Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2651 2025-11-19 16:39:14
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,568
Re: Miscellany
2451) Ozone
Gist
Ozone (O3) is a gas with a distinct odor that exists in two layers of the atmosphere: the protective stratospheric ozone layer and the harmful ground-level ozone. While stratospheric ozone is beneficial because it shields the Earth from the sun's harmful ultraviolet (UV) radiation, ground-level ozone is a major air pollutant and a key component of smog that can cause serious health problems. Ground-level ozone forms when pollutants from sources like car exhaust and industrial emissions react with sunlight.
Ozone (O3) is a gas with a chemical formula of O3, meaning it has three oxygen atoms instead of the two in the oxygen we breathe (O2). It can be "good" or "bad" depending on its location in the atmosphere: "good" stratospheric ozone forms a protective layer that shields Earth from the sun's harmful ultraviolet (UV) radiation, while "bad" ground-level ozone is a pollutant that can damage lungs and crops.
Summary
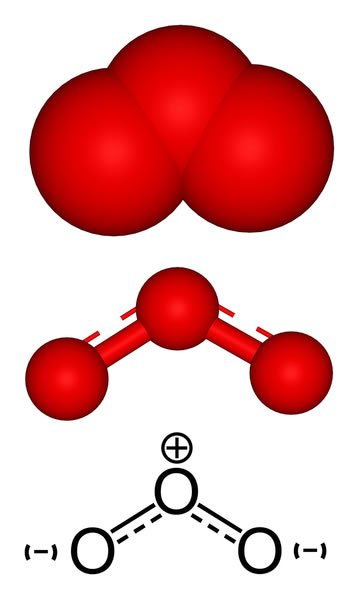
Ozone, also called trioxygen, is an inorganic molecule with the chemical formula O3. It is a pale-blue gas with a distinctively pungent odour. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O2, breaking down in the lower atmosphere to O2 (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.
Ozone's odour is reminiscent of chlorine, and detectable by many people at concentrations of as little as 0.1 ppm in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black solid. Ozone's instability with regard to more common dioxygen is such that both concentrated gas and liquid ozone may decompose explosively at elevated temperatures, physical shock, or fast warming to the boiling point. It is therefore used commercially only in low concentrations.
Ozone is a powerful oxidising agent (far more so than dioxygen) and has many industrial and consumer applications related to oxidation. This same high oxidising potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about 0.1 ppm. While this makes ozone a potent respiratory hazard and pollutant near ground level, a higher concentration in the ozone layer (from two to eight ppm) is beneficial, preventing damaging UV light from reaching the Earth's surface.
Details
Ozone, (O3), triatomic allotrope of oxygen (a form of oxygen in which the molecule contains three atoms instead of two as in the common form) that accounts for the distinctive odor of the air after a thunderstorm or around electrical equipment. The odor of ozone around electrical machines was reported as early as 1785; ozone’s chemical constitution was established in 1872. Ozone is an irritating pale blue gas that is explosive and toxic, even at low concentrations. It occurs naturally in small amounts in Earth’s stratosphere, where it absorbs solar ultraviolet radiation, which otherwise could cause severe damage to living organisms on Earth’s surface. Under certain conditions, photochemical reactions between nitrogen oxides and hydrocarbons in the lower atmosphere can produce ozone in concentrations high enough to cause irritation of the eyes and mucous membranes. Such ground-level ozone is considered a major air pollutant.
Ozone usually is manufactured by passing an electric discharge through a current of oxygen or dry air. The resulting mixtures of ozone and original gases are suitable for most industrial purposes, although purer ozone may be obtained from them by various methods; for example, upon liquefaction, an oxygen-ozone mixture separates into two layers, of which the denser one contains about 75 percent ozone. The extreme instability and reactivity of concentrated ozone makes its preparation both difficult and hazardous.
Ozone is 1.5 times as dense as oxygen; at −112 °C (−170 °F) it condenses to a dark blue liquid, which freezes at −251.4 °C (−420 °F). The gas decomposes rapidly at temperatures above 100 °C (212 °F) or, in the presence of certain catalysts, at room temperatures. Although it resembles oxygen in many respects, ozone is much more reactive; hence, it is an extremely powerful oxidizing agent, particularly useful in converting olefins into aldehydes, ketones, or carboxylic acids. Because it can decolorize many substances, it is used commercially as a bleaching agent for organic compounds; as a strong germicide it is used to sterilize drinking water as well as to remove objectionable odors and flavors.
Additional Information
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere (the stratosphere) and lower atmosphere (the troposphere). Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways.
Stratospheric ozone is formed naturally through the interaction of solar ultraviolet (UV) radiation with molecular oxygen (O2). The "ozone layer," approximately 6 through 30 miles above the Earth's surface, reduces the amount of harmful UV radiation reaching the Earth's surface.
Tropospheric or ground-level ozone – what we breathe – is formed primarily from photochemical reactions between two major classes of air pollutants, volatile organic compounds (VOC) and nitrogen oxides (NOx). These reactions have traditionally been viewed as depending upon the presence of heat and sunlight, resulting in higher ambient ozone concentrations in summer months. Within the last decade, however, high ozone concentrations have also been observed under specific circumstances in cold months, where a few high elevation areas in the Western U.S. with high levels of local VOC and NOx emissions have formed ozone when snow is on the ground and temperatures are near or below freezing. Ozone contributes to what we typically experience as "smog" or haze, which still occurs most frequently in the summertime, but can occur throughout the year in some southern and mountain regions.
Although some stratospheric ozone is transported into the troposphere, and some VOC and NOx occur naturally, the majority of ground-level ozone is the result of reactions of man-made VOC and NOx. Significant sources of VOC are chemical plants, gasoline pumps, oil-based paints, autobody shops, and print shops. Nitrogen oxides result primarily from high temperature combustion. Significant sources are power plants, industrial furnaces and boilers, and motor vehicles.
Ozone has two properties of interest to human health. First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin cancer and cataracts. Second, when inhaled, it reacts chemically with many biological molecules in the respiratory tract, leading to a number of adverse health effects. This course addresses this second property.
Ozone (O3) is a gas molecule composed of three oxygen atoms which occur both in the Earth's upper atmosphere and at ground level. There is both a "good" and "bad" Ozone.
Bad Ozone - Ground-level Ozone is an air pollutant that is harmful to breathe and damages crops, trees, and other vegetation. It is the main ingredient of urban smog. The Centers for Disease Control and Prevention (CDC) states that breathing ground-level ozone can be harmful to your health.
Good Ozone - Ozone is produced naturally in the stratosphere. But this "good" ozone layer is gradually being destroyed by man-made chemicals referred to as ozone-depleting substances (ODS). Essentially, Ozone is only good for our stratosphere, which is a layer of the earth's protective barrier.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2652 Yesterday 22:27:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,568
Re: Miscellany
2452) Seebeck Effect
Gist
Seebeck effect, production of an electromotive force (emf) and consequently an electric current in a loop of material consisting of at least two dissimilar conductors when two junctions are maintained at different temperatures. The conductors are commonly metals, though they need not even be solids. The German physicist Thomas Johann Seebeck discovered (1821) the effect. The Seebeck effect is used to measure temperature with great sensitivity and accuracy and to generate electric power for special applications.
Summary
The Seebeck effect is the direct conversion of temperature differences into electrical voltage, generated when two different conductors or semiconductors are joined to form a loop. This phenomenon creates a small voltage, called the Seebeck voltage, which can be used for practical applications like generating electricity from heat using thermoelectric generators or measuring temperature with thermocouples.
The Seebeck effect is the phenomenon where a temperature difference between two different conductors or semiconductors creates an electrical voltage. This direct conversion of thermal energy to electrical energy is the principle behind thermoelectric devices like thermocouples, which use a temperature gradient to generate a measurable voltage.
Details:
Key learnings:
Seebeck Effect Definition: The Seebeck effect is defined as the conversion of temperature differences into electric voltage, enabling various practical applications.
Temperature to Electricity: This effect generates electricity when there is a temperature difference across the junctions of two different materials.
Key Applications: Thermocouples and thermoelectric generators are primary applications, used for temperature measurement and converting waste heat into power.
Material Requirements: Effective materials for the Seebeck effect include metals with low Seebeck coefficients and semiconductors with higher coefficients for better performance.
Advantages and Challenges: While the Seebeck effect is reliable and can harness low-grade heat, finding materials with the right properties remains a significant challenge.
The Seebeck effect is a phenomenon that converts temperature differences into electric voltage and vice versa. It is named after Thomas Johann Seebeck, a German physicist who discovered it in 1821. The Seebeck effect is the basis of thermocouples, thermoelectric generators, and spin caloritronics.
Thomas Seebeck:
What is the Seebeck Effect?
The Seebeck effect is defined as the generation of an electric potential (or voltage) across two different conductors or semiconductors that are connected in a loop and have a temperature difference between their junctions. The voltage is proportional to the temperature difference and depends on the materials used.
For example, a thermocouple is a device that uses the Seebeck effect to measure temperature. It consists of two wires made of different metals (such as copper and iron) that are joined at both ends. One end is exposed to a hot source (such as a flame) and the other end is kept cold (such as in ice water). The temperature difference between the ends creates a voltage across the wires, which can be measured by a voltmeter.
The Seebeck effect also enables the generation of electricity from waste heat. In a thermoelectric generator, multiple thermocouples are linked either in series or parallel. These thermocouples have one side connected to a heat source—like an engine or furnace—and the other to a heat sink, such as air or water. This temperature differential generates a voltage capable of powering electrical devices, such as lights or fans.
How Does the Seebeck Effect Work?
Electrons, which are negatively charged and move freely in conductors and semiconductors, drive the Seebeck effect. When these materials are heated, the electrons gain energy and move from the hot area to the cooler one, generating an electric current as they travel.
However, different materials have different numbers and types of electrons available for conduction. Some materials have more electrons than others, and some have electrons with different spin orientations. Spin is a quantum property of electrons that makes them act like tiny magnets. When two materials with different electron characteristics are joined together, they form an interface where electrons can exchange energy and spin.
The Seebeck effect occurs when two such interfaces are subjected to a temperature difference. The electrons at the hot interface gain more energy and spin from the heat source and transfer them to the electrons at the cold interface through the loop. This creates an imbalance of charge and spin between the interfaces, resulting in an electric potential and a magnetic field. The electric potential drives an electric current through the loop, while the magnetic field deflects a compass needle placed near it.
What are the Applications of the Seebeck Effect?
The Seebeck effect has many applications in science, engineering, and technology. Some of them are:
* Thermocouples: These are devices that use the Seebeck effect to measure temperature with high accuracy and sensitivity. They are widely used in industries, laboratories, and households for various purposes, such as controlling ovens, monitoring engines, measuring body temperature, etc.
* Thermoelectric generators: These are devices that use the Seebeck effect to convert waste heat into electricity for special applications, such as powering spacecraft, remote sensors, medical implants, etc.
* Spin caloritronics: This is a branch of physics that studies how heat and spin interact in magnetic materials. The Seebeck effect plays an important role in this field, as it can create spin currents and voltages from temperature gradients. This can lead to novel devices for information processing and storage, such as spin batteries, spin transistors, spin valves, etc.
What are the Advantages and Limitations of the Seebeck Effect?
The Seebeck effect has some advantages and limitations that affect its performance and efficiency. Some of them are:
Advantages: The Seebeck effect is simple, reliable, and versatile. It does not require any moving parts or external power sources. It can operate over a wide range of temperatures and materials. It can generate electricity from low-grade heat sources that would otherwise be wasted.
Limitations: The Seebeck effect is limited by the availability and compatibility of materials. It requires materials with high electrical conductivity and low thermal conductivity to achieve high voltage and low heat loss. It also requires materials with different Seebeck coefficients to create a voltage difference. The Seebeck coefficient is a property that measures how much voltage is generated per unit temperature difference for a given material. The Seebeck coefficient depends on the type and concentration of charge carriers, their energy levels, and their interactions with the lattice. The Seebeck coefficient can vary with temperature, composition, and magnetic field. Finding materials with high and stable Seebeck coefficients is a challenge for thermoelectric applications.
What are the Types of Materials Used for the Seebeck Effect?
The materials used for the Seebeck effect can be classified into three categories: metals, semiconductors, and superconductors.
* Metals: Metals are good conductors of both electricity and heat. They have low Seebeck coefficients and high thermal conductivity, which makes them inefficient for thermoelectric applications. However, metals are easy to fabricate and connect, and they have high mechanical strength and stability. Metals are commonly used for thermocouples, where accuracy and durability are more important than efficiency. Some examples of metal pairs used for thermocouples are copper-constantan, iron-constantan, chromel-alumel, etc.
* Semiconductors: Semiconductors are materials that have an intermediate electrical conductivity that can be controlled by doping or applying an electric field. They have higher Seebeck coefficients and lower thermal conductivity than metals, which makes them more suitable for thermoelectric applications. However, semiconductors are more difficult to fabricate and connect, and they have lower mechanical strength and stability than metals. Semiconductors are commonly used for thermoelectric generators and coolers, where efficiency and performance are more important than accuracy and durability. Some examples of semiconductor pairs used for thermoelectric devices are bismuth telluride-antimony telluride, lead telluride-silicon germanium, etc.
* Superconductors: Superconductors are materials that have zero electrical resistance below a critical temperature. They have very high Seebeck coefficients and very low thermal conductivity, which makes them ideal for thermoelectric applications. However, superconductors are very rare and expensive, and they require very low temperatures to operate, which limits their practical use. Superconductors are mainly used for research purposes, such as studying the spin Seebeck effect, which is a phenomenon that involves the generation of a spin voltage from a temperature gradient in a magnetic material.
Conclusion
The Seebeck effect, which transforms temperature differences into electrical voltage, plays a crucial role in devices like thermocouples and thermoelectric generators. Its efficiency hinges on the materials used—specifically their conductivity and Seebeck coefficients. Despite challenges in material selection, its potential in various fields remains substantial.
Additional Information
The Seebeck effect is a phenomenon in which a temperature difference between two dissimilar electrical conductors or semiconductors produces a voltage difference between the two substances.
When heat is applied to one of the two conductors or semiconductors, heated electrons flow toward the cooler conductor or semiconductor. If the pair is connected through an electrical circuit, direct current (DC) flows through that circuit.
Seebeck effect: Key findings
The Seebeck effect refers to the buildup of electric potential which happens when there is a temperature gradient between different electrical conductors or semiconductors.
Here are some key findings of this phenomenon:
* The voltages produced by the Seebeck effect are small, usually only a few microvolts (millionths of a volt) per kelvin of temperature difference at the junction between the conductors or semiconductors.
* If the temperature difference is large enough, some Seebeck-effect devices can produce a few millivolts (thousandths of a volt).
* Numerous such devices can be connected in series to increase the output voltage or in parallel to increase the maximum deliverable current.
* Large arrays of Seebeck-effect devices can provide useful, small-scale electrical power if a large temperature difference is maintained across the junctions.
Seebeck effect: Explanation
In 1821, German physicist Thomas Seebeck discovered that when two wires made from dissimilar metals are joined at two ends to form a loop, and if the two junctions are maintained at different temperatures, a voltage develops in the circuit. This phenomenon is therefore named after him.
When heat is applied to one of the two conductors or semiconductors, that metal heats up. Consequently, the valence electrons present in this metal flow toward the cooler metal. This happens because electrons move to where energy (in this case, heat) is lower. If the metals are connected through an electrical circuit, direct current flows through the circuit.
However, this voltage is just a few microvolts per kelvin temperature difference. Thermal energy is continuously transferred from the warmer metal to the cooler metal until eventually, temperature equilibrium is obtained.
The Seebeck effect and its resultant thermoelectric effect is a reversible process. If the hot and cold junctions are interchanged, valence electrons will flow in the other direction, and also change the direction of the DC current.
Seebeck effect and thermocouples
The pair of metal wires forming the electrical circuit is known as a thermocouple. On a larger scale and due to the Seebeck effect, thermocouples are used to approximately measure temperature differences. They are also used to actuate electronic switches that can turn large systems on and off, a capability that is employed in thermoelectric cooling technology.
Seebeck used copper and bismuth in his experiment. Other common thermocouple metal combinations that are used today include the following:
* constantan and copper
* constantan and iron
* constantan and chromel
* constantan and alumel
Applications of Seebeck effect
There are many applications of the Seebeck effect. In addition to its use in thermocouples to measure temperature differences, the phenomenon is also used in the following ways:
* in thermopiles (that is, in a setting where a number of thermocouples are connected in series);
* in thermoelectric generators that function as heat engines;
* in power plants to convert waste heat into (extra) power;
* in automobiles as automotive thermoelectric generators, to increase fuel efficiency;
* in high-frequency electrical power sensors;
* to verify material degradation and radiation level, and to perform strength testing of radioactive materials (which vary with temperature over a given time period); and
* to actuate security alarms or switches.
Spin Seebeck effect
In 2008, physicists discovered the Spin Seebeck effect (SSE). This effect refers to the generation of a spin voltage caused by a temperature gradient in a ferromagnet. This gradient enables the thermal injection of spin currents from the ferromagnet into a nonmagnetic metal. This injection happens over a macroscopic scale of several millimeters.
SSE is seen when heat is applied to a magnetized metal. As a result, electrons rearrange themselves according to their spin. Unlike ordinary electron movement, this rearrangement does not create heat as a waste product.
The effect could lead to the development of smaller, faster and more energy-efficient microchips or switches, as well as spintronics devices.
Seebeck effect vs. Peltier effect
In 1834, Jean Peltier, a French watchmaker, discovered another second thermoelectric effect that was later named the Peltier effect. Peltier observed that when a current flows through a circuit containing a junction of two dissimilar metals -- similar to the setup in the Seebeck effect -- heat is either absorbed or liberated at the junction. This absorption or liberation depends on the pair of metals used and the direction of the current.
The Seebeck effect and Peltier effect both involve circuits made from dissimilar metals, as well as heat and electricity. Both are also reversible processes. But despite these similarities, there are some differences between these effects as well.
The Seebeck effect occurs when the two ends of a thermocouple are at different temperatures, which results in electricity flowing from the hot metal to the cold metal.
In the Peltier effect, a temperature difference is created between the junctions when electrical current flows across the terminals. In a copper-constantan thermocouple in which the current at the junction is flowing from copper (+) to constantan (-), heat will be absorbed. But if the direction of the current is reversed -- i.e., from constantan (-) to copper (+) -- it will result in heat liberation.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline