Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 Jokes » Mushroom Jokes - I » Today 00:04:10
- Jai Ganesh
- Replies: 0
Q: Why did the Fungi leave the party?
A: There wasn't mushroom.
* * *
Q: Why did the Mushroom get invited to all the parties?
A: 'Cuz he's a fungi!
* * *
Q: Why do Toadstools grow so close together?
A: They don't need Mushroom.
* * *
Q: What would a mushroom car say?
A: Shroom shroom!
* * *
Q: Which vegetable goes best with jacket potatoes?
A: Button Mushrooms.
* * *
#2 Re: This is Cool » Miscellany » Today 00:03:28
2516) Sodium Phosphate
Gist
Sodium phosphate is a versatile salt (H3PO4) used as a food additive (emulsifier, buffer, leavening agent), cleaning agent, and saline laxative for colonoscopies. It exists in mono-, di-, and tribasic forms, often used in processed foods and medical treatments. While generally safe, excessive consumption can cause serious side effects.
Consuming small amounts of foods that contain sodium phosphate is "most likely" not harmful to your health. However, since many people consume fast food, processed meats and packaged foods on a daily basis, there is concern that high levels of sodium phosphate can harm the body.
Summary
A sodium phosphate is a generic variety of salts of sodium (Na+) and phosphate. Phosphate also forms families or condensed anions including di-, tri-, tetra-, and polyphosphates. Most of these salts are known in both anhydrous (water-free) and hydrated forms. The hydrates are more common than the anhydrous forms.
Uses
Sodium phosphates have many applications in food and for water treatment. Sodium phosphates are often used as water-retaining agents for frozen food, thickening agents for processed food, and leavening agents for baked goods. It is also a source of the phosphate ion (an emulsifying agent) for processed cheese, where it chelates calcium, thereby allowing the casein in cheese to remain suspended and preventing separation during heating. They are also used to control pH of processed foods.
They are also used in medicine for constipation and to prepare the bowel for medical procedures, by acting as an osmotic laxative that draws water into the bowel.
Like other phosphate salts they are used in detergents to increase their activity in hard water. They are also used in water softeners in addition to regular sodium chloride.
They are also useful corrosion inhibitors for preventing rusting of metal pipes.
Adverse effects
Sodium phosphates are popular in commerce in part because they are inexpensive and because they are nontoxic at normal levels of consumption. However, oral sodium phosphates when taken at high doses for bowel preparation for colonoscopy may in some individuals carry a risk of kidney injury under the form of phosphate nephropathy. There are several oral phosphate formulations which are prepared extemporaneously. Oral phosphate prep drugs have been withdrawn in the United States, although evidence of causality is equivocal. Since safe and effective replacements for phosphate purgatives are available, several medical authorities have recommended general disuse of oral phosphates.
Details:
Description
Sodium phosphate is a colorless to white crystalline powder or granules. It is prepared by neutralization of phosphoric acid under controlled conditions with sodium hydroxide or sodium carbonate .
We are committed to bringing you Greener Alternative Products, which belong to one of the four categories of greener alternatives. Sodium phosphate supports cleaner technologies as a versatile buffering and stabilizing agent that helps control pH, mitigate corrosion and side reactions, and extend equipment/component lifetimes—reducing waste, chemical use, and energy demand across processes.
Application
Sodium phosphate is used as a:
* component in the preparation of solid crystals of calcium hydroxyapatite substituted by strontium
* block buffer to study segmental and subcellular distribution of CFTR in the kidney
(CFTR: The Cystic Fibrosis Transmembrane Conductance Regulator is a protein-coding gene and ion channel that transports chloride and bicarbonate ions across epithelial cell membranes, crucial for regulating fluid balance in the lungs and pancreas. Mutations in the CFTR gene cause cystic fibrosis, leading to thick, sticky mucus, chronic infections, and organ damage.)
Additional Information:
What is Sodium Phosphate?
Sodium phosphate also known as phospho soda with the formula Na3PO4 is a saline cathartic that is familiar to radiologists since it is often used as a cleansing agent prior to double contrast barium enema.
It is prepared by neutralization of phosphoric acid under controlled conditions with sodium hydroxide or sodium carbonate. Sodium phosphate cradles are the most widely recognized, yet there is broad utilization of potassium phosphate buffer and blends of sodium and potassium. Many compounds of pharmaceutical interest are formulated in sodium phosphate buffers.
Uses of Sodium Phosphate – Na3PO4
* Short-term, local treatment of inflammation with neomycin as bacterial prophylaxis.
* Used after glaucoma surgery or after cataract surgery.
* Used as a mild laxative, stimulates the emptying of the gall bladder.
* One of the most palatable of the saline laxatives. It is also used in the form of an oral solution as an antihypercalcemia.
* Used to control the pH of water hardness precipitation and control agent in mildly acidic solutions.
Frequently Asked Questions
Q1. Is sodium phosphate an acid or a base?
A1. Sodium phosphate is a salt obtained by the reaction of phosphoric acid and sodium hydroxide.
Q2. What is sodium phosphate used for in medicine?
A2. Sodium biphosphate and sodium phosphate are sources of phosphorus which is a material that occurs naturally and is essential in every cell in the body. Sodium biphosphate and sodium phosphate is a combination drug used in adults before a colonoscopy to relieve constipation and cleanse the intestines.
Q3. What happens if your phosphate levels are low?
A3. High phosphate levels seldom contribute to hypophosphatemia symptoms; rather symptoms generally arise from the underlying disorder that causes hypophosphatemia. Ultra low levels of phosphate can cause difficulty breathing, agitation, altered mental state, muscle weakness and muscle damage called rhabdomyolysis.
Q4. What is sodium phosphate monobasic monohydrate?
A4. Sodium Phosphate, monobasic (monohydrate) is a reagent commonly used in molecular biology, biochemistry, and chromatography with a very high buffering capacity. Monobasic sodium phosphate is extremely hygroscopic and soluble in water.
Q5. What is the difference between sodium phosphate monobasic and dibasic?
A5. Sodium phosphate monobasic has the chemical formula of NaH2PO4, and the chemical formula of Na2HPO4 has sodium phosphate dibasic. As sodium phosphate dibasic dissolves in water, the basicity in the medium is higher than when monobasic sodium phosphate dissolves in water.

#3 Re: Dark Discussions at Cafe Infinity » crème de la crème » Today 00:03:02
2453) Thomas Huckle Weller
Gist:
Work
Many infectious diseases are caused by viruses—very small biological particles. A virus lacks metabolism of its own and cannot multiply without infecting a living cell. For a long time the prevailing opinion was that viruses could not be cultured in a laboratory. However, in 1949 Frederick Robbins, John Enders, and Thomas Weller succeeded in culturing the virus that causes polio in human muscle and tissue in a laboratory setting. This became an important step on the road toward a vaccine against polio.
Summary
Thomas H. Weller (born June 15, 1915, Ann Arbor, Mich., U.S.—died Aug. 23, 2008, Needham, Mass.) was an American physician and virologist who was the corecipient (with John Enders and Frederick Robbins) of the Nobel Prize for Physiology or Medicine in 1954 for the successful cultivation of poliomyelitis virus in tissue cultures. This made it possible to study the virus “in the test tube”—a procedure that led to the development of polio vaccines.
After his education at the University of Michigan at Ann Arbor (A.B., 1936; M.S., 1937) and Harvard University (M.D., 1940), Weller became a teaching fellow at the Harvard Medical School (1940–42) and served in the U.S. Army Medical Corps during World War II. He was appointed assistant director of Enders’ infectious diseases laboratory at the Children’s Medical Center, Boston (1949–55), and, working with Enders and Robbins, soon achieved the propagation of poliomyelitis virus in laboratory suspensions of human embryonic skin and muscle tissue. He was also the first (with the American physician Franklin Neva) to achieve the laboratory propagation of rubella (German measles) virus and to isolate chicken pox virus from human cell cultures. Weller became professor of tropical public health at Harvard University in 1954 and from 1966 to 1981 served also as director of the Center for the Prevention of Infectious Diseases at the Harvard University School of Public Health. Weller’s autobiography, Growing Pathogens in Tissue Cultures: Fifty Years in Academic Tropical Medicine, Pediatrics, and Virology, was published in 2004.
Details
Thomas Huckle Weller (June 15, 1915 – August 23, 2008) was an American virologist. He, John Franklin Enders and Frederick Chapman Robbins were awarded a Nobel Prize in Physiology or Medicine in 1954 for showing how to cultivate poliomyelitis viruses in a test tube, using a combination of human embryonic skin and muscle tissue.
Biography
Weller was born and grew up in Ann Arbor, Michigan, where he graduated from Ann Arbor High School. He then went to the University of Michigan, where his father Carl Vernon Weller was a professor in the Department of Pathology. At Michigan, he studied medical zoology and received a B.S. and an M.S., with his masters thesis on fish parasites.
In 1936, Weller entered Harvard Medical School, and in 1939 began working under John Franklin Enders, with whom he would later (along with Frederick Chapman Robbins) share the Nobel Prize. It was Enders who got Weller involved in researching viruses and tissue-culture techniques for determining infectious disease causes. Weller received his MD in 1940, and went to work at Children's Hospital in Boston.
In 1942, during World War II, he entered the Army Medical Corps and was stationed at the Antilles Medical Laboratory in Puerto Rico, earning the rank of Major and heading the facility's Departments of Bacteriology, Virology and Parasitology. After the War, he returned to Children's Hospital in Boston, and it was there in 1947, that he rejoined Enders in the newly created Research Division of Infectious Diseases. After several leading positions, in July 1954, he was appointed head of the Tropical Public Health Department at the Harvard School of Public Health. Weller also served from 1953 to 1959 as director of the Commission on Parasitic Diseases of the American Armed Forces Epidemiological Board.
In addition to his work on polio, Weller also contributed to treating schistosomiasis, and Coxsackie viruses. He was also the first to isolate the virus responsible for chickenpox.
Awards
In 1954, Weller was awarded the George Ledlie prize in recognition of his research on rubella, polio and cytomegalovirus(CMV) viruses.
He was awarded the Nobel Prize in Physiology or Medicine in 1954 for his research on polio.
In 1996 he was awarded the Walter Reed Medal from the American Society of Tropical Medicine and Hygiene.
Personal life
In 1945, Weller married Kathleen Fahey, who died in 2011 aged 95. They had two sons and two daughters.

#4 Dark Discussions at Cafe Infinity » Comeback Quotes » Today 00:02:22
- Jai Ganesh
- Replies: 0
Comeback Quotes
1. I don't know, I am lucky. I always make a good comeback. - Hardik Pandya
2. When you are injured, you need to strengthen yourself very well to make a comeback very confidently. - P. V. Sindhu
3. Comebacks are not at all easy. After a major surgery, the difficult part is to conquer the inner demons. It's all in the mind. Only an individual can overcome his fears. - Rohit Sharma
4. I would like to thank all my tennis fans who were there from Day One when I was No. 1, through my stabbing, and my comeback. - Monica Seles
5. At one point, when I didn't make the 2007 World Cup squad, I was very, very frustrated. Then I became very hard on myself. Whenever I used to go to the nets, or when I trained in the gym, I was very hard on myself. I couldn't sleep; I used to think a lot. Very, very desperate to make a comeback. - Gautam Gambhir
6. For some reason, every time I peak in my career, I injure myself. So, I'm constantly on the comeback trail. - Sania Mirza
7. I am pleased about making a comeback in Bollywood, but then I really cannot think about leaving South Indian cinema. Whatever I am today is because of South films, and I cannot give up on that. - Tamannaah
8. I have never played cricket for selfish reasons like scoring 800-900 runs on flat tracks to make a comeback. - Gautam Gambhir
9. By making a comeback, I'm changing the attitude of people toward me. If I'd known that people would react so enthusiastically, I'd have done it years ago. - Mark Spitz
10. Looking back, yes, I made too many comebacks. But each comeback I was 100 percent sure that I would win. I never came back for the money, because I didn't need it. The adulation I was getting anyway in other spheres. But I'm a guy who likes to see how close he can get to the edge of the mountain - that's what makes me tick. - Sugar Ray Leonard
11. In the single group format, where each team plays against the other, it gives a chance to the established outfits to make a comeback if they falter in the early games. - Inzamam-ul-Haq
12. Wimbledon is not the easiest tournament in which to make a comeback. - Mats Wilander
#5 This is Cool » Mount Kilimanjaro » Yesterday 16:59:23
- Jai Ganesh
- Replies: 0
Mount Kilimanjaro
Gist
Mount Kilimanjaro, located in Tanzania, is Africa's highest peak (5,895 meters/19,341 feet) and the world's tallest free-standing mountain. It is a dormant stratovolcano with three volcanic cones—Kibo, Mawenzi, and Shira—and is famous for its snow-capped peak and five diverse ecological zones. Climbing is a popular non-technical trek, typically taking 5-9 days.
Kilimanjaro is famous as Africa's highest peak, the world's highest free-standing mountain, and a bucket-list destination for trekkers, known for its iconic snow-capped summit, diverse ecosystems, and status as one of the Seven Summits challenge. Its fame comes from being an accessible yet challenging climb, offering a unique journey through five distinct climate zones, making it a symbol of adventure and a visible indicator of climate change due to its shrinking glaciers.
Summary
Mount Kilimanjaro is a large dormant volcano in Tanzania. It is the highest mountain in Africa and the highest free-standing mountain above sea level in the world, at 5,895 m (19,341 ft) above sea level and 4,900 m (16,100 ft) above its plateau base. It is also the highest volcano in the Eastern Hemisphere and the fourth most prominent peak on Earth.
Kilimanjaro's southern and eastern slopes served as the home of the Chagga Kingdoms until their abolition in 1963 by Julius Nyerere. The origin and meaning of the name Kilimanjaro is unknown, but may mean "mountain of greatness" or "unclimbable". Although described in classical sources, German missionary Johannes Rebmann is credited as the first European to report the mountain's existence, in 1848. After several European attempts, Hans Meyer reached Kilimanjaro's highest summit in 1899.
The mountain was incorporated into Kilimanjaro National Park in 1973. As one of the Seven Summits, Kilimanjaro is a major hiking and climbing destination. There are seven established routes to Uhuru Peak, the mountain's highest point. Although not as technically challenging as similar mountains, the prominence of Kilimanjaro poses a serious risk of altitude sickness.
One of several mountains arising from the East African Rift, Kilimanjaro was formed from volcanic activity over 2 million years ago. Its slopes host montane forests and cloud forests. Multiple species are endemic to Mount Kilimanjaro, including the giant groundsel Dendrosenecio kilimanjari. The mountain possesses a large ice cap and the largest glaciers in Africa, including Credner Glacier, Furtwängler Glacier, and the Rebmann Glacier. This ice cap is rapidly shrinking, with over 80% lost in the 20th century. The cap is projected to disappear entirely by the mid-21st century.
Details
Mount Kilimanjaro is Africa’s tallest mountain and the world’s largest free-standing mountain.
Located in Tanzania, Mount Kilimanjaro is Africa’s tallest mountain at about 5,895 meters (19,340 feet). It is the largest free-standing mountain rise in the world, meaning it is not part of a mountain range.
Also called a stratovolcano (a term for a very large volcano made of ash, lava and rock), Kilimanjaro is made up of three cones: Kibo, Mawenzi and Shira. Kibo is the summit of the mountain and the tallest of the three volcanic formations. While Mawenzi and Shira are extinct, Kibo is considered dormant and could possibly erupt again. Scientists estimate that the last time it erupted was 360,000 years ago. The highest point on Kibo’s crater rim is called Uhuru, the Swahili word for “freedom.”
No one knows how Kilimanjaro got its name. It may come from the Swahili word Kilima (meaning “mountain”) and the KiChagga word Njaro (meaning “shining” or “whiteness”); the mountain is known for its snow-capped peak. Some local people living in the foothills of the mountain, including the Chagga and the Maasai, view it as the seat of God.
Unfortunately, the white snow that the mountain is named for may soon disappear. Over the last hundred years, all of Kilimanjaro’s glaciers have begun to retreat. Some have vanished altogether. Scientists have studied satellite images and learned that Kilimanjaro has lost more than 90 percent of its ice since 1900. Many experts are studying the causes of this catastrophic melt.
The people who live in the vicinity of Kilimanjaro are an important part of the mountain’s history. In 1889, local climber Yohani Kinyala Lauwo (also known as Mzee Lauwo) guided German geographer Hans Meyer and Austrian mountaineer Ludwig Purtscheller to the Kilimanjaro summit. Lauwo then became the first Tanzanian to reach the peak at the age of 18. Purtscheller and Meyer were the first Europeans to summit. Lauwo was a member of the Chagga tribe. The Chagga have lived on Kilimanjaro’s slopes for centuries. Lauwo went on to guide climbers to Kilimanjaro’s summit for more than 50 years, dying at the age of 125.
Kilimanjaro continues to be a popular hiking spot. This is partly because the hiking routes do not require as much equipment or experience as mountains of similar heights. Tens of thousands of climbers ascend the mountain each year. The climb is still dangerous, however, because of the risk of altitude sickness. Climbers can experience altitude sickness if they ascend too quickly, and it can be deadly if not treated right away.
In 1973, the mountain and its six surrounding forest corridors were named Kilimanjaro National Park. The park was named a United Nations Educational, Scientific and Cultural Organization (UNESCO) World Heritage site in 1987. These measures can help protect the area’s unique environment. A variety of animals live in the area surrounding the mountain, including the blue monkey (Cercopithecus mitis).
Additional Information
Kilimanjaro is a volcanic massif in northeastern Tanzania, near the Kenya border. Its central cone, Kibo, rises to 19,340 feet (5,895 metres) and is the highest point in Africa. Kilimanjaro lies about 100 miles (160 km) east of the East African Rift System and about 140 miles (225 km) south of Nairobi, Kenya. The massif extends approximately east-west for 50 miles (80 km) and consists of three principal extinct volcanoes: Kibo (centre), Mawensi (east), and Shira (west). Kibo, the youngest and highest, retains the form of a typical volcanic cone and crater and is linked by a 7-mile (11-km) saddle at about 15,000 feet (4,500 metres) with Mawensi (16,893 feet [5,149 metres]), which is the older core of a former summit. Shira ridge (13,000 feet [3,962 metres]) is a remnant of an earlier crater. Below the saddle, Kilimanjaro slopes in a typical volcanic curve to the plains below, which lie at an elevation of about 3,300 feet (1,000 metres). The breathtaking snow-clad dome of Kibo contains a caldera (crater) on its southern side that is 1.2 miles (2 km) across and some 980 feet (300 metres) deep, with an inner cone that displays residual volcanic activity. Mawensi’s cone is highly eroded, jagged, and precipitous and is cleft east and west by gorges. Only Kibo retains a permanent ice cap. Mawensi has semipermanent ice patches and substantial seasonal snow.
The mountain and its surrounding forests were designated a game reserve in the early part of the 20th century. In 1973 Mount Kilimanjaro National Park was established to protect the mountain above the tree line as well as the six forest corridors that extend downslope through the montane forest belt. The park was designated a UNESCO World Heritage site in 1987.
Kilimanjaro has a succession of vegetation zones consisting of (from base to summit) the semiarid scrub of the surrounding plateau; the massif’s cultivated, well-watered southern slopes; dense cloud forest; open moorland; alpine desert; and moss and lichen communities. Two notable species that grow in the moorlands are the giant lobelia (Lobelia deckenii) and the giant groundsel (Senecio johnstonii cottonii). The forests of the southern slopes and surrounding areas are home to elephants, buffalo, and eland (oxlike antelopes). Smaller mammals inhabiting the forests include black and white colobus monkeys, blue monkeys, and bushbuck and duikers (small African antelopes). The forests also host a rich variety of birdlife, including the rare Abbot’s starling.
The Kilimanjaro formations became known to Europeans when they were reached in 1848 by the German missionaries Johannes Rebmann and Johann Ludwig Krapf, although the news that there were snow-capped mountains so close to the Equator was not believed until more than a decade later. The Kibo summit was first reached in 1889 by the German geographer Hans Meyer and the Austrian mountaineer Ludwig Purtscheller. The Kilimanjaro region is one of Tanzania’s leading producers of mild coffee, barley, wheat, and sugar; other crops include sisal, corn (maize), beans, bananas, wattle bark (Acacia), cotton, pyrethrum, and potatoes. The region is populated by the Chaga (Chagga), Pare, Kahe, and Mbugu peoples. The town of Moshi, at the southern foot of Kilimanjaro, is the chief trading centre and base for ascent. As Kibo’s peak can be reached without the aid of mountaineering equipment, thousands of hikers attempt the ascent each year.

#6 Science HQ » Radius (Bone) » Yesterday 16:23:04
- Jai Ganesh
- Replies: 0
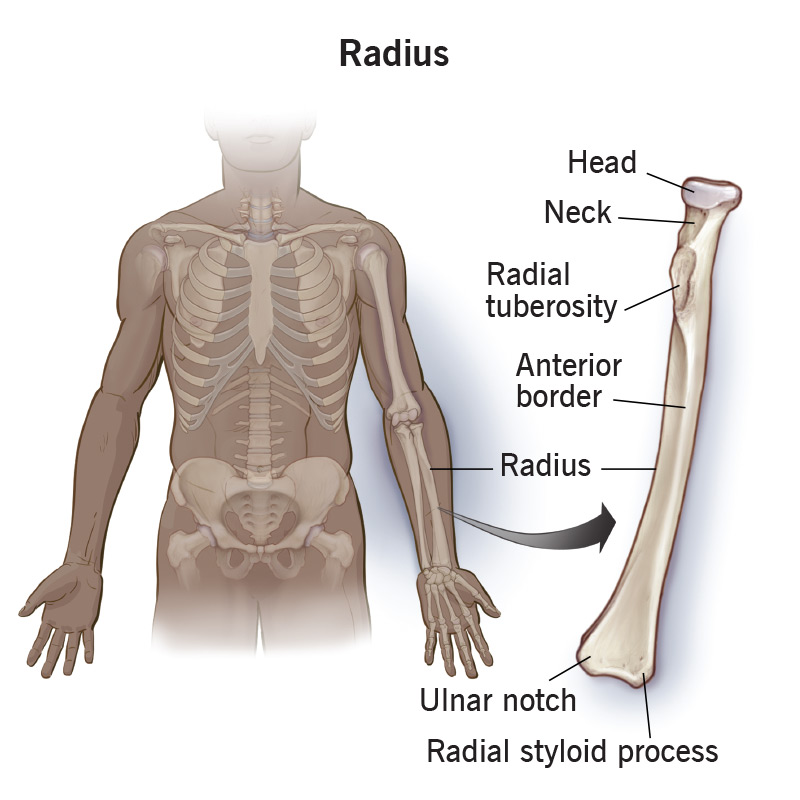
Radius (Bone)
Gist
The radius (or radial bone) is one of two long bones in the forearm, extending from the lateral (thumb) side of the elbow to the wrist. It runs parallel to the ulna, is shorter and thicker than the ulna, and is crucial for rotating the forearm (pronation/supination) and supporting wrist movement.
The radius is located in the forearm and provides articulation at the elbow and wrist joints. The radius extends from the elbow to the wrist. The ulna lies adjacent to the radius and the two bones work together to move the arm at the wrist and elbow. The humerus is the solitary long bone of the upper arm.
Summary
The radius is a long bone in the forearm. It lies laterally and parallel to ulna, the second of the forearm bones. The radius pivots around the ulna to produce movement at the proximal and distal radio-ulnar joints.
The radius articulates in four places:
* Elbow joint – Partly formed by an articulation between the head of the radius, and the capitulum of the humerus.
* Proximal radioulnar joint – An articulation between the radial head, and the radial notch of the ulna.
* Wrist joint – An articulation between the distal end of the radius and the carpal bones.
* Distal radioulnar joint – An articulation between the ulnar notch and the head of the ulna.
Proximal End
The proximal end of the radius articulates in both the elbow and proximal radioulnar joints.
Important bony landmarks include the head, neck and radial tuberosity:
Head of radius – A disk shaped structure, with a concave articulating surface. It is thicker medially, where it takes part in the proximal radioulnar joint.
Neck – A narrow area of bone, which lies between the radial head and radial tuberosity.
Radial tuberosity – A bony projection, which serves as the place of attachment of the biceps brachii muscle.
Shaft
The radial shaft expands in diameter as it moves distally. Much like the ulna, it is triangular in shape, with three borders and three surfaces.
In the middle of the lateral surface, there is a small roughening for the attachment of the pronator teres muscle.
Distal End
In the distal region, the radial shaft expands to form a rectangular end. The lateral side projects distally as the styloid process. In the medial surface, there is a concavity, called the ulnar notch, which articulates with the head of ulna, forming the distal radioulnar joint.
The distal surface of the radius has two facets, for articulation with the scaphoid and lunate carpal bones. This makes up the wrist joint.
Details
The radius or radial bone (pl.: radii or radiuses) is one of the two large bones of the forearm, the other being the ulna. It extends from the lateral side of the elbow to the thumb side of the wrist and runs parallel to the ulna. The ulna is longer than the radius, but the radius is thicker. The radius is a long bone, prism-shaped and slightly curved longitudinally.
The radius is part of three joints: the elbow and the wrist, both of which are synovial joints; and the radioulnar joint, which is a syndesmosis. At the elbow, it joins with the capitulum of the humerus, and in a separate region, with the ulna at the radial notch. At the wrist, the radius forms a joint with the ulna bone. The radioulnar joint allows for supination and pronation of the forearm.
The corresponding bone in the leg is the tibia.
Structure
The long narrow medullary cavity is enclosed in a strong wall of compact bone. It is thickest along the interosseous border and thinnest at the extremities, same over the cup-shaped articular surface (fovea) of the head.
The trabeculae of the spongy tissue are somewhat arched at the upper end and pass upward from the compact layer of the shaft to the fovea capituli (the humerus's cup-shaped articulatory notch); they are crossed by others parallel to the surface of the fovea. The arrangement at the lower end is somewhat similar. It is missing in radial aplasia.
The radius has a body and two extremities. The upper extremity of the radius consists of a somewhat cylindrical head articulating with the ulna and the humerus, a neck, and a radial tuberosity. The body of the radius is self-explanatory, and the lower extremity of the radius is roughly quadrilateral in shape, with articular surfaces for the ulna, scaphoid and lunate bones. The distal end of the radius forms two palpable points, radially the styloid process and Lister's tubercle on the ulnar side. Along with the proximal and distal radioulnar articulations, an interosseous membrane originates medially along the length of the body of the radius to attach the radius to the ulna.
Additional Information
Your radius is one of the bones in your forearm. It helps you move your arm and wrist. When you injure your radius, it’s likely the muscles and nerves attached to it will be damaged, too.
Overview:
What is the radius?
The radius is one of the bones in your forearm. It helps you move your arm and wrist. Your radius also supports lots of important muscles, tendons, ligaments and blood vessels.
If you experience a fractured (broken) radius, you might need surgery to repair your bone and physical therapy to help you regain your strength and ability to move.
Your radius — like all bones — can be affected by osteoporosis.
Because your radius is connected to so many muscles and nerves, injuries to one often can affect the others.
Function:
What does the radius do?
Your radius has several important jobs, including:
* Helping your forearm and wrist move, flex and rotate.
* Holding seven muscles in place.
* Stabilizing the rest of your arm, wrist and hand.
Anatomy:
Where is the radius located?
Your radius is one of two bones in your forearm. The other is your ulna. The radius is opposite your ulna, on the lateral (thumb) side of your forearm. Your radius rotates over your ulna when you stretch your arm straight out in front of you with your palms facing down. They’re more parallel to each other when you hold your arms straight out with your palms face up.
What does the radius look like?
Your radius has a small end where it meets your humerus (upper arm bone), a long shaft in the middle that’s slightly curved and a wider end that meets your wrist. It’s thicker and slightly shorter than your ulna.
Even though it’s one long bone, your radius is made up of several parts. These include:
Radius proximal aspect
The upper (proximal) end of your radius connects to your humerus. The proximal end (aspect) contains the:
* Head.
* Neck.
* Radial tuberosity.
Radius shaft
The shaft is the long middle portion of the radius that supports the weight of your forearm and gives it its shape.
Radius distal aspect
The lower (distal) end of your radius forms the top of your wrist joint. It’s wider than the rest of your radius where it meets your scaphoid and lunate (wrist or carpal bones). The distal end of the radius includes:
* Styloid process.
* Ulnar notch.
All these parts and labels are usually more for your healthcare provider to use as they describe where you’re having pain or issues. If you ever break your radius — a radial fracture — your provider might use some of these terms to describe where your bone was damaged.
How big is the radius?
Your radius is the third longest bone in your arm and is one of the longest bones in your body. Most adults’ radius bones are around 10 inches long.
Conditions and Disorders:
What are the common conditions and disorders that affect the radius?
The most common issues that affect the radius are fractures, osteoporosis and damage to nerves or muscles around it.
Radius fractures
A bone fracture is the medical term for a broken bone. The most common causes of radius fractures include:
* Sports injuries.
* Car accidents.
* Falls.
Symptoms of a fracture include:
* Pain.
* Swelling.
* Tenderness.
* Inability to move your arm like you usually can.
* Bruising or discoloration.
* A deformity or bump that’s not usually on your body.
Some fractures that affect the radius include:
* Colles fractures.
* Smith fractures.
* Greenstick fractures.
* Growth plate fractures.
Go to the emergency room right away if you’ve experienced a trauma or think you have a fracture.
Osteoporosis
Osteoporosis weakens bones, making them more susceptible to sudden and unexpected fractures. It usually doesn’t cause any symptoms you can notice, so many people don’t know they have osteoporosis until after it causes them to break a bone.
Females and adults older than 65 have an increased risk for developing osteoporosis. Talk to your provider about a bone density test that can catch osteoporosis before it causes a fracture.
Nursemaid elbow
Nursemaid elbow is a common injury for young children. You might also see it referred to as a “pulled elbow.” It’s a partially dislocated radius where it meets a child’s elbow. It happens because kids’ ligaments are looser than adults’. Pulling on your child’s arm or hand causes nursemaid elbow. It usually happens accidentally after motions like tugging your child’s arm so they don’t walk into the street, or playfully lifting them up by their hands.
What tests are done on the radius?
The most common test done to check the health of your radius is a bone density test. It’s sometimes called a DEXA or DXA scan. A bone density test uses low levels of X-rays to measure how strong your bones are. It’s a way for your provider to track bone loss as you age.
If you’ve experienced a humeral fracture, your provider or surgeon might need imaging tests, including:
* X-rays.
* Magnetic resonance imaging (MRI).
* CT scan.
What are common treatments done to the radius?
Your radius won’t need treatment unless you’ve experienced a fracture or injury or have osteoporosis.
Radius fracture treatment
How your fracture is treated depends on which type it is and what caused it. You’ll need some form of immobilization like a splint or cast. You might need surgery to realign (set) your bone to its correct position so it can heal.
Osteoporosis treatment
Treatments for osteoporosis can include exercise, vitamin and mineral supplements and medications.
Your provider will help you develop a treatment plan that’s customized for you and your bone health.
#7 Re: Jai Ganesh's Puzzles » General Quiz » Yesterday 15:36:08
Hi,
#10789. What does the term in Biology Hormone mean?
#10780. What does the term in Biology Host mean?
#8 Re: Jai Ganesh's Puzzles » English language puzzles » Yesterday 15:23:27
Hi,
#5985. What does the noun havoc mean?
#5986. What does the noun haystack mean?
#9 Re: Jai Ganesh's Puzzles » Doc, Doc! » Yesterday 15:12:48
Hi,
#2589. What does the medical term Episcleral layer mean?
#10 Re: Jai Ganesh's Puzzles » 10 second questions » Yesterday 14:48:16
Hi,
#9875.
#11 Re: Jai Ganesh's Puzzles » Oral puzzles » Yesterday 14:36:26
Hi,
#6368.
#12 Re: Exercises » Compute the solution: » Yesterday 14:10:48
Hi,
2729.
#13 Re: This is Cool » Miscellany » Yesterday 00:08:41
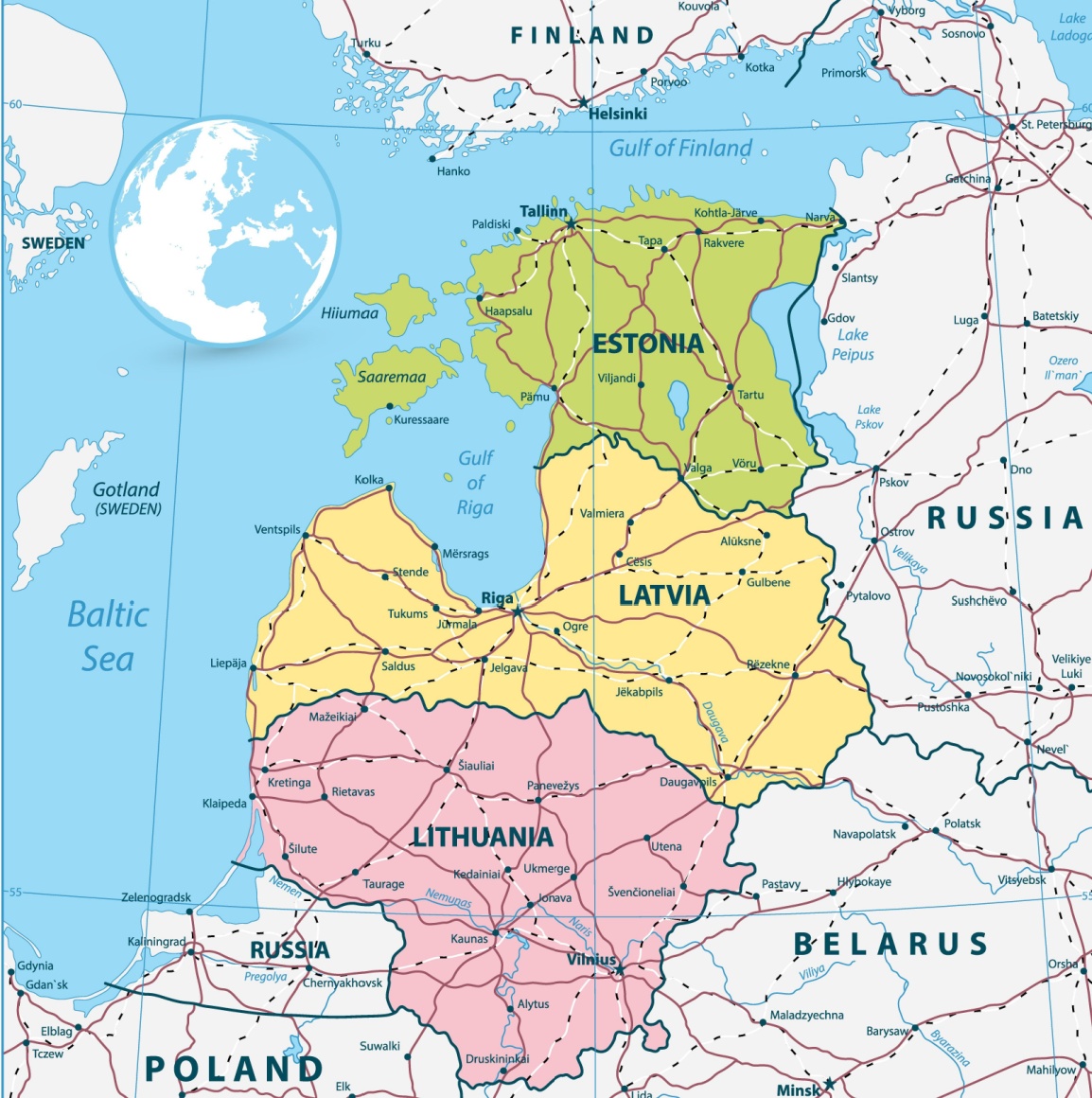
2515) Baltic States
Gist
The Baltic states—Estonia, Latvia, and Lithuania—are three sovereign nations in Northern Europe on the eastern coast of the Baltic Sea. Gaining independence from the Soviet Union in 1991, they are now parliamentary republics, members of the EU, NATO, and the OECD, featuring high-income economies, high living standards, and a shared history.
The Baltic States receive their name from the Baltic Sea, which borders them. Lithuania, Estonia, and Latvia are bounded on the west and north by the Baltic Sea.
Summary
The Baltic states[a] or the Baltic countries is a geopolitical term encompassing Estonia, Latvia, and Lithuania. All three countries are members of NATO, the European Union, the Eurozone, and the OECD. The three sovereign states on the eastern coast of the Baltic Sea are sometimes referred to as the "Baltic nations", less often and in historical circumstances also as the "Baltic republics", the "Baltic lands", or simply the Baltics.
All three Baltic countries are classified as high-income economies by the World Bank and maintain a very high Human Development Index. The three governments engage in intergovernmental and parliamentary cooperation. There is also frequent cooperation in foreign and security policy, defence, energy, and transportation.
NATO: North Atlantic Treaty Organization.
OECD: Organisation for Economic Co-operation and Development.
Geography
The Baltic States cover an area of 175,228 square kilometres (67,656 sq mi) (roughly twice the size of mainland Portugal), with a population of 6,132,500 (2024). Bordered by the Baltic Sea to the west and the north, they share borders with Russia, Belarus, and Poland. The Kaliningrad Oblast, formerly known as Königsberg in Germany, is landlocked between Lithuania and Poland and belongs to Russia.
The terrain of this region is relatively flat, punctuated by numerous lakes and ponds, particularly in the north, and hills in Lithuania.
General statistics
All three are unitary republics, which simultaneously joined the European Union on 1 May 2004, share EET/EEST time zone schedules and the euro currency.
EET: Eastern European Time.
EEST: Eastern European Summer Time.
Details
Baltic states are the northeastern region of Europe containing the countries of Estonia, Latvia, and Lithuania, on the eastern shores of the Baltic Sea.
The Baltic states are bounded on the west and north by the Baltic Sea, which gives the region its name, on the east by Russia, on the southeast by Belarus, and on the southwest by Poland and an exclave of Russia. The underlying geology is sandstone, shale, and limestone, evidenced by hilly uplands that alternate with low-lying plains and bear mute testimony to the impact of the glacial era. In fact, glacial deposits in the form of eskers, moraines, and drumlins occur in profusion and tend to disrupt the drainage pattern, which results in frequent flooding. The Baltic region is dotted with more than 7,000 lakes and countless peat bogs, swamps, and marshes. A multitude of rivers, notably the Neman (Lithuanian: Nemunas) and Western Dvina (Latvian: Daugava), empty northwestward into the Baltic Sea.
The climate is cool and damp, with greater rainfall in the interior uplands than along the coast. Temperatures are moderate in comparison with other areas of the East European Plain, such as in neighbouring Russia. Despite its extensive agriculture, the Baltic region remains more than one-third forested. Trees that adapt to the often poorly drained soil are common, such as birches and conifers. Among the animals that inhabit the region are elk, boar, roe deer, wolves, hares, and badgers.
The Latvian and Lithuanian peoples speak languages belonging to the Baltic branch of the Indo-European linguistic family and are commonly known as Balts. The Estonian (and Livonian) peoples, who are considered Finnic peoples, speak languages of the Finno-Ugric family and constitute the core of the southern branch of the Baltic Finns. Culturally, the Estonians were strongly influenced by the Germans, and traces of the original Finnish culture have been preserved only in folklore. The Latvians also were considerably Germanized, and the majority of both the Estonians and the Latvians belong to the Lutheran church. However, most Lithuanians, associated historically with Poland, are Roman Catholic.
The vast majority of ethnic Estonians, Latvians, and Lithuanians live within the borders of their respective states. In all three countries virtually everyone among the titular nationalities speaks the native tongue as their first language, which is remarkable in light of the massive Russian immigration to the Baltic states during the second half of the 20th century. Initially, attempts to Russify the Baltic peoples were overt, but later they were moderated as Russian immigration soared and the sheer weight of the immigrant numbers simply served to promote this objective in less-blatant ways. Independence from the Soviet Union in 1991 allowed the Baltic states to place controls on immigration, and, in the decade following, the Russian presence in Baltic life diminished. At the beginning of the 21st century, the titular nationalities of Lithuania and Estonia accounted for about four-fifths and two-thirds of the countries’ populations, respectively, while ethnic Latvians made up just less than three-fifths of their nation’s population. Around this time, Poles eclipsed Russians as the largest minority in Lithuania. Urban dwellers constitute more than two-thirds of the region’s population, with the largest cities being Vilnius and Kaunas in southeastern Lithuania, the Latvian capital of Riga, and Tallinn on the northwestern coast of Estonia. Life expectancy in the Baltic states is comparatively low by European standards, as are the rates of natural increase, which were negative in all three countries at the beginning of the 21st century, owing in part to an aging population. Overall population fell in each of the Baltic states in the years following independence, primarily because of the return emigration of Russians to Russia, as well as other out-migration to western Europe and North America. In some cases, Russians took on the nationalities of their adopted Baltic countries and were thus counted among the ethnic majorities.
After the breakup of the Soviet Union, the Baltic states struggled to make a transition to a market economy from the system of Soviet national planning that had been in place since the end of World War II. A highly productive region for the former U.S.S.R., the Baltic states catered to economies of scale in output and regional specialization in industry—for example, manufacturing electric motors, machine tools, and radio receivers. Latvia, for example, was a leading producer of Soviet radio receivers. Throughout the 1990s privatization accelerated, national currencies were reintroduced, and non-Russian foreign investment increased.
Agriculture remains important to the Baltic economy, with potatoes, cereal grains, and fodder crops produced and dairy cattle and pigs raised. Timbering and fisheries enjoy modest success. The Baltic region is not rich in natural resources. Though Estonia is an important producer of oil shale, a large share of mineral and energy resources is imported. Low energy supplies, inflationary prices, and an economic collapse in Russia contributed to an energy crisis in the Baltics in the 1990s. Industry in the Baltic states is prominent, especially the production of food and beverages, textiles, wood products, and electronics and the traditional stalwarts of machine building and metal fabricating. The three states have the highest productivity of the former constituent republics of the Soviet Union.
Shortly after attaining independence, Estonia, Latvia, and Lithuania abandoned the Russian ruble in favour of new domestic currencies (the kroon, lats, and litas, respectively), which, as they strengthened, greatly improved foreign trade. The main trading partners outside the region are Russia, Germany, Finland, and Sweden. The financial stability of the Baltic nations was an important prerequisite to their entering the European Union and the North Atlantic Treaty Organization in 2004. Estonia, Latvia, and Lithuania each adopted the euro as its common currency in 2011, 2014, and 2015, respectively.
This article covers the history of the region from antiquity to the post-Soviet period. Additional information on the region’s physical and human geography can be found in the article Europe. Area 67,612 square miles (175,116 square km). Pop. (2020 est.) 5,841,000.
Additional Information:
What are the Baltic States?
The term Baltic States refers to a group of independent countries that border the Baltic Sea. The Baltic States are composed of three countries: Lithuania, Estonia, and Latvia. Combined, the countries are no more significant in size than the state of Missouri.
The Baltic States or Baltic countries are located in the northeastern region of Europe. These countries gained independence from the Russian Empire in the 1910s and regained independence from the Soviet Union in the 1990s.
While the population of the Baltic states is quite small, each country has a major city as its capital: Tallinn in Estonia; Rīga in Latvia; and Vilnius in Lithuania. Each city has an old town, with historic buildings reflecting their German heritage. Rīga also has a large number of art nouveau buildings. The legacy of the Soviet Union is gradually being erased, although many Soviet-era apartment blocks remain. The capital cities are the seat of government, with many administrative offices in them. Economic activity is intense: Rīga and Tallinn are both important port cities, while Vilnius has emerged as a major financial center. In recent years, the Baltic capitals have become tourist destinations as foreigners have come to appreciate their attractions.
Song Traditions
One of the most significant cultural traditions in the Baltic states is the singing of folk songs, of which there are thousands. Traditional dance is also important. Children begin to learn singing and dance at an early age, often becoming part of ensembles that perform publicly. Mass song and dance festivals, involving tens of thousands of people, are held on a regular basis, with a great deal of audience participation. The importance of these festivals has been officially recognized by UNESCO, which declared them to be Masterpieces of the Oral and Intangible Heritage of Humanity. This year, many festivities, including folk singing and dancing, will be held in all three Baltic states as part of the celebration of the 100th anniversary of independence for Estonia, Latvia, and Lithuania. The tradition of song and dance festivals has been maintained by Baltic communities in the United States, Canada, and elsewhere.
Celebrating Nature
A reverence for nature has always been at the heart of traditional Baltic culture, and it continues in the present day. The celebration of the mid-summer solstice is one of the most important holidays on the calendar, and all three countries basically shut down for two days. People go to the countryside, where they build bonfires and sing songs until late in the night. The natural splendor of the Baltic countries was endangered by pollution and development in the Soviet period, but now environmental protection is a high priority. The region’s natural beauty is a primary attraction for visitors from all over the world.
Innovation and Development
The traditional economies of the Baltic countries were based on timber, dairy products, and agriculture. These all remain important, but after the end of the Soviet period, Estonia, Latvia, and Lithuania have undergone a major economic transformation as new sectors have been developed in the areas of computer software, electronics, and ICT (Information and Communications Technology) products. Of the three Baltic states, Estonia has taken the lead in this process, with its development of commercial services such as Skype and governmental programs related to building e-society infrastructure. Latvia and Lithuania have also invested heavily in the electronics sphere. All three Baltic countries have developed close connections with Silicon Valley, with many Baltic companies basing their operations here. It is likely that e-commerce will continue to grow in the Baltic states as their highly educated professional classes make more innovations.
#14 Re: Dark Discussions at Cafe Infinity » crème de la crème » Yesterday 00:07:46
2452) Frederick Chapman Robbins
Gist:
Work
Many infectious diseases are caused by viruses—very small biological particles. A virus lacks metabolism of its own and cannot multiply without infecting a living cell. For a long time the prevailing opinion was that viruses could not be cultured in a laboratory. However, in 1949 Thomas Weller, John Enders, and Frederick Robbins succeeded in culturing the virus that causes polio in human muscle and tissue in a laboratory setting. This became an important step on the road toward a vaccine against polio.
Summary
Frederick Chapman Robbins (born August 25, 1916, Auburn, Alabama, U.S.—died August 4, 2003, Cleveland, Ohio) was an American pediatrician and virologist who received (with John Enders and Thomas Weller) the 1954 Nobel Prize for Physiology or Medicine for successfully cultivating poliomyelitis virus in tissue cultures. This accomplishment made possible the production of polio vaccines, the development of sophisticated diagnostic methods, and the isolation of new viruses.
A graduate of Harvard University Medical School (1940), Robbins served in the United States, Italy, and North Africa during World War II (1942–46) as chief of the U.S. Army’s 15th medical general laboratory virus and rickettsia section, where he investigated epidemics of infectious hepatitis, typhus, and Q fever.
After joining Enders and Weller at the Children’s Hospital in Boston in 1948, Robbins helped solve the difficult problem of propagating viruses—then known to grow only in living organisms—in laboratory suspensions of actively metabolizing cells in nutrient solutions. At that time it was believed that the virus responsible for poliomyelitis grew and multiplied only in mammalian nerve tissue, which is highly difficult to maintain outside the living animal. By 1952 Robbins and his colleagues had succeeded in cultivating the virus in mixtures of human embryonic skin and muscle tissue suspended in cell cultures, dramatically demonstrating that the polio virus subsists in extraneural tissue, only later attacking the lower part of the brain and sections of the spinal cord.
Robbins served as director of the department of pediatrics and contagious diseases at the Cleveland Metropolitan General Hospital (1952–66) and as professor of pediatrics (1952–80) and dean (1966–80) at the Case Western Reserve University School of Medicine, Cleveland, Ohio. He later served as president of the Institute of Medicine of the National Academy of Sciences (1980–85).
Details
Frederick Chapman Robbins (August 25, 1916 – August 4, 2003) was an American pediatrician and virologist. He was born in Auburn, Alabama, and grew up in Columbia, Missouri, attending David H. Hickman High School.
He received the Nobel Prize in Physiology or Medicine in 1954 along with John Franklin Enders and Thomas Huckle Weller, making Robbins the only Nobel laureate born in Alabama. The award was for breakthrough work in isolating and growing the poliovirus in tissue culture, paving the way for vaccines developed by Jonas Salk and Albert Sabin. He attended the University of Missouri and Harvard University.
In 1952, he was appointed professor of pediatrics at Case Western Reserve University. Robbins was elected a fellow of the American Academy of Arts and Sciences in 1962. From 1966 to 1980, Robbins was dean of the School of Medicine at Case Western. He was elected to the American Philosophical Society in 1972. In 1980, he assumed the presidency of the National Academy of Sciences' Institute of Medicine. He had been a member of the National Academy of Sciences since 1972. Five years later, in 1985, Robbins returned to Case Western Reserve as dean emeritus and distinguished university professor emeritus. He continued to be a fixture at the medical school until his death in 2003. The medical school's Frederick C. Robbins Society is named in his honor. His wife, Alice N. Robbins, died in 2016. She was the daughter of Nobel laureate John Howard Northrop.
Robbins received the Benjamin Franklin Medal for Distinguished Achievement in the Sciences of the American Philosophical Society in 1999.

#15 Jokes » Miscellaneous Food Jokes - IV » Yesterday 00:06:46
- Jai Ganesh
- Replies: 0
Q: What kind of witch crafts food?
A: A sand-witch.
* * *
Q: How does butter play baseball?
A: It butters up.
* * *
Q: Why do they call it Fast Food?
A: Because if you don't eat it really fast, you might actually taste it.
* * *
Q: What happens when you use pickles for a ping pong game?
A: You get a volley of the Dills.
* * *
#16 Dark Discussions at Cafe Infinity » Come and Go Quotes » Yesterday 00:06:19
- Jai Ganesh
- Replies: 0
Come and Go Quotes
1. Yes, you can lose somebody overnight, yes, your whole life can be turned upside down. Life is short. It can come and go like a feather in the wind. - Shania Twain
2. I don't give up. I'm a plodder. People come and go, but I stay the course. - Kevin Costner
3. I like having the dough to come and go as I please. - Bruce Willis
4. People say that artists come and go but let me tell you I am not an artist. I am first a human being and if you are a good human being, you have achieved everything no matter how big actor you are. - Dharmendra
5. People come and go in your life. It is up to you choose how you want to associate with that person. It is up to you to learn and imbibe things from such a person. - Rekha
6. The role has to excite me as an actor. It is like a train journey: you know where you have to reach, and all things that happen in between are like stations that come and go. Of course, there are films like 'Welcome,' which I did only for money. - Nana Patekar
7. Money and other tangibles will come and go; they shouldn't be the foundation of your marriage. - Hema Malini
8. I see people every day who think they're the be all and end all of the industry. I've seen so many people come and go, but the industry doesn't revolve around one person. - Farah Khan.
#17 This is Cool » Detergent » 2026-03-07 17:57:05
- Jai Ganesh
- Replies: 0
Detergent
Gist
A detergent is a cleaning agent, often called a "surfactant," that uses molecules with both water-loving (polar) heads and oil-loving (non-polar) tails to lift dirt, grease, and oil from surfaces, suspending them in water so they can be rinsed away. Unlike traditional soaps, detergents are synthetic, work effectively in hard water, and are formulated into various products like laundry liquids, dishwasher tabs, and shampoos, containing additional ingredients for foaming, softening, or scent
Detergent products on the market can be categorized into powder detergents, liquid detergents, detergent bars, detergent pods, etc. The primary purpose is to remove dirt, stains, sweat, oils, pathogens, and other contaminants from fabrics, leaving them clean, fresh, and often pleasantly scented.
Summary
A detergent is a product for cleaning that contains surfactants plus other components. Detergents comprise surfactants as main functional components to remove hydrophobic grease or dirt by dispersing them in water. They often further comprise water (to facilitate application), builders (to soften water), enzymes (for breaking down proteins, fats, or starches), and dyes or fragrances (to improve the user's sensory experience).
Common surfactants used in detergents are alkylbenzene sulfonates, which are soap-like compounds that are more soluble than soap in hard water, because the polar sulfonate is less likely than the polar carboxylate of soap to bind to calcium and other ions found in hard water.
Definitions
The word detergent is derived from the Latin adjective detergens, from the verb detergere, meaning to wipe or polish off. Detergent can be defined as a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. However, conventionally, detergent is used to mean synthetic cleaning compounds as opposed to soap (a salt of the natural fatty acid), even though soap is also a detergent in the true sense. In domestic contexts, the term detergent refers to household cleaning products such as laundry detergent or dish detergent, which are in fact complex mixtures of different compounds, not all of which are by themselves detergents. Detergency is the ability to remove unwanted substances termed 'soils' from a substrate (e.g., clothing).
Details
Key Takeaways:
* Laundry detergents clean fabrics. Surfactants are the key ingredient that allows detergents to remove dirt and stains by interacting with both oil and water.
* Modern detergents contain a mixture of surfactants, enzymes and other ingredients to tackle various stains and fabric care needs.
* The choice between powder and liquid detergents often comes down to personal preference, although environmental considerations and packaging waste are increasingly important factors for consumers.
Nothing beats the feeling of putting on a clean T-shirt, especially after a shower. It leaves you feeling fresh and ready to take on the day. And where do these clean T-shirts come from? Ah, yes -- the laundry, that household chore that never seems to go off of your to-do list. Even the numbers agree: The average American family does about 300 loads of laundry per year [source: Wall Street Journal].
You may find yourself constantly putting clothes and sheets in the wash because, put simply, people are dirty. We sweat, shed skin cells and come into contact with food, dirt and many more particles every day. Consequently, we need a way to effectively get clothes and fabrics clean to maintain personal hygiene and keep up the appearance of garments. But what exactly is going on in that washing machine to get our clothes and fabrics clean? The secret is laundry detergent.
Prior to the invention of laundry detergents, Americans used soap flakes to wash clothes. However, in the 1930s, the first laundry detergent, Dreft by Procter & Gamble, hit the market. Later, in 1943, Procter & Gamble produced Tide, which could get out tougher stains due to the use of some very unique chemical ingredients. Since the 1930s and 1940s, laundry detergent has become a household necessity that is used around the world to clean fabrics. So how exactly do laundry detergents get your clothes clean? In this article we are going to get to the bottom of what happens when detergents enter your washing machine.
Surfactants: Laundry Detergent's Cleaning Power
All laundry detergent ingredients have a job to do, but the one group that's really crucial to getting your clothes clean are surfactants. The word surfactant stems from the combination of words "surface-active agents." Surface-active agents get their name from their unique chemical structure, which allows them to interact with two different types of surfaces, such as oil and water. The tail of a surfactant molecule is hydrophobic, or not attracted to water. What the hydrophobic end is attracted to is grease and dirt. The head of the surfactant molecule, on the other hand, is hydrophilic -- it's attracted to water [source: Silberberg].
So when a greasy piece of clothing is immersed in water with detergents containing surfactant, the tail of the surfactant molecules attach to the grease, and the head end of the molecule is attracted to the water. When the washing machine agitates the clothes, the molecules form tiny spheres, which stay suspended in the water and are rinsed away when the water is drained. Therefore, the prime benefit of surfactants is their ability to draw grime out of clothing while making sure it doesn't return to the fabrics.
Essentially, there are four main types of surfactants, with the first three used the most in laundry detergents, and their actions depend on their interactions with ions. Ions are charged particles due to the gain or loss of electrons. Ions can be positive such as calcium, Ca2+, or negative such as chloride, Cl-.
* Anionic surfactants are negatively charged in solution. However, they do not work as well by themselves in hard water. This is because hard water has many positively charged ions presents such as calcium (Ca2+) and magnesium (Mg2+). Since anionic surfactants are negative they are attracted to the positive ions and bind, making them unable to bind to other molecules in solution.
* Nonionic surfactants have no charge. Therefore, they are not as easily impaired under hard water conditions, since they are not attracted to the positive ions.
* Cationic surfactants are positively charged in solution. They help the anionic surfactant molecules pack in at the water/dirt interface thereby allowing the anionic surfactants to pull more dirt away.
* Amphoteric or zwitterionic surfactants are both positively and negatively charged. These surfactants are very mild and are often found in gentler cleansers such as hand soaps, shampoos and cosmetics. [source: Silberberg].
Additional Components of Laundry Detergent
Although surfactants are at the heart of laundry detergent's ability to clean fabrics, other ingredients can help detergents clean better, brighten clothes or smell better. As described previously, some types of surfactants typically do not work well in hard water due to the excess positive ions present. Additives called builders can help detergents to work better under hard water conditions. Builders accomplish this feat by removing calcium (Ca2+) and magnesium (Mg2+) ions in hard water by binding to them. This allows the surfactants, especially anionic surfactants, to bind to more grime, rather than the positively charged ions in the wash water. Builders also are bases, so they work to neutralize acid and can help disrupt chemical bonds. Another benefit of adding builders to laundry detergents is that manufacturers can use less surfactant, since the builders make the surfactant more efficient. Some examples of builders include sodium tripolyphosphate (STTP) and zeolites [source: EPA].
Detergents can also include components that make clothes whiter or brighter. The most common whitening agents are bleaches. Bleaches contain peroxides, which can oxidize fabrics [source: EPA]. Fluorescent whiteners and brighteners are also added to some laundry detergents because they minimize the yellowing of fabrics. These additives work by absorbing ultraviolet light and emitting back visible blue light, which can mask the yellow that may make colors appear faded and whites appear dingy.
Enzymes are naturally occurring biologic agents present in many detergents in varying concentrations. These enzymes are typically classified into the following categories and are similar to the enzymes used by your body to digest food:
Proteases: help break down proteins
Lipases: help break down fat
Amylases: help break down starches [source: Basketter]
These enzymes help break down food particles that are present on clothing by catalyzing, or speeding up, the decomposition process. A point to consider is that enzymes are biological products that can break down over time. Therefore, detergents can also contain enzyme stabilizers, which protect the enzymes and help them function.
Some other components include fragrance and coloring, which give laundry detergents their distinctive scents and appearance. Detergents sometimes contain trace amounts of dye, which is not enough to dye your actual clothing. However, on top of making your laundry detergent more visually appealing, dyes can show you when there is still detergent left on your clothes after the wash cycle.
Lastly, fillers help dilute and distribute the active ingredients to their proper dosages. Powder and liquid detergents use different fillers. The major filler in powder detergents is sodium sulphate, which provides the granular powdery texture. The primary filler in liquid detergents is water.
Powder vs. Liquid Detergents
Laundry detergent manufacturers have come a long way since the first box of Tide was produced more than 60 years ago. Currently, the two main types of laundry detergent are powders and liquids. For the most part, powder and liquid detergents share the same active ingredients except for the filler used. Additionally, powder and liquid detergents both have pros and cons, and since they have similar cleaning power, people usually choose which type to use based on personal preference.
Here are some of the advantages and disadvantages of using powdered detergents:
Pro: They're generally cheaper.
Pro: The cardboard packaging is more eco-friendly.
Con: Some people think they don't dissolve as well in water. This may have been a problem with some of the first powdered detergents, but these days, most powders are designed to readily dissolve in water.
Con: Sodium sulphate can wreak havoc on septic systems.
Con: Powders contain more chemicals compared with liquids, due to the filler.
People may or may not use liquid detergents for an entirely different set of reasons:
Pro: The detergent is already pre-dissolved.
Pro: You can pre-treat stains by pouring it directly onto clothes.
Con: They're usually more expensive than powdered detergent.
Con: They have plastic packaging, which is less eco-friendly.
Environmental Considerations with Laundry Detergent
Even though detergents do a tremendous job of getting rid of the dirt and grime in our fabrics, at what cost does this come? Considering the toxicities of their chemical ingredients and carbon cost of production, it's not surprising that some people have concerns about the impacts of laundry detergents on the environment.
Their carbon footprint alone is significant by many people's standards. Carbon footprints are an indicator of the amount of carbon dioxide (CO2) produced while making, shipping and using a product. According to the Wall Street Journal, the carbon footprint of using UK detergent brand Tesco, varies from 1.3 pounds (0.6 kilograms) to 1.9 pounds (0.9 kilograms) per load, depending on the form of the detergent that's used. To put this in perspective, it is estimated that for every mile an average car travels, 1 pound (0.5 kilograms) of CO2 is emitted. Recall that American families on average do 300 loads of laundry per year. This means that the carbon footprint of laundry detergents for one year of laundry is approximately 480 pounds (218 kilograms) per year, or about 10 pounds (4.5 kilograms) per week. So, while this may not seem like a lot, especially if your car produces about 5 tons of CO2 per year, this number only reflects the laundry detergent. It does not factor in the extra energy requirements of running the washer and dryer [source: Wall Street Journal].
Now, add to that the toxic effects of the chemical components in detergents. According to the EPA, some of the major concerns about the chemical ingredients used in laundry detergents include the following:
* Toxicity to aquatic organisms and algae
* Persistence in the environment
* Eutrophication of fresh water, particularly by phosphate-based detergents (now, phosphates have been replaced by zeolites which may be alleviating this problem)
* Health problems in people, such as cancer [source: EPA]
Another concern relating to laundry detergent is that it can make the wash water acidic, and depending on where that water runs to, it could further impact the environment, having effects similar to acid rain.
Green Laundry Detergent Options
Given some of these environmental considerations about laundry detergents, there are some greener options available to today's consumer. Most detergents marketed as environmentally friendly don't include perfumes or dyes, and they're typically phosphate free, biodegradable, and they haven't been tested on animals.
One eco-conscious option is detergent designed to work well in cold water. On average, 80 to 85 percent of the total energy used washing a load of clothes goes to heating up the water [source: Sabaliunas et al.]. Washing in cold water saves energy, which can translate to savings on your household energy bills, too.
Another environmental approach is to use concentrated formulas, which cuts down on packaging and on the amount of water it takes to make the detergent. According to Proctor & Gamble spokeswoman Carol Berning, concentrated detergents require "less plastic for bottles, less corrugated cardboard for crating, and less gasoline used, because we need less trucks to move the shipments" [source: Consumer Reports]. The cold water and concentrated options that different companies manufacture may be one step to greener washing practices. However, even in these forms, the detergents still contain some potentially environmentally hazardous chemicals.
An additional green choice -- for the benefit of the environment and you wallet -- could be making your own laundry detergents. There are a variety of recipes out there, with the common ingredients of water, bar soap, borax and washing soda. Some environmental benefits of making your own laundry detergent are that they typically use fewer chemicals and additives, and they can save on packaging. However, be aware that clothing washed with homemade detergent may also require bleaching, and it may not get stains out as well as some of the commercially produced detergents.
Clearly, detergents are chemically complex products that are continually being improved upon, whether it is boosting their stain-fighting powers or making them greener.
Additional Information
Soap and detergent are substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as the human skin, textiles, and other solids. When soap and water are not available for hand washing or when repeated hand washing compromises the natural skin barrier (e.g., causing scaling or fissures to develop in the skin), hand sanitizers—coming in foam, gel, or liquid form—have been recommended.
The seemingly simple process of cleaning a soiled surface is, in fact, complex and consists of the following physical-chemical steps:
* Wetting of the surface and, in the case of textiles, penetration of the fibre structure by wash liquor containing the detergent. Detergents (and other surface-active agents) increase the spreading and wetting ability of water by reducing its surface tension—that is, the affinity its molecules have for each other in preference to the molecules of the material to be washed.
* Absorption of a layer of the soap or detergent at the interfaces between the water and the surface to be washed and between the water and the soil. In the case of ionic surface-active agents (explained below), the layer formed is ionic (electrically polar) in nature.
* Dispersion of soil from the fibre or other material into the wash water. This step is facilitated by mechanical agitation and high temperature; in the case of hand soap, soil is dispersed in the foam formed by mechanical action of the hands.
* Preventing the soil from being deposited again onto the surface cleaned. The soap or detergent accomplishes this by suspending the dirt in a protective colloid, sometimes with the aid of special additives. In a great many soiled surfaces the dirt is bound to the surface by a thin film of oil or grease. The cleaning of such surfaces involves the displacement of this film by the detergent solution, which is in turn washed away by rinse waters. The oil film breaks up and separates into individual droplets under the influence of the detergent solution. Proteinic stains, such as egg, milk, and blood, are difficult to remove by detergent action alone. The proteinic stain is nonsoluble in water, adheres strongly to the fibre, and prevents the penetration of the detergent. By using proteolytic enzymes (enzymes able to break down proteins) together with detergents, the proteinic substance can be made water-soluble or at least water-permeable, permitting the detergent to act and the proteinic stain to be dispersed together with the oily dirt. The enzymes may present a toxic hazard to some persons habitually exposed.
If detached oil droplets and dirt particles did not become suspended in the detergent solution in a stable and highly dispersed condition, they would be inclined to flocculate, or coalesce into aggregates large enough to be redeposited on the cleansed surface. In the washing of fabrics and similar materials, small oil droplets or fine, deflocculated dirt particles are more easily carried through interstices in the material than are relatively large ones. The action of the detergent in maintaining the dirt in a highly dispersed condition is therefore important in preventing retention of detached dirt by the fabric.
In order to perform as detergents (surface-active agents), soaps and detergents must have certain chemical structures: their molecules must contain a hydrophobic (water-insoluble) part, such as a fatty acid or a rather long chain carbon group, such as fatty alcohols or alkylbenzene. The molecule must also contain a hydrophilic (water-soluble) group, such as ―COONa, or a sulfo group, such as ―OSO3Na or ―SO3Na (such as in fatty alcohol sulfate or alkylbenzene sulfonate), or a long ethylene oxide chain in nonionic synthetic detergents. This hydrophilic part makes the molecule soluble in water. In general, the hydrophobic part of the molecule attaches itself to the solid or fibre and onto the soil, and the hydrophilic part attaches itself to the water.
Four groups of surface-active agents are distinguished:
* Anionic detergents (including soap and the largest portion of modern synthetic detergents), which produce electrically negative colloidal ions in solution.
* Cationic detergents, which produce electrically positive ions in solution.
* Nonionic detergents, which produce electrically neutral colloidal particles in solution.
* Ampholytic, or amphoteric, detergents, which are capable of acting either as anionic or cationic detergents in solution depending on the pH (acidity or alkalinity) of the solution.
The first detergent (or surface-active agent) was soap. In a strictly chemical sense, any compound formed by the reaction of a water-insoluble fatty acid with an organic base or an alkali metal may be called a soap. Practically, however, the soap industry is concerned mainly with those water-soluble soaps that result from the interaction between fatty acids and alkali metals. In certain cases, however, the salts of fatty acids with ammonia or with triethanolamine are also used, as in shaving preparations.

#18 Science HQ » Ulna (Bone) » 2026-03-07 17:10:00
- Jai Ganesh
- Replies: 0
Ulna (Bone)
Gist
The ulna is the longer, larger medial bone of the forearm, running parallel to the radius from the elbow to the wrist. It acts as a stable hinge for the elbow via the olecranon process and facilitates forearm rotation. It is essential for elbow stability, forearm rotation (pronation/supination), and serves as a major site for muscle attachment.
What is the ulna? The ulna is the longer of the two bones in your forearm. It helps you move your arm, wrist and hand. Your ulna also supports lots of important muscles, tendons, ligaments and blood vessels.
Summary
The ulna or ulnar bone (pl.: ulnae or ulnas) is a long bone in the forearm stretching from the elbow to the wrist. It is on the same side of the forearm as the little finger, running parallel to the radius, the forearm's other long bone. Longer and thinner than the radius, the ulna is considered to be the smaller long bone of the lower arm. The corresponding bone in the lower leg is the fibula.
Structure
The ulna is a long bone found in the forearm that stretches from the elbow to the wrist, and when in standard anatomical position, is found on the medial side of the forearm. It is broader close to the elbow, and narrows as it approaches the wrist.
Close to the elbow, the ulna has a bony process, the olecranon process, a hook-like structure that fits into the olecranon fossa of the humerus. This prevents hyperextension and forms a hinge joint with the trochlea of the humerus. There is also a radial notch for the head of the radius, and the ulnar tuberosity to which muscles attach.
Close to the wrist, the ulna has a styloid process.
Details:
Description
The ulna is one of two bones that make up the forearm, the other being the radius. It forms the elbow joint with the humerus and also articulates with the radius both proximally and distally. It is located in the medial forearm when the arm is in the anatomical position. It is the larger of the two forearm bones. Ulna assists in pronation and supination of the forearm and hand.
Structure
The ulna is a long bone larger proximally than distally.
Proximal ulna
The proximal ulna is hook-like in form which articulates with the trochlea of the humerus to create the hinge joint of the Elbow.
The articulation is formed of the olecranon and the coronoid process.
Olecranon
This is a large, curved bony prominence which is accepted into the olecranon fossa, located on the humerus, during elbow extension.
The olecranon forms the upper part of the semi-lunar notch which is a smooth, large depression and articulates with the humeral trochlea during elbow flexion and extension.
Coronoid process
The coronoid process is a horizontal, bony projection which attaches directly onto the ulnar shaft. It is received into the coronoid fossa of the humerus in elbow flexion. The coronoid process also forms the lower part of the semi-lunar notch.
On the lateral side of the coronoid process is the radial notch where the head of the radius sits.
Head of the ulna
The lateral, distal end of the ulna is the head of the ulna. It articulates with the ulnar notch on the radius and with the triangular articular disc in the Wrist Joint.
Shaft of the Ulna
The shaft of ulna is triangular in shape and it consists of three borders and three surfaces. It's width is decreased as it moves towards distal end.
The three surfaces are:
* Anterior surface
* Posterior Surface
* Medial Surface
The three borders are:
* Interosseous Border
* Anterior Border
* Posterior Border
Distal End
The head of Ulna has convex articular surface on its lateral side in order to articulate with ulnar notch of radius. It forms the distal radio-ulnar joint.
The styloid process has attachment of ulnar collateral ligament.
Articulations:
Elbow
The ulna articulates with the humerus at its most proximal point forming the elbow in a hinge joint. It is the trochlea of the humerus which sits in the semi-lunar notch of the ulna to form this joint.
Radio-ulnar joints
The ulna articulates with the radius proximally and distally to produce pronation (from the proximal joint) and supination (from the distal joint) of the forearm.
The ulna also articulates with the radius in a syndesmosis joint via its interosseous membrane. Which runs the length of the shaft of the ulna from the radial notch proximally to the head of the ulna. This also acts to compartmentalise dorsal and volar sides of the forearm.
Muscle attachments:
Olecranon process
Triceps - inserts onto the posterior of the olecranon process.
Anconeus - inserts onto lateral aspect
Flexor Carpi Ulnaris - origin:posterior, also shares an origin from humeral medial epicondyle
Coronoid process
Brachialis - inserts to anterior, inferior coronoid process
Pronator teres - originates medial surface, also from humeral medial epicondyle
Flexor Digitorum Superficialis - originates medial surface, also from humeral medial epicondyle
Shaft of ulna
All of the following arise from the shaft of the ulna:
Flexor Digitorum Profundus
Pronator quadratus
Extensor carpi ulnaris - also shared with lateral epicondyle of humerus
Abductor pollicis longus - also originates from interosseous membrane
Extensor Pollicis Longus - also originates from interosseous membrane
Extensor indicis - also originates from interosseous membrane
Clinical relevance
Like any other joint in the body the ulna can be affected by osteoarthritis in the elbow joint.
Ulnar fractures
Typically occur as a result of fragility and normally following a fall such as a fall on an outstretched hand (FOOSH)
Monteggia fracture - fracture of the proximal 1/3 of the ulnar shaft accompanied by the dislocation of the radial head.
Hume fracture - fracture of the olecranon accompanied by anterior dislocation of the radial head.
Galezzi's fracture - fracture to the distal radius accompanied by ulnar head dislocation at distal radio-ulna joint.
Additional Information
The ulna is one of the bones in your forearm. It helps you move your arm, wrist and hand. If osteoporosis weakens your bones, you have an increased risk for fractures you might not even know about. Talk to a healthcare provider about a bone density test.
Overview:
The ulna is the longer of the two bones in your forearm.
What is the ulna?
The ulna is the longer of the two bones in your forearm. It helps you move your arm, wrist and hand. Your ulna also supports lots of important muscles, tendons, ligaments and blood vessels.
If you experience a fractured (broken) ulna, you might need surgery to repair your bone and physical therapy to help you regain your strength and ability to move.
Your ulna — like all bones — can be affected by osteoporosis.
Because your ulna is connected to so many muscles and nerves, injuries to one can often affect the others.
Function:
What does the ulna do?
Your ulna has several important jobs, including:
* Helping your forearm and wrist move, flex and rotate.
* Holding more than a dozen muscles in place.
* Stabilizing the rest of your arm, wrist and hand.
* Helping your elbow and wrist move.
Anatomy:
Where is the ulna located?
The ulna is one of two bones in your forearm. The other is your radius. The ulna is on the medial (pinkie) side of your forearm.
What does the ulna look like?
The ulna has a notched end where it meets your humerus (upper arm bone), a long shaft in the middle that’s slightly curved and a narrow end that meets your wrist. It’s slightly longer than the radius.
Even though it’s one long bone, your ulna is made up of several parts. These include:
Ulna proximal aspect
The upper (proximal) end of your ulna connects to your humerus. It forms the bony point of your elbow. The ulna’s proximal end (aspect) contains the:
* Olecranon.
* Trochlear notch.
* Coronoid process.
* Radial notch.
* Ulnar tuberosity.
Ulna shaft
The shaft is the long middle portion of the ulna that supports the weight of your forearm and gives it its shape.
Ulna distal aspect
The lower (distal) end of your ulna forms part of your wrist joint. It ends in a rounded bump called the styloid process — the bump above your wrist on your pinkie side. The distal end is sometimes referred to as the head of your ulna.
Your healthcare provider may use all these parts and labels as they describe where you’re having pain or issues. If you ever break your ulna (an ulnar fracture), your provider might use some of these terms to describe where your bone was damaged.
How big is the ulna?
The ulna is the second longest bone in your arm. It’s one of the longest bones in your body. Most adults’ ulna bones are around 11 inches (in.) long.
Conditions and Disorders:
What are the common conditions and disorders that affect the ulna?
The most common issues that affect the ulna are fractures, osteoporosis and damage to the ulnar nerve. Common conditions include:
* Osteoarthritis.
* Ulnar wrist pain.
* Ulnar nerve entrapment.
Ulna fractures
A bone fracture is the medical term for a broken bone. The most common causes of ulna fractures include:
* Sports injuries.
* Fall.
* Car accidents.
Symptoms of a fracture include:
* Pain.
* Swelling.
* Tenderness.
* Inability to move your arm like you usually can.
* Bruising or discoloration.
* A deformity or bump that’s not usually on your body.
Go to the emergency room right away if you’ve experienced trauma or think you have a fracture.
Osteoporosis
Osteoporosis weakens bones, making them more susceptible to sudden and unexpected fractures. It usually doesn’t cause any symptoms you can notice, so many people don’t know they have osteoporosis until after it causes them to break a bone.
Females and adults older than 65 have an increased risk for developing osteoporosis. Talk to your provider about a bone density test that can catch osteoporosis before it causes a fracture.
What tests are done on the ulna?
The most common test done to check the health of your ulna is a bone density test. It’s sometimes called a DEXA or DXA scan. A bone density test uses low levels of X-rays to measure how strong your bones are. It’s a way for your provider to track bone loss as you age.
If you’ve experienced a humeral fracture, your provider or surgeon might need imaging tests, including:
* X-rays.
* Magnetic Resonance Imaging (MRI).
* CT scan.
What are common treatments for the ulna?
Usually, your ulna won’t need treatment unless you’ve experienced a fracture or other injury to your arm. You might need treatment if you’ve been diagnosed with osteoporosis.
Ulna fracture treatment
How your fracture is treated depends on which type it is and what caused it. You’ll need some form of immobilization like a splint or cast. You might need surgery to realign (set) your bone to its correct position so it can heal.
Osteoporosis treatment
Treatments for osteoporosis can include exercise, vitamin and mineral supplements and medications.
Your provider will help you develop a treatment plan that’s customized for you and your bone health.

#19 Re: Jai Ganesh's Puzzles » General Quiz » 2026-03-07 16:24:47
Hi,
#10787. What does the term in Biology Histology mean?
#10788. What does the term in Biology Hodgkin–Huxley model mean?
#20 Re: Jai Ganesh's Puzzles » English language puzzles » 2026-03-07 16:12:08
Hi,
#5983. What does the verb (used with object) gall mean?
#5984. What does the noun galleon mean?
#21 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2026-03-07 15:56:26
Hi,
#2588. What does the medical term Peristalsis mean?
#22 Re: Jai Ganesh's Puzzles » 10 second questions » 2026-03-07 15:40:41
Hi,
#9874.
#23 Re: Jai Ganesh's Puzzles » Oral puzzles » 2026-03-07 15:27:56
Hi,
#6367.
#24 Re: Exercises » Compute the solution: » 2026-03-07 15:09:57
Hi,
2728.
#25 Re: This is Cool » Miscellany » 2026-03-07 00:03:52
2514) Arctic Wolf
Gist:
Do Arctic wolves still exist?
They were listed as vulnerable from 1982 to 1995, but their status was upgraded to least concern in 1996 and has remained the same ever since. Thanks to their remote, icy habitat, Arctic wolves live far away from any human populations.
Are Arctic wolves friendly to humans?
The Arctic wolf is relatively unafraid of people, and can be coaxed to approach people in some areas.
Summary
The Arctic fox (Vulpes lagopus), also known as the white fox, polar fox, or snow fox, is a small species of fox native to the Arctic regions of the Northern Hemisphere and common throughout the Arctic tundra. It is well adapted to living in cold environments, and is known for its thick, warm fur that can be used as camouflage against snow in the winter. It has a large and fluffy tail. In the wild, most individuals do not live past their first year but some exceptional ones survive up to 11 years. Its body length ranges from 46 to 68 cm (18 to 27 in), with a generally rounded body shape to minimize the escape of body heat.
The Arctic fox preys on many small creatures such as lemmings, voles, ringed seal pups, fish, waterfowl, and seabirds. It also eats carrion, berries, seaweed, and insects and other small invertebrates. Arctic foxes form monogamous pairs during the breeding season and they stay together to raise their young in complex underground dens. Occasionally, other family members may assist in raising their young. Natural predators of the Arctic fox include golden eagles, Arctic wolves, polar bears, wolverines, red foxes, and brown bears.
Details
The Arctic wolf (Canis lupus arctos), also known as the white wolf, polar wolf, and the Arctic grey wolf, is a subspecies of grey wolf native to the High Arctic tundra of Canada's Queen Elizabeth Islands, from Melville Island to Ellesmere Island. Unlike some populations that move between tundra and forest regions, Arctic wolves spend their entire lives north of the northern treeline. Their southward distribution is limited to the northern fringes of the Middle Arctic tundra on the southern half of Prince of Wales and Somerset Islands.
It is a medium-sized subspecies, distinguished from the northwestern wolf by its smaller size, whiter colouration, narrower braincase, and larger carnassials. Since 1930, there has been a progressive reduction in size in Arctic wolf skulls, which is likely the result of wolf-dog hybridization.
Taxonomy
In 1935, the British zoologist Reginald Pocock attributed the subspecies name Canis lupus arctos (Arctic wolf) to a specimen from Melville Island in the Queen Elizabeth Islands, Canada. He wrote that similar wolves could be found on Ellesmere Island. He also attributed the name Canis lupus orion to a Greenland wolf specimen from Cape York, northwest Greenland. Both wolves are recognized as separate subspecies of Canis lupus in the taxonomic authority Mammal Species of the World (2005).
A study by Chambers et al. (2012) using autosomal microsatellite DNA and Mitochondrial DNA data indicate that the Arctic wolf has no unique haplotypes which suggests that its colonization of the Arctic Archipelago from the North American mainland was relatively recent, and thus not sufficient to warrant subspecies status. During a meeting assembled in 2014 by the National Center for Ecological Analysis and Synthesis of the United States Fish and Wildlife Service, one speaker, Robert K. Wayne, mentioned he disagreed with the conclusion that a subspecies had to be genetically distinct, believing that different subspecies could slowly grade into each other - suggesting that although it was impossible to determine if an individual wolf was one subspecies or the next using DNA, the population of Arctic wolves as a whole could be distinguished by the looking at the proportions of single-nucleotide polymorphisms (SNP): i.e. Arctic wolves could be distinguished by having three wolves in the putative population with a specific SNP, whereas another subspecies could be distinguished by having 20 wolves with that SNP. Wayne furthermore stated that he believed the habitat in which the wolf happened to be found was a good enough characteristic to distinguish a subspecies.
Behaviour
The Arctic wolf is relatively unafraid of people, and can be coaxed to approach people in some areas. The wolves on Ellesmere Island do not fear humans, which is thought to be due to them seeing humans so little, and they will approach humans cautiously and curiously. Otto Sverdrup wrote that during the Fram expedition, a pair of wolves shadowed one of his teammates, who kept them at a distance by waving his ski pole. In 1977, a pair of scientists were approached by six wolves on Ellesmere Island, with one animal leaping at one of the scientists and grazing a cheek. A number of incidents involving aggressive wolves have occurred in Alert, Nunavut, where the wolves have lived in close proximity to the local weather station for decades and became habituated to humans. One of these wolves attacked 3 people, was shot, and tested positive for rabies.
Very little is known about the movement of the Arctic wolves, mainly due to climate. The only time at which the wolf migrates is during the wintertime when there is complete darkness for 24 hours. This makes Arctic wolf movement hard to research. About 2,250 km (1,400 mi) south of the High Arctic, a wolf movement study took place in the wintertime in complete darkness, when the temperature was as low as −53 °C (−63 °F). The researchers found that wolves prey mainly on the muskoxen. There is no available information of the wolves' movements where the muskoxen were.
Diet
In the wild, Arctic wolves primarily prey on muskoxen and Arctic hares. They have also been found to prey on lemmings, caribou, Arctic foxes, birds, and beetles. It has been also found that Arctic wolves scavenge through garbage. This sort of food source will not always be found in the Arctic wolf's diet because of regional and seasonal availability. There is some debate whether the muskox or the Arctic hare is the primary prey for the hare-wolf-muskox predator-prey system. Evidence suggests that muskoxen provide long-term viability and other ungulates do not appear in the wolf's diet. Evidence suggesting that Arctic wolves depend more on hares claims that the mature wolf population paralleled the increase of hares rather than muskoxen availability. However, the degree of reliance between the two sources of food is uncertain and the amount of consumption between the two species also depends on the season and year. Debate continues when seasonal variations and the diet of young wolves is discussed. According to one study, muskox calves serve as a primary food source because the needs of pups are greater but another study suggests that "when hares were much more plentiful (Mech, 2000), wolves commonly fed them to their pups during summer." These differences may be attributed to location as well. Polar bears are rarely encountered by wolves, though there are two records of wolf packs killing polar bear cubs.
Conservation
The Arctic wolf is least concern, but it does face threats. In 1997, there was a decline in the Arctic wolf population and its prey, muskoxen (Ovibos moschatus), and Arctic hares (Lepus arcticus). This was due to unfavourable weather conditions during the summers for four years. Arctic wolf populations recovered the next summer when weather conditions returned to normal.
Additional Information
Arctic wolves have white fur year-round which allows them to blend into their snowy surroundings. Their coat is long and silky with soft, thick under fur. This is shed in the spring and the coat becomes shorter and less dense. The lengthy tail is bushy, and the legs are long giving it a lanky appearance. The feet are large and digitate with non-retractable claws. The forefeet have five toes and the hind feet have four. The skull is broad and the face and ears are well defined. Ears are slightly rounded and the face is less pointed than other species of wolf. This wolf is a subspecies of the gray wolf (Canis lupus). The year-round white coats and slightly shorter ears and noses distinguish them from the other subspecies of Canis lupus. They are also slightly smaller in stature. Male Arctic wolves generally weigh between 34 – 46 kg, and females between 36 – 38 kg.
Distribution
This sub-species lives primarily in the Arctic, the region located above 67 degrees north latitude. This is the area along the northern edge of the North American continent and northward to the North Pole, as well as along the eastern and northern shores of Greenland.
Habitat
The land in the Arctic is covered with snow and ice for most of the year except for brief periods during the summer months. Due to scarcity of grazing plants and resulting low density of prey species, wolves roam over large areas hunting for food.
Diet
They are predatory carnivores. They hunt in packs for caribou and musk-oxen. They also consume Arctic hares, ptarmigan, lemmings, and other small animals including nesting birds.
Reproduction
These wolves live in groups of seven to ten individuals. There is a highly complex social order within wolf packs and each pack has a dominant male and female, who bond for life. Mating between the pair takes place during the breeding season of January through to March. The gestation period for the pregnant alpha female is from 53 to 61 days. Permafrost in the Arctic makes it difficult for the wolves to dig dens. Instead, their dens are often rock outcroppings, caves, or shallow depressions in the tundra soil. The mother gives birth to two or three pups in late May to early June. Litter size is smaller for Arctic wolves. Pups are born blind and deaf. They have soft, fuzzy dark hair with small, droopy ears and blunt muzzles. They are entirely dependent on their mother; she is the only one who has contact with them at this time. She in turn relies on her mate to bring her food. At about ten days the pups’ eyes open and at three weeks they can hear. After a month the pups are able to eat meat. From then on the whole pack shares the job of feeding them, bringing meat which they regurgitate for the pups. Each member of the pack will affectionately lick, nuzzle, and sniff each pup. They become caregivers - providing food, play, and protection. Pups respond with squeaks and tail wags. They nibble and lick the feeder’s muzzle to stimulate regurgitation. They leave the den after eight to ten weeks to discover the outside world. As pups they are already establishing, through play, their future roles within the pack. When the pack hunts, one adult member will remain as a puppy sitter. Pups have grown strong enough to travel at six months of age, and from that time will join the other members of the pack learning survival skills. They become sexually mature at two to three years of age.
Adaptation
Wolves communicate with each other in a variety of ways. Clear communication is a key element to the success of a cooperative pack.
Body language: They have a rich vocabulary of visual signs that communicate social rank, mood, and intentions. Subtle changes in tail and ear positions, of body and head angle, making and breaking eye contact, and facial expressions are just a few. Tail movements for example are related to various feelings: friendliness, aggression, fear, status, social tension, threat, and submission are all communicated via tail position.
Vocalization: Wolves howl for many reasons, to assemble the pack before and after hunts, to locate members of the pack over distances, to warn neighboring packs of their presence, and apparently just for the fun of it. They often howl at a rendezvous site. The wolf’s howl can be heard up to 5 km away. They also growl, snarl, whine, yip, whimper, and bark.
Signs: They use scent marking to communicate their presence and territory boundaries to other wolves. This can be either urine or feces left on rocks and snow banks along their hunting trails. Wolves have a very good sense of smell. They can detect prey 1.6 km away and can sense an animal three days after it has gone.
Wolves are very social animals and live in family packs for protection and for hunting.
Pack structure: There is an alpha pair in each pack. The alpha male is the leader. Only the alpha couple breeds and with only one litter per pack. The number of pups is low in relationship to the number of adults. Wolves have concern for other wolves in the pack, with the younger members feeding and protecting the older wolves. All adults co-operate in feeding and educating the young. Wolf pack members protect each other.
Hunting: Life in a pack is extremely important to the survival of Arctic wolves. They live in very harsh conditions. Hunting together allows them to kill larger prey including musk oxen and caribou. A successful hunt depends on the cooperative efforts of the entire pack.
Wolves demonstrate intelligence in choosing prey: they look for old, sick, or weak animals that are easier to catch. Wolves work hard for their prey; they kill only one of every ten large mammals they chase. They eat all of the catch, including the bones. They have 42 teeth. Four large canine teeth are used for hanging onto and biting through flesh. Molars at the back are specialized shearing teeth referred to as carnassials. When hunting alone a wolf catches smaller animals.
Arctic wolves are well adapted to icy conditions. White fur allows them to blend into snowy surroundings. To help reduce heat loss their ears are small and rounded, the muzzle is short, and the fur is dense. Legs are shorter than other subspecies. They have tufts of hair between the pads of their feet.
Threats to Survival
Polar bears sometimes prey on Arctic wolves. Global climate change may affect these wolves. Exploration of the natural resources such as oil and gas will no doubt have an impact.
