potassium ions and nitrate ions. Uses of potassium nitrate include the manufacture of fertilizers, pesticides, glass, fireworks, explosives, and rocket fuels. It is also used as a food preservative, and when added to meat it causes a reaction between the myoglobin and hemoglobin in the blood, making the meat appear red in colour. It is also used as an additive in some toothpastes to help with tooth sensitivity. Potassium nitrate is toxic to humans in high levels, so its use is carefully controlled when human consumption is involved.
It is found in impure form, often called saltpetre (also called nitre), its name derived from the Latin words sal patrae, meaning “salt of the rock,” as it is often found as a white material deposited on the surface of rocks. Saltpetre can form on the surface of soil in various warm-climate locations, including in Egypt, Spain, and Iran. In such places, feces, urine, and decaying plants react with moisture and an alkaline soil to create nitrates. These nitrates dissolve in rainwater, and white deposits of potassium nitrate are left behind when this water evaporates. Many caves throughout the world have large deposits of saltpetre due to large amounts of bat guano and urine found there.
History and production
In the 9th century Chinese chemists discovered that a mixture of potassium nitrate, sulfur, and charcoal would spontaneously produce smoke and flames. By the 11th century the Chinese were incorporating the mixture, gunpowder, into smoke bombs to help fight off enemies, and in the 13th and 14th centuries they used this explosive power to propel objects at their foes with guns.
Historically in the United States, in the early to mid-19th century, caves in Kentucky, Tennessee, and West Virginia were extensively mined for saltpetre that was used to manufacture gunpowder. As more applications of potassium nitrate were discovered, the demand for the chemical compound increased. The increased demand shifted its production from the caves, where only a finite amount of the chemical could be manufactured, to industrial labs, with a much higher ability for production. The most common method of industrial production uses potassium chloride in a double displacement reaction with nitric acid.
Chemical properties
Potassium nitrate has a molar mass of 101.10 grams per mole. It has a boiling point of 400 °C (752 °F), a melting point of 334 °C (633 °F), and a density of 2.11 grams per cubic centimetre at 25 °C (70 °F). It is soluble in water at 38.3 grams per 100 millilitres at 25 °C but is only slightly soluble in most alcohols. It is insoluble in ethanol.
While not itself combustible, potassium nitrate accelerates the burning of combustible materials. Potassium nitrate is a strong oxidizer and when heated decomposes to potassium nitrite and oxygen, which helps the combustion process in explosives. For this reason, potassium nitrate is referred to as an oxidizing agent. It has multiple uses including in the manufacture of fertilizers, medicine, gunpowder, fireworks, and explosives.
Modern industrial production
In the United States more than 200,000 tonnes of potassium nitrate are manufactured annually, almost 90 percent of the production being used in fertilizers. The remaining 10 percent is used in processes such as the manufacture of matches and fireworks and of glass and ceramics. Annual worldwide production is in excess of 30 million tonnes, Russia leading the way in production at just over one-half of this annual total.
Additional Information
Potassium nitrate is a manufactured fertilizer for supplying nitrogen and potassium. It is made from potassium chloride and a source of nitrate, such as sodium nitrate, ammonium nitrate, or nitric acid. Potassium nitrate is sold as a water-soluble, crystalline material for hydroponics and in a prilled form for soil application. Sales of potassium nitrate account for only a small portion of the global potassium fertilizer market as a fertilizer for special uses.
#280 Dark Discussions at Cafe Infinity » Coaching Quotes - I » 2025-10-27 16:04:26
- Jai Ganesh
- Replies: 0
Coaching Quotes - I
1. Coaching is a very different skill. You need patience, you need a lot of organisation. I don't have any. - Wasim Akram
2. I coach for my dad's academy. Sometimes it's just about being there - it's not just the coaching - it's seeing that you are there to inspire or they are trying to impress you. - Moeen Ali
3. Well my thoughts on American swimming are that our prospects look favorable, but we may not have as strong a showing in the gold medal count as in previous Olympics. But I am not coaching. - Mark Spitz
4. One must understand that shooting is a very individual sport and see what sort of coaching possibilities exist in the country and what their standards are. One of the issues that has been faced earlier by shooting athletes is that we have one odd national coach for whom it's impossible to give that sort of attention to say, a group of 30. - Abhinav Bindra
5. It's important, according to me, to train in small doses so as to not lose the joy of playing chess. I personally think too many coaching and training classes may take away a child's interest in the game itself. The essential thing to do is practise often and, in case of a doubt, to consult a trainer. - Viswanathan Anand
6. We are not coaching on a daily basis because we often travel with our charity and commercial interests. - Nadia Comaneci
7. Geez, I just played cricket because I loved the game. I never thought about it much, never really had any formal coaching. - Steve Waugh
8. Obviously I was disappointed when it fell into disuse, because it was my own track named after me, but I am sure all those youngsters we lost will be coming back, and I certainly intend to be down here as much as I can, coaching and advising. - Linford Christie.
#281 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-27 15:44:33
Hi,
#10633. What does the term in Geography Chaparral mean?
#10634. What does the term in Geography Nautical chart mean?
#282 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-27 15:18:04
Hi,
#5829. What does the adjective hawkish mean?
#5830. What does the adjective hazy mean?
#283 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-27 14:33:37
Hi,
#2508. What does the medical term Idiopathic pulmonary fibrosis mean?
#284 Jokes » Banana Jokes - IV » 2025-10-27 14:20:18
- Jai Ganesh
- Replies: 0
Q: What's worst than a monkey eating bananas?
A: A monkey going bananas.
* * *
Q: What's yellow and always points to the north?
A: A magnetic banana.
* * *
Q: What is yellow and goes bzzzzzz?
A: An electric banana.
* * *
Q: How do monkeys get down the stairs?
A: They slide down the banana-ster!
* * *
Q: What did the banana say to the monkey?
A: Nothing, bananas can't talk!
* * *
#285 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-27 14:06:00
Hi,
#9777.
#286 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-27 13:58:02
Hi,
#6282.
#287 Re: Exercises » Compute the solution: » 2025-10-27 13:37:55
Hi,
2629.
#288 This is Cool » Sodium Hydroxide » 2025-10-26 22:52:58
- Jai Ganesh
- Replies: 0
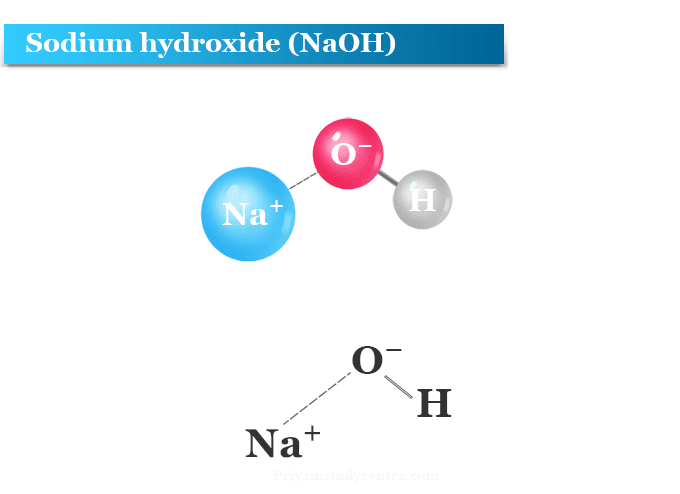
Sodium Hydroxide
Gist
Sodium hydroxide (NaOH), also known as lye or caustic soda, is a strong, white, and corrosive ionic compound used in making soap, detergents, and as a drain cleaner. It is a highly reactive and deliquescent solid that absorbs moisture and carbon dioxide from the air and generates significant heat when dissolved in water. Sodium hydroxide is also used in various industries, such as food processing and the paper industry.
Sodium hydroxide is a versatile chemical with widespread uses in manufacturing soaps, detergents, paper, and rayon. It's also used in petroleum refining, food processing (like peeling fruits and vegetables), water treatment, and as a powerful drain cleaner because it breaks down fats and grease. Industrially, it plays a key role in processes like aluminum extraction, metal cleaning, and producing other chemicals.
Summary
Sodium hydroxide (NaOH) is a corrosive white crystalline solid that contains the Na+ (sodium) cation and the OH− (hydroxide) anion. It readily absorbs moisture until it dissolves. Sodium hydroxide is the most widely used industrial alkali and is often used in drain and oven cleaners. It is highly corrosive to animal and vegetable tissue. The alkaline solutions it forms when dissolved in water neutralize acids in various commercial processes. In petroleum refining, it removes sulfuric and organic acids. In soapmaking, it acts on natural fats or oils, such as tallow or vegetable oil, to produce sodium fatty acid salt (soap) and glycerin (or glycerol); this saponification reaction is the basis for all soapmaking. In papermaking, sodium hydroxide is used to break down wood into pulp. Solutions of NaOH are used in the treatment of cellulose and in the manufacture of many chemicals.
Details
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.
Sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins at ambient temperatures, and may cause severe chemical burns at high concentrations. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates NaOH·nH2O. The monohydrate NaOH·H2O crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound.
As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.
Sodium hydroxide is used in many industries: in the making of wood pulp and paper, textiles, drinking water, soaps and detergents, and as a drain cleaner. Worldwide production in 2022 was approximately 83 million tons.[15]
Properties:
Physical properties
Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °C (604 °F) without decomposition and boils at 1,388 °C (2,530 °F). It is highly soluble in water, with a lower solubility in polar solvents such as ethanol and methanol. Sodium hydroxide is insoluble in ether and other non-polar solvents.
Similar to the hydration of sulfuric acid, dissolution of solid sodium hydroxide in water is a highly exothermic reaction where a large amount of heat is liberated, posing a threat to safety through the possibility of splashing. The resulting solution is usually colorless and odorless. As with other alkaline solutions, it feels slippery with skin contact due to the process of saponification that occurs between NaOH and natural skin oils.
Viscosity
Concentrated (50%) aqueous solutions of sodium hydroxide have a characteristic viscosity, 78 mPa·s, that is much greater than that of water (1.0 mPa·s) and near that of olive oil (85 mPa·s) at room temperature. The viscosity of aqueous NaOH, as with any liquid chemical, is inversely related to its temperature, i.e., its viscosity decreases as temperature increases, and vice versa. The viscosity of sodium hydroxide solutions plays a direct role in its application as well as its storage.
Hydrates
Sodium hydroxide can form several hydrates NaOH·nH2O, which result in a complex solubility diagram that was described in detail by Spencer Umfreville Pickering in 1893. The known hydrates and the approximate ranges of temperature and concentration (mass percent of NaOH) of their saturated water solutions are:
* Heptahydrate, NaOH·7H2O: from −28 °C (18.8%) to −24 °C (22.2%).
* Pentahydrate, NaOH·5H2O: from −24 °C (22.2%) to −17.7 °C (24.8%).
* Tetrahydrate, NaOH·4H2O, α form: from −17.7 °C (24.8%) to 5.4 °C (32.5%).
* Tetrahydrate, NaOH·4H2O, β form: metastable.
* Trihemihydrate, NaOH·3.5H2O: from 5.4 °C (32.5%) to 15.38 °C (38.8%) and then to 5.0 °C (45.7%).
* Trihydrate, NaOH·3H2O: metastable.
* Dihydrate, NaOH·2H2O: from 5.0 °C (45.7%) to 12.3 °C (51%).
* Monohydrate, NaOH·H2O: from 12.3 °C (51%) to 65.10 °C (69%) then to 62.63 °C (73.1%).
Early reports refer to hydrates with n = 0.5 or n = 2/3, but later careful investigations failed to confirm their existence.
The only hydrates with stable melting points are NaOH·H2O (65.10 °C) and NaOH·3.5H2O (15.38 °C). The other hydrates, except the metastable ones NaOH·3H2O and NaOH·4H2O (β) can be crystallized from solutions of the proper composition, as listed above. However, solutions of NaOH can be easily supercooled by many degrees, which allows the formation of hydrates (including the metastable ones) from solutions with different concentrations.
For example, when a solution of NaOH and water with 1:2 mole ratio (52.6% NaOH by mass) is cooled, the monohydrate normally starts to crystallize (at about 22 °C) before the dihydrate. However, the solution can easily be supercooled down to −15 °C, at which point it may quickly crystallize as the dihydrate. When heated, the solid dihydrate might melt directly into a solution at 13.35 °C; however, once the temperature exceeds 12.58 °C it often decomposes into solid monohydrate and a liquid solution. Even the n = 3.5 hydrate is difficult to crystallize, because the solution supercools so much that other hydrates become more stable.
A hot water solution containing 73.1% (mass) of NaOH is a eutectic that solidifies at about 62.63 °C as an intimate mix of anhydrous and monohydrate crystals.
A second stable eutectic composition is 45.4% (mass) of NaOH, that solidifies at about 4.9 °C into a mixture of crystals of the dihydrate and of the 3.5-hydrate.
The third stable eutectic has 18.4% (mass) of NaOH. It solidifies at about −28.7 °C as a mixture of water ice and the heptahydrate NaOH·7H2O.
When solutions with less than 18.4% NaOH are cooled, water ice crystallizes first, leaving the NaOH in solution.
The α form of the tetrahydrate has density 1.33 g/{cm}^3. It melts congruously at 7.55 °C into a liquid with 35.7% NaOH and density 1.392 g/{cm}^3, and therefore floats on it like ice on water. However, at about 4.9 °C it may instead melt incongruously into a mixture of solid NaOH·3.5H2O and a liquid solution.
The β form of the tetrahydrate is metastable, and often transforms spontaneously to the α form when cooled below −20 °C. Once initiated, the exothermic transformation is complete in a few minutes, with a 6.5% increase in volume of the solid. The β form can be crystallized from supercooled solutions at −26 °C, and melts partially at −1.83 °C.
The "sodium hydroxide" of commerce is often the monohydrate (density 1.829 g/cm3). Physical data in technical literature may refer to this form, rather than the anhydrous compound.
Crystal structure
NaOH and its monohydrate form orthorhombic crystals with the space groups Cmcm (oS8) and Pbca (oP24), respectively. The monohydrate cell dimensions are a = 1.1825, b = 0.6213, c = 0.6069 nm. The atoms are arranged in a hydrargillite-like layer structure, with each sodium atom surrounded by six oxygen atoms, three each from hydroxide ions and three from water molecules. The hydrogen atoms of the hydroxyls form strong bonds with oxygen atoms within each O layer. Adjacent O layers are held together by hydrogen bonds between water molecules
Additional Information
Caustic soda is the chemical compound sodium hydroxide (NaOH). This compound is an alkali – a type of base that can neutralize acids and is soluble in water. Today caustic soda can be manufactured in the form of pellets, flakes, powders, solutions and more.
What is caustic soda used for?
Caustic soda has become a common ingredient in the production of many everyday items. Commonly known as lye, it has been used to make soap for centuries, and its ability to dissolve grease makes it a common ingredient in oven cleaners and products used to unclog drains.
Caustic soda is often used to manufacture cleaning products like soaps and detergents.
Sodium hydroxide also plays a key role in processing wood pulp to create paper and cardboard boxes, which have become increasingly essential over the course of the global COVID-19 pandemic as medical supplies are shipped long distances.
The chemical compound is also used to break down the sedimentary rock that aluminum is extracted from. The mineral then goes on to be used in a number of items like construction materials, automobiles and consumer goods like food packaging and soda cans.
One perhaps unexpected use for caustic soda is in the manufacturing of pharmaceuticals like blood thinners and cholesterol medication.
A versatile water treatment product, sodium hydroxide is often used to maintain the safety and cleanliness of pools by removing harmful metals like lead and copper. As a base, sodium hydroxide lowers acidity, regulating water's pH. Additionally, the compound can be used to create sodium hypochlorite, which further disinfects water.
A co-product of the chlorine manufacturing process, caustic soda has been used for decades to create products that enhance our lives every day.
#289 Re: Exercises » Compute the solution: » 2025-10-26 21:13:22
Hi,
Good work!
2628.
#290 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-26 18:17:24
Hi,
#5827. What does the adjective insipid mean?
#5828. What does the adjective insinuating mean?
#291 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-26 17:51:54
Hi,
#10631. What does the term in Geography Chain mean?
#10632. What does the term in Geography Channel mean?
#292 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-26 17:00:49
2375) Salvador Luria
Gist:
Work
Bacteriophages are viruses that attach themselves to bacteria, emptying their genetic material into them, which leads to the rapid spawning of new phage inside the bacteria. By applying genetic concept and developing statistical approaches in their studies of bacteriophages, Max Delbrück, Salvador Luria, and Alfred Hershey were able to shed new light on a range of unanswered questions within genetics. For example, in 1943 Luria and Delbrück proved through statistical investigations that bacteria, like more complex organisms, develop via mutations.
Summary
Salvador Luria (born Aug. 13, 1912, Turin, Italy—died Feb. 6, 1991, Lexington, Mass., U.S.) was an Italian-born American biologist who, along with Max Delbrück and Alfred Day Hershey, won the Nobel Prize for Physiology or Medicine in 1969 for research on bacteriophages, viruses that infect bacteria.
Luria graduated from the University of Turin in 1935 and became a radiology specialist. He fled Italy for France in 1938 and went to the United States in 1940 after learning the techniques of phage research at the Pasteur Institute in Paris. Soon after his arrival, he met Delbrück, through whom he became involved with the American Phage Group, an informal scientific organization devoted to solving the problems of viral self-replication. Working with a member of the group in 1942, Luria obtained one of the electron micrographs of phage particles, which confirmed earlier descriptions of them as consisting of a round head and a thin tail.
In 1943 Luria and Delbrück published a paper showing that, contrary to the current view, viruses undergo permanent changes in their hereditary material. That same year he and Delbrück devised the fluctuation test, which provided experimental evidence that phage-resistant bacteria were the result of spontaneous mutations rather than a direct response to changes in the environment. In 1945 Hershey and Luria demonstrated the existence not only of such bacterial mutants but also of spontaneous phage mutants.
Luria became Sedgwick professor of biology at the Massachusetts Institute of Technology in 1964. In 1974 he became director of the Center for Cancer Research at MIT. He was an author of a college textbook, General Virology (1953), and a popular text for the general reader, Life: The Unfinished Experiment (1973).
Details
Salvador Edward Luria (August 13, 1912 – February 6, 1991) was an Italian microbiologist, later a naturalized U.S. citizen. He won the Nobel Prize in Physiology or Medicine in 1969, with Max Delbrück and Alfred Hershey, for their discoveries on the replication mechanism and the genetic structure of viruses. Salvador Luria also showed that bacterial resistance to viruses (phages) is genetically inherited.
Biography:
Early life
Luria was born Salvatore Luria in Turin, Italy to an influential Italian Sephardi Jewish family. His parents were Davide and Ester (Sacerdote) Luria. He attended the medical school at the University of Turin studying with Giuseppe Levi. There, he met two other future Nobel laureates: Rita Levi-Montalcini and Renato Dulbecco. He graduated from the University of Turin in 1935 and never got a master's degree or a PhD as they were not contemplated by the Italian high educational system (which, on the other hand, was very selective). From 1936 to 1937, Luria served his required time in the Italian army as a medical officer. He then took classes in radiology at the University of Rome. Here, he was introduced to Max Delbrück's theories on the gene as a molecule and began to formulate methods for testing genetic theory with the bacteriophages, viruses that infect bacteria.
In 1938, he received a fellowship to study in the United States, where he intended to work with Delbrück. Soon after Luria received the award, Benito Mussolini's fascist regime banned Jews from academic research fellowships. Without funding sources for work in the U.S. or Italy, Luria left his home country for Paris, France in 1938. As the Nazi German armies invaded France in 1940, Luria fled on bicycle to Marseille where he received an immigration visa to the United States.
Phage research
Luria arrived in New York City on September 12, 1940, and soon changed his first and middle names. With the help of physicist Enrico Fermi, whom he knew from his time at the University of Rome, Luria received a Rockefeller Foundation fellowship at Columbia University. He soon met Delbrück and Hershey, and they collaborated on experiments at Cold Spring Harbor Laboratory and in Delbrück's lab at Vanderbilt University.
His famous experiment with Delbrück in 1943, known as the Luria–Delbrück experiment, demonstrated statistically that inheritance in bacteria must follow Darwinian rather than Lamarckian principles and that mutant bacteria occurring randomly can still bestow viral resistance without the virus being present. The idea that natural selection affects bacteria has profound consequences, for example, it explains how bacteria develop antibiotic resistance.
Luria and Latarjet in 1947 published a quantitative analysis on the effect of ultraviolet irradiation on bacteriophage multiplication during intracellular growth. During the early course of infection they found an increase in bacteriophage resistance to ultraviolet irradiation and then later a decrease. At the time this pattern, known as the Luria-Laterjet effect, was published little was known about the central role of DNA in biology. Later work established that multiple specific DNA repair pathways, encoded by the infecting bacteriophage, contribute to the increase in UV resistance early in infection.
From 1943 to 1950, he worked at Indiana University. His first graduate student was James D. Watson, who went on to discover the structure of DNA with Francis Crick. In January 1947, Luria became a naturalized citizen of the United States.
In 1950, Luria moved to the University of Illinois Urbana–Champaign. In the early 1950s, Luria and Giuseppe Bertani discovered the phenomenon of host-controlled restriction and modification of a bacterial virus: a culture of E. coli can significantly reduce the production of phages grown in other strains; however, once the phage become established in that strain, they also become restricted in their ability to grow in other strains. It was later discovered by other researchers that bacteria produce enzymes that cut viral DNA at particular sequences but not the bacteria's own DNA, which is protected by methylation. These enzymes became known as restriction enzymes and developed into one of the main molecular tools in molecular biology.
Luria won the Nobel Prize in Physiology or Medicine in 1969, with Max Delbrück and Alfred Hershey, for their discoveries on the replication mechanism and the genetic structure of viruses.
Later work
In 1959, he became chair of Microbiology at the Massachusetts Institute of Technology (MIT). At MIT, he switched his research focus from phages to cell membranes and bacteriocins.[citation needed] While on sabbatical in 1963 to study at the Institut Pasteur in Paris, he found that bacteriocins impair the function of cell membranes. Returning to MIT, his lab discovered that bacteriocins achieve this impairment by forming holes in the cell membrane, allowing ions to flow through and destroy the electrochemical gradient of cells. In 1972, he became chair of The Center for Cancer Research at MIT. The department he established included future Nobel Prize winners David Baltimore, Susumu Tonegawa, Phillip Allen Sharp and H. Robert Horvitz.
In addition to the Nobel Prize, Luria received a number of awards and recognitions. He was elected to the American Academy of Arts and Sciences in 1959. He was named a member of the National Academy of Sciences in 1960. In 1964, he was elected to the American Philosophical Society. From 1968 to 1969, he served as president of the American Society for Microbiology. In 1969, he was awarded the Louisa Gross Horwitz Prize from Columbia University together with Max Delbrück, co-winner with Luria of the Nobel Prize in Physiology or Medicine in 1969. In the U.S. he won the 1974 National Book Award in Science for his popular science book Life: the Unfinished Experiment and received the National Medal of Science in 1991.
Political activism
Throughout his career, Luria was an outspoken political advocate. He joined with Linus Pauling in 1957 to protest against nuclear weapon testing. Luria was an opponent of the Vietnam War and a supporter of organized labor. In the 1970s, he was involved in debates over genetic engineering, advocating a compromise position of moderate oversight and regulation rather than the extremes of a complete ban or full scientific freedom. Due to his political involvement, he was blacklisted from receiving funding from the National Institutes of Health for a short time in 1969.
Noam Chomsky describes him as a friend, and claims that Luria attempted to influence Jewish American writer Elie Wiesel's public stance on Israel.
Death
Luria died in Lexington, Massachusetts of a heart attack on 6 February 1991 at the age of 78.

#293 Re: This is Cool » Miscellany » 2025-10-26 16:38:35
2427) Tartaric Acid
Gist
Tartaric acid is a naturally occurring, white crystalline organic acid found in fruits like grapes, bananas, and tamarinds. It is used as a food additive in baking powder to create a leavening reaction that makes baked goods rise. It also has antioxidant properties and a tangy flavor, with industrial uses including metal polishing and dyeing.
Tartaric acid has numerous uses in the food industry as an acidulant and flavor enhancer, especially in wine, baked goods, and candies. It also has pharmaceutical applications as an excipient in effervescent tablets and for flavor in medicines. Other uses include cosmetics for its exfoliating properties, industrial applications like metal cleaning and tanning leather, and as a leavening agent in baking powder.
Summary
Tartaric acid is a dicarboxylic acid, one of the most widely distributed of plant acids, with a number of food and industrial uses. Along with several of its salts, cream of tartar (potassium hydrogen tartrate) and Rochelle salt (potassium sodium tartrate), it is obtained from by-products of wine fermentation. In a partially purified form, tartar was known to the ancient Greeks and Romans; the free acid was first isolated in 1769 by Swedish chemist Carl Wilhelm Scheele. The lees, or sediments, and other waste products from fermentation are heated and neutralized with calcium hydroxide; the precipitated calcium tartrate is then treated with sulfuric acid to produce free tartaric acid. Rochelle salt is prepared from the crude crystalline potassium acid salt, called argol, by neutralization with sodium carbonate. Purified cream of tartar comes chiefly from the filtrates from production of the acid and Rochelle salt. A third salt, tartar emetic (antimony potassium tartrate), is made from the potassium acid salt and antimony oxide.
Three stereoisomeric forms of tartaric acid exist: (1) dextrorotatory tartaric acid (d-tartaric acid) found in grapes and several other fruits, (2) levorotatory tartaric acid (l-tartaric acid) obtained chiefly by resolution of racemic tartaric acid, and (3) a meso or achiral form. Racemic tartaric acid (an equal mixture of d- and l-tartaric acid) is prepared commercially by the molybdenum- or tungsten-catalyzed oxidation of maleic anhydride with hydrogen peroxide.
Study of the crystallographic, chemical, and optical properties of the tartaric acids by French chemist and microbiologist Louis Pasteur laid the basis for modern ideas of stereoisomerism.
The various tartaric acids and the common tartrate salts are all colourless, crystalline solids readily soluble in water. Tartaric acid is widely used as an acidulant in carbonated drinks, effervescent tablets, gelatin desserts, and fruit jellies. It has many industrial applications—e.g., in cleaning and polishing metals, in calico printing, in wool dyeing, and in certain photographic printing and development processes. Rochelle salt is used in silvering mirrors, in processing cheese, and in compounding mild cathartics. Cream of tartar is incorporated into baking powders, hard candies, and taffies; and it is employed in the cleaning of brass, the electrolytic tinning of iron and steel, and the coating of other metals with gold and silver. Tartar emetic is used as an insecticide and a dyeing mordant.
Details
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid.
History
Tartaric acid has been known to winemakers for centuries– its crude crystalline form as found off top of wine barrels were called tartarum (rendered tartre by Chaucer) or "wine stone". However, chemical extraction and purification was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele.
Tartaric acid played an important role in the discovery of chemical chirality. This property of tartaric acid was first observed in 1832 by Jean Baptiste Biot, who observed its ability to rotate polarized light. Louis Pasteur continued this research in 1847 by investigating the shapes of sodium ammonium tartrate crystals, which he found to be chiral. By manually sorting the differently shaped crystals, Pasteur was the first to produce a pure sample of levotartaric acid.
Tartaric acid in wine
Tartaric acid may be most immediately recognizable to wine drinkers as the source of "wine diamonds", the small potassium bitartrate crystals that sometimes form spontaneously on the cork or bottom of the bottle. These "tartrates" are harmless, despite sometimes being mistaken for broken glass, and are prevented in many wines through cold stabilization (which is not always preferred since it can change the wine's profile). The tartrates remaining on the inside of aging barrels were at one time a major industrial source of potassium bitartrate.
Tartaric acid plays an important role chemically, lowering the pH of fermenting "must" to a level where many undesirable spoilage bacteria cannot live, and acting as a preservative after fermentation. In the mouth, tartaric acid provides some of the tartness in the wine, although citric and malic acids also play a role.
Tartaric acid in fruits
Grapes and tamarinds have the highest levels of tartaric acid concentration. Other fruits with tartaric acid are bananas, avocados, prickly pear fruit, apples, cherries, papayas, peaches, pears, pineapples, strawberries, mangoes and citrus fruits.
Trace amounts of tartaric acid have been found in cranberries and other berries.
Tartaric acid is also present in the leaves and pods of Pelargonium plants and beans.
Applications
Tartaric acid and its derivatives have a plethora of uses in the field of pharmaceuticals. For example, it has been used in the production of effervescent salts, in combination with citric acid, to improve the taste of oral medications. The potassium antimonyl derivative of the acid known as tartar emetic is included, in small doses, in cough syrup as an expectorant.
Tartaric acid also has several applications for industrial use. The acid has been observed to chelate metal ions such as calcium and magnesium. Therefore, the acid has served in the farming and metal industries as a chelating agent for complexing micronutrients in soil fertilizer and for cleaning metal surfaces consisting of aluminium, copper, iron, and alloys of these metals, respectively.
Additional Information
Tartaric acid is the other major grape acid, along with malic acid. Unlike malic acid, the concentration of tartaric acid does not decline markedly during grape ripening. In addition, tartaric acid is metabolized by few microorganisms. Thus, it is usually the preferred acid added to increase the acidity of high pH wines. Regrettably, this carries the risk of increasing bitartrate instability.
Tartaric acid is synthesized in many plants, but accumulates in significant quantities in only a few genera, most significantly, members of the Vitaceae. It is so characteristic of V. vinifera that its presence in neolithic vessels in the Near East can be taken as evidence of wine production. The acid commonly collects as a potassium salt in leaves and grapes. As wines age, dissolved tartrates crystallize and tend to precipitate. Because chilling speeds the process, wines often are cooled near the end of maturation to enhance early tartrate precipitation and avoid crystal deposition in the bottle. Nevertheless, crystals may continue to form after bottling. This partially occurs due to the conversion of the natural (l form) of tartaric acid to the d isomer. The calcium salt of both isomers is about one-eighth as soluble as the l-tartrate salt alone. Therefore, most wines form a salt deposit when aged sufficiently long.

#294 Dark Discussions at Cafe Infinity » Coach Quotes - V » 2025-10-26 15:36:12
- Jai Ganesh
- Replies: 0
Coach Quotes - V
1. You can never guarantee the wins but you can guarantee that you give it 100%. That way you can always look back and feel comfortable, as a player or a coach. - Ivan Lendl
2. I think one of the things about being a good coach is to recognise when you have given all that you can. In fact there should be some sort of unspoken law that says that a coach cannot have anyone for three or four years - if you have not passed on most of the stuff you know in that time, then you are not doing a good job. - Daley Thompson
3. My first and only experience in baseball, the coach signed me up; he didn't tell me there's a thing called the curveball. I didn't know that. So the ball's coming at me and I start backing out, and then it broke inside. And the umpire says, 'Strike one!' And I'm saying, 'How is that a strike? It almost hit me!' - Magic Johnson
4. My focus had always been the on-side. My coach wanted me to work on the offside strokes since he was convinced of my ability and timing on the leg side. I worked hard and firmed up my defensive technique. I am happy getting runs all around the wicket now, and getting a lot of boundaries. No one calls me a 'leggie batsman' anymore. - Virat Kohli
5. I was too kind of brave and proud to want a dialect coach because I thought that showed weakness in my armor. But then you just learn it's a more efficient way of doing it. A dialect coach is really important because it takes a certain technical responsibility off your shoulders. - Russell Crowe
6. I would love to be a coach, mentor, or a batting consultant. I would love to commentate in Hindi, as most people who watch the game are more comfortable with Hindi in India rather than English. - Virender Sehwag
7. Every coach desires to bring in his own support staff. - Virender Sehwag.
#295 Jokes » Banana Jokes - III » 2025-10-26 15:14:03
- Jai Ganesh
- Replies: 0
Q: What did the banana do when he saw a monkey?
A: The banana split!
* * *
Q: What is yellow on the inside and green on the outside?
A: A banana dressed up as a cucumber !
* * *
Q: What would you call two banana skins?
A: A pair of slippers!
* * *
Q: What do you do if you see a blue banana?
A: Try to cheer it up.
* * *
Q: What's yellow and writes?
A: A ball-point banana.
* * *
#296 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-26 14:37:39
Hi,
#2507. What does the medical term Aortic valve replacement mean?
#297 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-26 14:15:34
Hi,
#9776.
#298 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-26 14:04:29
Hi,
#6281.
#299 Re: Exercises » Compute the solution: » 2025-10-26 13:57:15
Hi,
2627.
#300 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-25 18:33:05
2374) Alfred Hershey
Gist:
Work
Bacteriophages are viruses that attach themselves to bacteria, emptying their genetic material into them, which leads to the rapid spawning of new phage inside the bacteria. By applying genetic concept and developing statistical approaches in their studies of bacteriophages, Max Delbrück, Salvador Luria, and Alfred Hershey were able to shed new light on a range of unanswered questions within genetics. For example, in 1952 Hershey and Martha Chase were able to demonstrate that DNA was transferred from bacteriophages to bacteria, a discovery that confirmed DNA as the bearer of genetic information.
Summary
A.D. Hershey (born Dec. 4, 1908, Owosso, Mich., U.S.—died May 22, 1997, Syosset, N.Y.) was an American biologist who, along with Max Delbrück and Salvador Luria, won the Nobel Prize for Physiology or Medicine in 1969. The prize was given for research done on bacteriophages (viruses that infect bacteria).
Hershey earned a doctorate in chemistry from Michigan State College (now Michigan State University) in 1934 and then took a position at Washington University School of Medicine in St. Louis, Mo. He joined the staff of the Genetics Research Unit of the Carnegie Institution of Washington in 1950 after giving up his position as professor at Washington University. In 1963 he became director of the Genetics Research Unit.
Hershey, Delbrück, and Luria began exchanging information on phage research in the early 1940s. In 1945 Hershey and Luria, working independently, demonstrated the occurrence of spontaneous mutation in both the bacteriophages and the host. The next year, Hershey and Delbrück independently discovered the occurrence of genetic recombination in phages—i.e., that different strains of phages inhabiting the same bacterial cell can exchange or combine genetic material. Delbrück incorrectly interpreted his results as specifically induced mutations, but Hershey and one of his students proved that the results they had obtained were recombinations by showing that the genetic processes in question correspond with the crossing-over of parts of similar chromosomes observed in cells of higher organisms.
Hershey is most noted for the so-called blender experiment that he performed with Martha Chase in 1952. By showing that phage DNA is the principal component entering the host cell during infection, Hershey proved that DNA, rather than protein, is the genetic material of the phage.
Details
Alfred Day Hershey (December 4, 1908 – May 22, 1997) was an American Nobel Prize–winning bacteriologist and geneticist.
Early years
Hershey was born in Owosso, Michigan to Robert Day and Alma Wilbur Hershey. He earned a B.S. in chemistry in 1930, and Ph.D. in bacteriology in 1934 from Michigan State University. Shortly after, Hershey accepted a faculty position at Washington University in St. Louis, serving as an instructor of bacteriology and immunology from 1934 to 1950.
Bacteriophage research
At Washington University, Hershey worked closely with department head Jacques Bronfenbrenner to investigate bacteriophages, or phages—viruses that infect and replicate inside bacteria. Hershey's work on the factors impacting the virus' ability to infect its targets brought him to the attention of fellow phage researchers Max Delbrück and Salvador Luria.
The Phage Group
In 1943, Delbrück invited Hershey to Vanderbilt University to discuss his phage research. Together, with Luria, they would form the core of an informal network of researchers called "the Phage group". Three years later, Hershey and Delbrück would independently discover that different strains of bacteriophage can both exchange genetic material when infecting the same bacterial cell. This process results in hybrid phages containing genetic material from both sources, which Hershey referred to as "genetic recombination".
Hershey left Washington University in 1950 for the Department of Genetics of the Carnegie Institution of Washington, a predecessor of Cold Spring Harbor Laboratory. Two years later, he and Martha Chase would conduct the famous Hershey–Chase, or "Waring Blender" experiment. Their work confirmed that DNA, not protein, was the genetic material of life.
Later years and death
In 1962, Hershey was named director of the Department of Genetics, a position he held until his retirement in 1970. He would live on the grounds of Cold Spring Harbor Laboratory (CSHL) for the rest of his life.
Hershey's work with bacteriophage would earn him a share of the 1969 Nobel Prize in Physiology or Medicine with Delbrück and Luria, "for their discoveries concerning the replication mechanism and the genetic structure of viruses."
Although officially retired from scientific research, Hershey would continue to pursue new projects. In 1971, he edited The Bacteriophage λ, an extensive volume on the subject, published by CSHL Press that same year. In 1981, Hershey became a founding member of the World Cultural Council.
Hershey died from congestive heart failure on May 22, 1997 at his home in Laurel Hollow, New York. He was 88 years old. At the time, he was survived by his wife Harriet Davidson (1918–2000) and their only child, Peter Manning Hershey (1956–1999).
Following his death, Frank Stahl, a member of The Phage Group, wrote: "The Phage Church, as we were sometimes called Phage group, was led by the Trinity of Delbrück, Luria, and Hershey. Delbrück's status as founder and his ex cathedra manner made him the pope, of course, and Luria was the hard-working, socially sensitive priest-confessor. And Al (Hershey) was the saint."
