Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2626 2025-10-25 18:03:08
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,248
Re: Miscellany
2426) Naphtha
Gist
Naphtha is a flammable liquid mixture of hydrocarbons, primarily derived from crude oil distillation, that serves as a crucial feedstock and fuel in various industries. It is used to produce high-octane gasoline, petrochemicals for plastics, fertilizers, and is also used as a solvent and for fuels like lighter fluid or camp stoves. Due to its flammability, it must be handled with care, and its vapor is heavier than air and can travel to an ignition source.
The main uses of crude oil naphtha fall into the general areas of (i) precursor to gasoline and other liquid fuels, (ii) solvents or diluents for paints, (iii) dry-cleaning solvents, (iv) solvents for cutback asphalt, (v) solvents in rubber industry, and (vi) solvents for industrial extraction processes.
Summary
Naphtha is any of various volatile, highly flammable liquid hydrocarbon mixtures used chiefly as solvents and diluents and as raw materials for conversion to gasoline. Naphtha was the name originally applied to the more volatile kinds of petroleum issuing from the ground in the Baku district of Azerbaijan and Iran. As early as the 1st century ad, naphtha was mentioned by the Greek writer Dioscorides and the Roman writer Pliny the Elder. Alchemists used the word principally to distinguish various mobile liquids of low boiling point, including certain ethers and esters.
In modern usage the word naphtha is usually accompanied by a distinctive prefix. Coal-tar naphtha is a volatile commercial product obtained by the distillation of coal tar. Shale naphtha is obtained by the distillation of oil produced from bituminous shale by destructive distillation. Petroleum naphtha is a name used primarily in the United States for petroleum distillate containing principally aliphatic hydrocarbons and boiling higher than gasoline and lower than kerosene.
Details
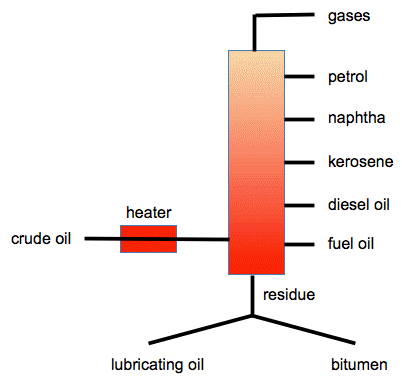
Naphtha is a flammable liquid hydrocarbon mixture. Generally, it is a fraction of crude oil, but it can also be produced from natural-gas condensates, petroleum distillates, and the fractional distillation of coal tar and peat. In some industries and regions, the name naphtha refers to crude oil or refined petroleum products such as kerosene or diesel fuel.
Naphtha is also known as Shellite in Australia.
Modern period
Since the 19th century, solvent naphtha has denoted a product (xylene or trimethylbenzenes) derived by fractional distillation from petroleum; these mineral spirits, also known as "Stoddard Solvent," were originally the main active ingredient in Fels Naptha laundry soap. The naphtha in Fels Naptha was later removed as a cancer risk.
The usage of the term "naphtha" during this time typically implies petroleum naphtha, a colorless liquid with a similar odor to gasoline. However, "coal tar naphtha," a reddish brown liquid that is a mixture of hydrocarbons (toluene, xylene, and cumene, etc.), could also be intended in some contexts.
Petroleum
In older usage, "naphtha" simply meant crude oil, but this usage is now obsolete in English. There are a number of cognates to the word in different modern languages, typically signifying "petroleum" or "crude oil."
The Ukrainian & Belarusian word (nafta), Lithuanian, Latvian, & Estonian "nafta," and the Persian naft mean "crude oil." The Russian word (neft') means "crude oil," but (nafta) is a synonym of ligroin. Also, in Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Finland, Italy, Serbia, Slovenia, and Macedonia nafta (нафта in Cyrillic) is colloquially used to indicate diesel fuel and crude oil. In the Czech Republic and Slovakia, nafta was historically used for both diesel fuel and crude oil, but its use for crude oil is now obsolete and it generally indicates diesel fuel. In Bulgarian, nafta means diesel fuel, while neft, as well as petrol (петрол in Cyrillic), means crude oil. Nafta is also used in everyday parlance in Argentina, Uruguay and Paraguay to refer to gasoline/petrol. Similarly, in Flemish, the word naft(e) is used colloquially for gasoline. In Poland, the word nafta means kerosene, and colloquially crude oil (the technical name for crude oil is ropa naftowa, also colloquially used for diesel fuel as ropa).
Types
Naphtha has been divided into two types by many sources in order to differentiate between common grades more clearly:
One source distinguishes by boiling point as well as carbon atom count per molecule:
* Light naphtha is the fraction boiling between 30 and 90 °C (86 and 194 °F) and consists of molecules with 5–6 carbon atoms.
* Heavy naphtha boils between 90 and 200 °C (194 and 392 °F) and consists of molecules with 6–12 carbon atoms.
Chemistry of Hazardous Materials differentiates light and heavy based on the carbon atom count and hydrocarbon structure:
* Light [is] a mixture consisting mainly of straight-chained and cyclic aliphatic hydrocarbons having from five to six carbon atoms per molecule.
* Heavy [is] a mixture consisting mainly of straight-chained and cyclic aliphatic hydrocarbons having from seven to nine carbon atoms per molecule.
Some sources also define petroleum naphtha, which contains both heavy and light naphtha, and typically consists of 15-30% of crude oil by weight.
Uses:
Heavy crude oil dilution
Naphtha is used to dilute heavy crude oil to reduce its viscosity and enable/facilitate transport; undiluted heavy crude cannot normally be transported by pipeline, and may also be difficult to pump onto oil tankers. Other common dilutants include natural-gas condensate and light crude. However, naphtha is a particularly efficient dilutant and can be recycled from diluted heavy crude after transport and processing. The importance of oil dilutants has increased as global production of lighter crude oils has fallen and shifted to exploitation of heavier reserves.
Fuel
Light naphtha is used as a fuel in some commercial applications. One notable example is wick-based cigarette lighters, such as the Zippo, which draw "lighter fluid"—naphtha—into a wick from a reservoir to be ignited using the flint and wheel.
It is also a fuel for camping stoves and oil lanterns, known as "white gas", where naphtha's low boiling point makes it easy to ignite. Naphtha is sometimes preferred over kerosene because it clogs fuel lines less. The outdoor equipment manufacturer MSR published a list of trade names and translations to help outdoor enthusiasts obtain the correct products in various countries.
Naphtha was also historically used as both a fuel and a working fluid in some small boats where steam technology was impractical; most were built to circumvent safety laws relating to traditional steam launches.
As an internal combustion engine fuel, petroleum naphtha has seen very little use and suffers from lower efficiency and low octane ratings, typically 40 to 70 RON. It can be used to run unmodified diesel engines, though it has a longer ignition-delay than diesel. Naphtha tends to be noisy in combustion due to the high pressure rise rate. There is a possibility of using naphtha as a low-octane base fuel in an octane-on-demand concept, with the engine drawing a high-octane mix only when needed. Naptha benefits from lesser emissions in refinement: fuel energy losses from "well-to-tank" are 13%; lower than the 22% losses for petroleum.
Plastics
Naphtha is a crucial component in the production of plastics.
Additional Information
Naphtha is a term used to refer to a group of volatile, flammable mixtures of liquid hydrocarbons that are used mainly as solvents, diluents, or raw materials for gasoline conversion. It is a lightweight petrochemical feedstock that is separated from crude oil in the fractional distillation process along with kerosene and jet fuel.
There are many specific types of naphtha that vary in the amounts and types of hydrocarbons contained in their unique blend. Refineries can produce various forms of naphtha, and each has specific guidelines in how it should be handled and stored. Generally speaking, the flammability and volatility of naphtha should be taken into consideration as they are significant safety hazards.
Uses and Safety
As mentioned above, naphtha is commonly used as a solvent. It is used in hydrocarbon cracking, laundry soaps, and cleaning fluids. Naphtha is also used to make varnishes, and sometimes is used as a fuel for camp stoves and as a solvent (diluent) for paint. Although naphtha has many uses, some forms of it can be dangerous. Many kinds of naphtha can cause skin irritation, upset stomachs, and other health problems if people are exposed to them. Some forms are also carcinogens, and thus inhalation or ingestion of the chemical should be avoided.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2627 2025-10-26 16:38:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,248
Re: Miscellany
2427) Tartaric Acid
Gist
Tartaric acid is a naturally occurring, white crystalline organic acid found in fruits like grapes, bananas, and tamarinds. It is used as a food additive in baking powder to create a leavening reaction that makes baked goods rise. It also has antioxidant properties and a tangy flavor, with industrial uses including metal polishing and dyeing.
Tartaric acid has numerous uses in the food industry as an acidulant and flavor enhancer, especially in wine, baked goods, and candies. It also has pharmaceutical applications as an excipient in effervescent tablets and for flavor in medicines. Other uses include cosmetics for its exfoliating properties, industrial applications like metal cleaning and tanning leather, and as a leavening agent in baking powder.
Summary
Tartaric acid is a dicarboxylic acid, one of the most widely distributed of plant acids, with a number of food and industrial uses. Along with several of its salts, cream of tartar (potassium hydrogen tartrate) and Rochelle salt (potassium sodium tartrate), it is obtained from by-products of wine fermentation. In a partially purified form, tartar was known to the ancient Greeks and Romans; the free acid was first isolated in 1769 by Swedish chemist Carl Wilhelm Scheele. The lees, or sediments, and other waste products from fermentation are heated and neutralized with calcium hydroxide; the precipitated calcium tartrate is then treated with sulfuric acid to produce free tartaric acid. Rochelle salt is prepared from the crude crystalline potassium acid salt, called argol, by neutralization with sodium carbonate. Purified cream of tartar comes chiefly from the filtrates from production of the acid and Rochelle salt. A third salt, tartar emetic (antimony potassium tartrate), is made from the potassium acid salt and antimony oxide.
Three stereoisomeric forms of tartaric acid exist: (1) dextrorotatory tartaric acid (d-tartaric acid) found in grapes and several other fruits, (2) levorotatory tartaric acid (l-tartaric acid) obtained chiefly by resolution of racemic tartaric acid, and (3) a meso or achiral form. Racemic tartaric acid (an equal mixture of d- and l-tartaric acid) is prepared commercially by the molybdenum- or tungsten-catalyzed oxidation of maleic anhydride with hydrogen peroxide.
Study of the crystallographic, chemical, and optical properties of the tartaric acids by French chemist and microbiologist Louis Pasteur laid the basis for modern ideas of stereoisomerism.
The various tartaric acids and the common tartrate salts are all colourless, crystalline solids readily soluble in water. Tartaric acid is widely used as an acidulant in carbonated drinks, effervescent tablets, gelatin desserts, and fruit jellies. It has many industrial applications—e.g., in cleaning and polishing metals, in calico printing, in wool dyeing, and in certain photographic printing and development processes. Rochelle salt is used in silvering mirrors, in processing cheese, and in compounding mild cathartics. Cream of tartar is incorporated into baking powders, hard candies, and taffies; and it is employed in the cleaning of brass, the electrolytic tinning of iron and steel, and the coating of other metals with gold and silver. Tartar emetic is used as an insecticide and a dyeing mordant.
Details
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid.
History
Tartaric acid has been known to winemakers for centuries– its crude crystalline form as found off top of wine barrels were called tartarum (rendered tartre by Chaucer) or "wine stone". However, chemical extraction and purification was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele.
Tartaric acid played an important role in the discovery of chemical chirality. This property of tartaric acid was first observed in 1832 by Jean Baptiste Biot, who observed its ability to rotate polarized light. Louis Pasteur continued this research in 1847 by investigating the shapes of sodium ammonium tartrate crystals, which he found to be chiral. By manually sorting the differently shaped crystals, Pasteur was the first to produce a pure sample of levotartaric acid.
Tartaric acid in wine
Tartaric acid may be most immediately recognizable to wine drinkers as the source of "wine diamonds", the small potassium bitartrate crystals that sometimes form spontaneously on the cork or bottom of the bottle. These "tartrates" are harmless, despite sometimes being mistaken for broken glass, and are prevented in many wines through cold stabilization (which is not always preferred since it can change the wine's profile). The tartrates remaining on the inside of aging barrels were at one time a major industrial source of potassium bitartrate.
Tartaric acid plays an important role chemically, lowering the pH of fermenting "must" to a level where many undesirable spoilage bacteria cannot live, and acting as a preservative after fermentation. In the mouth, tartaric acid provides some of the tartness in the wine, although citric and malic acids also play a role.
Tartaric acid in fruits
Grapes and tamarinds have the highest levels of tartaric acid concentration. Other fruits with tartaric acid are bananas, avocados, prickly pear fruit, apples, cherries, papayas, peaches, pears, pineapples, strawberries, mangoes and citrus fruits.
Trace amounts of tartaric acid have been found in cranberries and other berries.
Tartaric acid is also present in the leaves and pods of Pelargonium plants and beans.
Applications
Tartaric acid and its derivatives have a plethora of uses in the field of pharmaceuticals. For example, it has been used in the production of effervescent salts, in combination with citric acid, to improve the taste of oral medications. The potassium antimonyl derivative of the acid known as tartar emetic is included, in small doses, in cough syrup as an expectorant.
Tartaric acid also has several applications for industrial use. The acid has been observed to chelate metal ions such as calcium and magnesium. Therefore, the acid has served in the farming and metal industries as a chelating agent for complexing micronutrients in soil fertilizer and for cleaning metal surfaces consisting of aluminium, copper, iron, and alloys of these metals, respectively.
Additional Information
Tartaric acid is the other major grape acid, along with malic acid. Unlike malic acid, the concentration of tartaric acid does not decline markedly during grape ripening. In addition, tartaric acid is metabolized by few microorganisms. Thus, it is usually the preferred acid added to increase the acidity of high pH wines. Regrettably, this carries the risk of increasing bitartrate instability.
Tartaric acid is synthesized in many plants, but accumulates in significant quantities in only a few genera, most significantly, members of the Vitaceae. It is so characteristic of V. vinifera that its presence in neolithic vessels in the Near East can be taken as evidence of wine production. The acid commonly collects as a potassium salt in leaves and grapes. As wines age, dissolved tartrates crystallize and tend to precipitate. Because chilling speeds the process, wines often are cooled near the end of maturation to enhance early tartrate precipitation and avoid crystal deposition in the bottle. Nevertheless, crystals may continue to form after bottling. This partially occurs due to the conversion of the natural (l form) of tartaric acid to the d isomer. The calcium salt of both isomers is about one-eighth as soluble as the l-tartrate salt alone. Therefore, most wines form a salt deposit when aged sufficiently long.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2628 Yesterday 17:08:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,248
Re: Miscellany
2428) Potassium Nitrate
Gist
Potassium nitrate (KNO3) is a chemical compound, also known as saltpeter or nitre, with applications in agriculture, industry, and medicine. It is an ionic salt of potassium and nitrate ions, appearing as a white crystalline powder that is soluble in water. Key properties include a density of approximately 2.11 g/{cm}^{3}, a melting point of 334 degrees Centigrade, and a boiling point of 400 degrees Centigrade before it decomposes.
Potassium nitrate (KNO3) has a wide range of uses, including as a component in fertilizers for plant growth, a food preservative, and in the production of fireworks and explosives. It is also used in specialty toothpastes to treat sensitive teeth, to remove tree stumps by accelerating decomposition, and in some industrial processes like solar power storage and glass manufacturing.
Summary
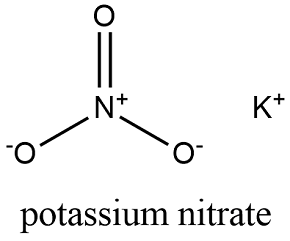
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula KNO3. It is a potassium salt of nitric acid. This salt consists of potassium cations K+ and nitrate anions NO(−3), and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or nitre outside the United States). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the United States).
Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color.
Details
Potassium nitrate (KNO3) is an ionic white crystalline salt made up of potassium ions and nitrate ions. Uses of potassium nitrate include the manufacture of fertilizers, pesticides, glass, fireworks, explosives, and rocket fuels. It is also used as a food preservative, and when added to meat it causes a reaction between the myoglobin and hemoglobin in the blood, making the meat appear red in colour. It is also used as an additive in some toothpastes to help with tooth sensitivity. Potassium nitrate is toxic to humans in high levels, so its use is carefully controlled when human consumption is involved.
It is found in impure form, often called saltpetre (also called nitre), its name derived from the Latin words sal patrae, meaning “salt of the rock,” as it is often found as a white material deposited on the surface of rocks. Saltpetre can form on the surface of soil in various warm-climate locations, including in Egypt, Spain, and Iran. In such places, feces, urine, and decaying plants react with moisture and an alkaline soil to create nitrates. These nitrates dissolve in rainwater, and white deposits of potassium nitrate are left behind when this water evaporates. Many caves throughout the world have large deposits of saltpetre due to large amounts of bat guano and urine found there.
History and production
In the 9th century Chinese chemists discovered that a mixture of potassium nitrate, sulfur, and charcoal would spontaneously produce smoke and flames. By the 11th century the Chinese were incorporating the mixture, gunpowder, into smoke bombs to help fight off enemies, and in the 13th and 14th centuries they used this explosive power to propel objects at their foes with guns.
Historically in the United States, in the early to mid-19th century, caves in Kentucky, Tennessee, and West Virginia were extensively mined for saltpetre that was used to manufacture gunpowder. As more applications of potassium nitrate were discovered, the demand for the chemical compound increased. The increased demand shifted its production from the caves, where only a finite amount of the chemical could be manufactured, to industrial labs, with a much higher ability for production. The most common method of industrial production uses potassium chloride in a double displacement reaction with nitric acid.
Chemical properties
Potassium nitrate has a molar mass of 101.10 grams per mole. It has a boiling point of 400 °C (752 °F), a melting point of 334 °C (633 °F), and a density of 2.11 grams per cubic centimetre at 25 °C (70 °F). It is soluble in water at 38.3 grams per 100 millilitres at 25 °C but is only slightly soluble in most alcohols. It is insoluble in ethanol.
While not itself combustible, potassium nitrate accelerates the burning of combustible materials. Potassium nitrate is a strong oxidizer and when heated decomposes to potassium nitrite and oxygen, which helps the combustion process in explosives. For this reason, potassium nitrate is referred to as an oxidizing agent. It has multiple uses including in the manufacture of fertilizers, medicine, gunpowder, fireworks, and explosives.
Modern industrial production
In the United States more than 200,000 tonnes of potassium nitrate are manufactured annually, almost 90 percent of the production being used in fertilizers. The remaining 10 percent is used in processes such as the manufacture of matches and fireworks and of glass and ceramics. Annual worldwide production is in excess of 30 million tonnes, Russia leading the way in production at just over one-half of this annual total.
Additional Information
Potassium nitrate is a manufactured fertilizer for supplying nitrogen and potassium. It is made from potassium chloride and a source of nitrate, such as sodium nitrate, ammonium nitrate, or nitric acid. Potassium nitrate is sold as a water-soluble, crystalline material for hydroponics and in a prilled form for soil application. Sales of potassium nitrate account for only a small portion of the global potassium fertilizer market as a fertilizer for special uses.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline