Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-11-03 21:45:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,733
Carbonic Acid
Carbonic Acid
Gist
Carbonic acid (H2CO3) is a weak acid formed when carbon dioxide (CO2)) dissolves in water. It is an unstable compound that can dissociate into a hydrogen ion (H+) and a bicarbonate ion (HCO3-), and it plays a critical role in regulating blood pH in the human body. Carbonic acid is also found in carbonated beverages and is responsible for weathering limestone.
Carbonic acid is used in the food and beverage industry for carbonating soft drinks, wine, and sparkling water; in medicine for its role as a blood buffer and for certain topical treatments; and in industrial applications for pH control in water treatment, as a cleaning agent, and in the production of other chemicals.
Summary
Carbonic acid is a chemical compound with the chemical formula H2CO3. The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters.
In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis.
Terminology in biochemical literature
In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula H2CO3. Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid.
In physiology, carbon dioxide excreted by the lungs may be called volatile acid or respiratory acid.
Details
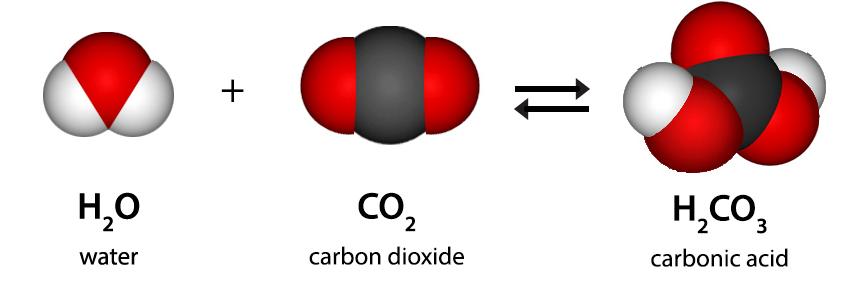
Carbonic acid, (H2CO3) is a compound of the elements hydrogen, carbon, and oxygen. It is formed in small amounts when its anhydride, carbon dioxide (CO2), dissolves in water.
The predominant species are simply loosely hydrated CO2 molecules. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be formed—namely, hydrogen carbonates.
However, the acid-base behaviour of carbonic acid depends on the different rates of some of the reactions involved, as well as their dependence on the pH of the system.
Between pH values of 8 and 10, all the above equilibrium reactions are significant.
Carbonic acid plays a role in the assembly of caves and cave formations like stalactites and stalagmites. The largest and most common caves are those formed by dissolution of limestone or dolomite by the action of water rich in carbonic acid derived from recent rainfall. The calcite in stalactites and stalagmites is derived from the overlying limestone near the bedrock/soil interface. Rainwater infiltrating through the soil absorbs carbon dioxide from the carbon dioxide-rich soil and forms a dilute solution of carbonic acid. When this acid water reaches the base of the soil, it reacts with the calcite in the limestone bedrock and takes some of it into solution. The water continues its downward course through narrow joints and fractures in the unsaturated zone with little further chemical reaction. When the water emerges from the cave roof, carbon dioxide is lost into the cave atmosphere, and some of the calcium carbonate is precipitated. The infiltrating water acts as a calcite pump, removing it from the top of the bedrock and redepositing it in the cave below.
Carbonic acid is important in the transport of carbon dioxide in the blood. Carbon dioxide enters blood in the tissues because its local partial pressure is greater than its partial pressure in blood flowing through the tissues. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). Blood acidity is minimally affected by the released hydrogen ions because blood proteins, especially hemoglobin, are effective buffering agents. (A buffer solution resists change in acidity by combining with added hydrogen ions and, essentially, inactivating them.) The natural conversion of carbon dioxide to carbonic acid is a relatively slow process; however, carbonic anhydrase, a protein enzyme present inside the red blood cell, catalyzes this reaction with sufficient rapidity that it is accomplished in only a fraction of a second. Because the enzyme is present only inside the red blood cell, bicarbonate accumulates to a much greater extent within the red cell than in the plasma. The capacity of blood to carry carbon dioxide as bicarbonate is enhanced by an ion transport system inside the red blood cell membrane that simultaneously moves a bicarbonate ion out of the cell and into the plasma in exchange for a chloride ion. The simultaneous exchange of these two ions, known as the chloride shift, permits the plasma to be used as a storage site for bicarbonate without changing the electrical charge of either the plasma or the red blood cell. Only 26 percent of the total carbon dioxide content of blood exists as bicarbonate inside the red blood cell, while 62 percent exists as bicarbonate in plasma; however, the bulk of bicarbonate ions is first produced inside the cell, then transported to the plasma. A reverse sequence of reactions occurs when blood reaches the lung, where the partial pressure of carbon dioxide is lower than in the blood.
Additional Information
Carbonic Acid is an inorganic, weak, and unstable acid. The molecule of Carbonic Acid consists of one carbon atom, two hydrogen atoms, and three oxygen atoms. Carbonic Acid is also referred to as a respiratory acid as it is the only acid that is exhaled in the gaseous state by human lungs.
Carbonic acid is studied majorly in various fields of science because of its vast applications and uses. It helps improve marine life due to its natural process of ocean acidification. It is essential for the human body as well.
What is Carbonic Acid?
Carbonic acid is a chemical compound made of carbon dioxide, oxygen, and hydrogen as its elements. It is a weak acid with the chemical formula H2CO3. It is formed when carbon dioxide is dissolved in water. Carbonic acid is a diprotic acid, which means it can form two types of salts: carbonate and bicarbonate. Carbonic Acid is also known as dihydrogen carbonate (because it is made of two hydrogen atoms and a carbonate ion), aerial acid (or acid of air), Oxidocarboxylic acid, Hydroxyformic acid, etc.
It was discovered by the French chemist Antoine Lavoisier in the late 18th century. He found that when carbon dioxide reacts with water an acidic solution is formed which he named as carbonic acid.
Formula of Carbonic Acid
The compound of carbonic acid consists of one carbon atom, two hydrogen atoms, and three oxygen atoms. It can also be represented as OC(OH)2 due to the presence of a carbon-oxygen double bond.
Structure of Carbonic Acid
Carbonic acid consists of a carboxyl functional group along with a hydroxyl group. The structure of carbonic acids consists of one carbon-oxygen double bond and further, this carbon is single bonded with two hydroxyl groups (OH).
Preparation of Carbonic Acid
When strong acid reacts with calcium carbonate, carbon dioxide gas is evolved which when dissolved in water, Carbonic acid is formed.
Properties of Carbonic Acid
Carbonic acid has a variety of properties which are classified into physical and chemical properties. The physical and chemical properties of carbonic acid are described below:
Physical Properties of Carbonic Acid
* State: Carbonic Acid generally occurs as a solution, but according to recent research it was found that Carbonic acid is also being prepared in a solid state by NASA scientists.
* Odor: Carbonic Acid are generally odorless.
* pKa value: The pKa value of Carbonic acid is 6.35. The pKa value is inversely proportional to the acidity of the compound. Hence, carbonic acid is termed under weak acid.
* Boiling Point: The boiling point of carbonic acid is around 127° Celsius.
* Melting Point: The melting point of carbonic acid is around -53° Celsius.
* Density: Carbonic acid has a density of around 1.668 grams per cubic centimeter.
* Molecular weight: The molecular weight of Carbonic acid is 62.024 g/mol.
* Conjugate base: The conjugate bases of Carbonic acid are bicarbonate and carbonate.
Chemical Properties of Carbonic Acid
* Stability: Carbonic acids are unstable under normal circumstances. However, they can be stabilized under high pressure and high temperatures.
* pH: Being a weak acidic, carbonic acid has a pH value of around 4.68
* Diprotic acid: It is a diprotic acid, i.e. it can form two types of salts namely carbonate and bicarbonate.
* Dissociation reaction: In the presence of water, Carbonic acid can partially dissociate into bicarbonate ions (HCO3-) and hydrogen ions (H+).
* Reaction with base: Carbonic acids form bicarbonate salt when reacted with a moderate amount of base and it form carbonate salts if reacted with excess amount of base.
* Decomposition reaction: Carbonic acid can easily decompose into carbon dioxide and water. However, this reaction is reversible under suitable conditions.
Uses of Carbonic Acid
* Carbonated Drinks: Carbonic acid provides the fizzy and bubbly taste in carbonated drinks like sparkling water, sodas, soft drinks, beer, champagne and other carbonated beverages
* Buffering System: Carbonic acid acts as a buffer system in human bodies which helps in maintaining the pH balance in the blood.
* pH regulation: Carbonic acid is also useful in regulating the pH level of water in swimming pools and in regulating pH balance in various other industrial processes.
* Medicinal uses: It is used in medications to balance acid-base levels in the body and in ointments to treat various fungal infections like ringworm. It is also consumed to induce vomiting (when required).
* pH indicator: Carbonic acid is used as a pH indicator to check whether the solution is acidic or basic.
* Precipitation: Carbonic acid is used as a solvent in the precipitation of various ammonium salts like ammonium persulfate.
Cleansing agent: Carbonic acid is also used as a cleaning agent to clean contact lenses.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1