Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2024-04-28 17:34:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Shielding Effect
Shielding Effect
Summary
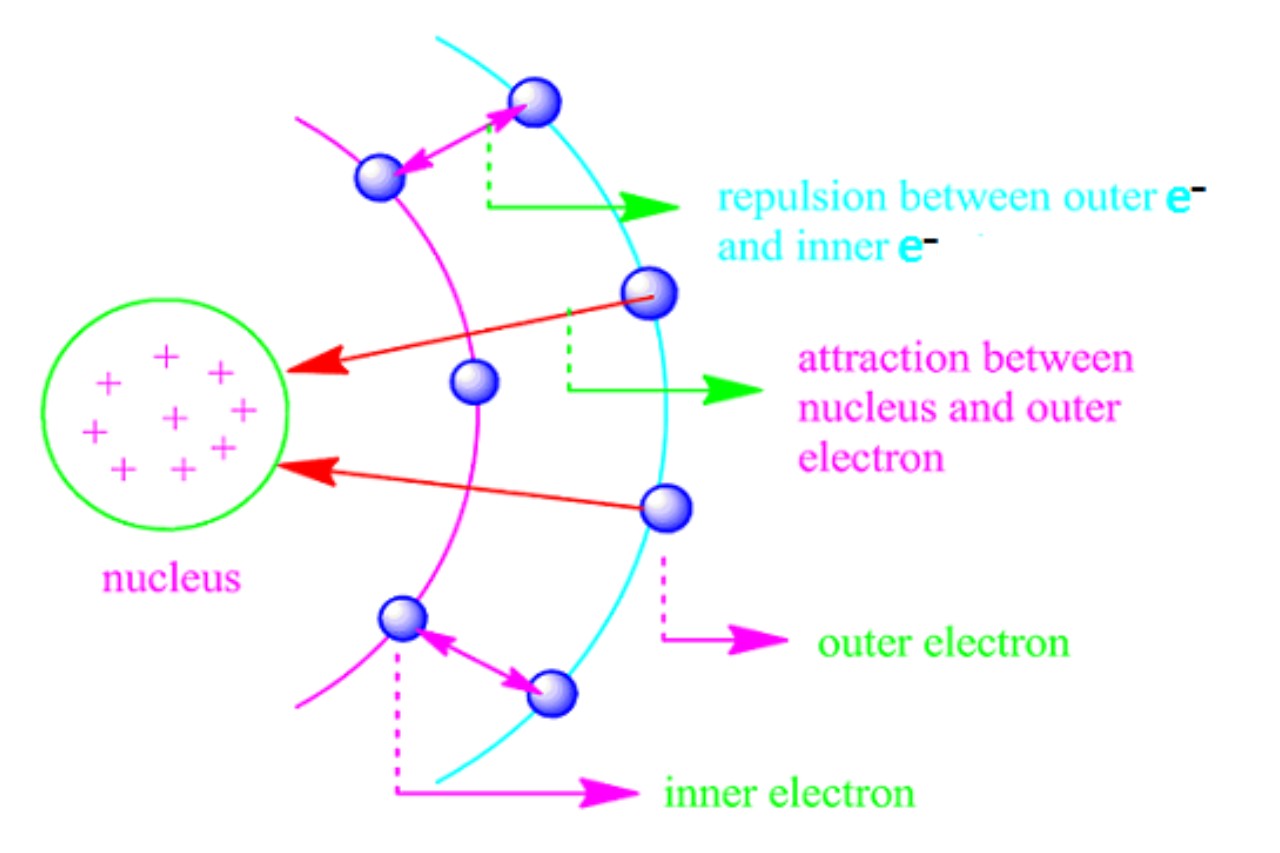
In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more than one electron. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces on the electrons in the atom. It is a special case of electric-field screening. This effect also has some significance in many projects in material sciences.
Details
Shielding Effect means the blocking of valence shell electrons attracted by the nucleus due to the presence of inner-shell electrons. It occurs when electrons closer to the nucleus “shield” electrons farther away from the positive charge of the nucleus, reducing the effective nuclear charge experienced by the valence electrons.
The shielding effect is significant in understanding trends in atomic properties, such as ionization energies and electronegativity. In this article, we look into what is a shielding effect, its definition, order, effective nuclear charge, formula, Slater’s rule, etc.
What is the Shielding Effect?
Shielding effect describes how electrons closer to the nucleus “shield” the electrons farther away from the positive charge of the nucleus. This phenomenon occurs because inner-shell electrons reduce the effective nuclear charge experienced by valence electrons. The more core electrons an atom has, the stronger the shielding effect, as they block some of the positive charge from reaching the valence electrons.
This effect is crucial in understanding various atomic properties, such as ionization energies and electronegativity. As you move down the periodic table, the shielding effect becomes stronger due to the addition of more core electrons, while it remains constant as you move across the same period since the number of core electrons stays the same.
Shielding Effect Definition
Shielding effect is a phenomena due to which the electrostatic attraction between nucleus and valence cell reduces because of the presence of inner-shell electrons.
Order of Screening Effect
The order of screening effect of electrons from s, p, d, to f orbitals follows a specific pattern based on their proximity to the nucleus and shielding capabilities.
The screening effect describes the decrease in attraction between an electron and the nucleus as the number of electron shells increases. Here is the order of screening effect from greatest to smallest:
s-orbital: Electrons in the s-orbital exhibit the highest screening effect due to their close proximity to the nucleus and spherical shape. They shield other electrons effectively.
p-orbital: Following the s-orbital, electrons in the p-orbital provide a moderate screening effect.
d-orbital: Electrons in the d-orbital have less shielding capability compared to s and p orbitals.
f-orbital: Electrons in the f-orbital exhibit the least screening effect among the orbitals.
This order is determined by factors such as electron penetration towards the nucleus, shielding by core electrons, and electron-electron repulsion.
Effective Nuclear Charge
The effective nuclear charge refers to the actual positive charge experienced by an electron in a multi-electron atom. It is influenced by factors such as the number of protons in the nucleus (atomic number) and the shielding effect of inner electrons.
The trend for the effective nuclear charge is that it increases with decreasing atomic radius and increasing ionization energy across a period in the periodic table. This increase results from the stronger pull of the nucleus on valence electrons due to a decrease in atomic size and an increase in atomic charge.
Effective nuclear charge plays a crucial role in understanding various properties of elements, such as ionization energies and chemical reactivity.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1