Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-10-26 22:52:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,012
Sodium Hydroxide
Sodium Hydroxide
Gist
Sodium hydroxide (NaOH), also known as lye or caustic soda, is a strong, white, and corrosive ionic compound used in making soap, detergents, and as a drain cleaner. It is a highly reactive and deliquescent solid that absorbs moisture and carbon dioxide from the air and generates significant heat when dissolved in water. Sodium hydroxide is also used in various industries, such as food processing and the paper industry.
Sodium hydroxide is a versatile chemical with widespread uses in manufacturing soaps, detergents, paper, and rayon. It's also used in petroleum refining, food processing (like peeling fruits and vegetables), water treatment, and as a powerful drain cleaner because it breaks down fats and grease. Industrially, it plays a key role in processes like aluminum extraction, metal cleaning, and producing other chemicals.
Summary
Sodium hydroxide (NaOH) is a corrosive white crystalline solid that contains the Na+ (sodium) cation and the OH− (hydroxide) anion. It readily absorbs moisture until it dissolves. Sodium hydroxide is the most widely used industrial alkali and is often used in drain and oven cleaners. It is highly corrosive to animal and vegetable tissue. The alkaline solutions it forms when dissolved in water neutralize acids in various commercial processes. In petroleum refining, it removes sulfuric and organic acids. In soapmaking, it acts on natural fats or oils, such as tallow or vegetable oil, to produce sodium fatty acid salt (soap) and glycerin (or glycerol); this saponification reaction is the basis for all soapmaking. In papermaking, sodium hydroxide is used to break down wood into pulp. Solutions of NaOH are used in the treatment of cellulose and in the manufacture of many chemicals.
Details
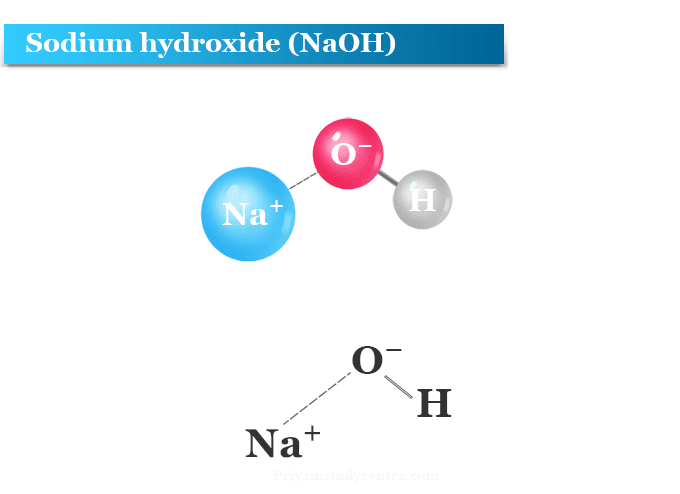
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.
Sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins at ambient temperatures, and may cause severe chemical burns at high concentrations. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates NaOH·nH2O. The monohydrate NaOH·H2O crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound.
As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.
Sodium hydroxide is used in many industries: in the making of wood pulp and paper, textiles, drinking water, soaps and detergents, and as a drain cleaner. Worldwide production in 2022 was approximately 83 million tons.[15]
Properties:
Physical properties
Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °C (604 °F) without decomposition and boils at 1,388 °C (2,530 °F). It is highly soluble in water, with a lower solubility in polar solvents such as ethanol and methanol. Sodium hydroxide is insoluble in ether and other non-polar solvents.
Similar to the hydration of sulfuric acid, dissolution of solid sodium hydroxide in water is a highly exothermic reaction where a large amount of heat is liberated, posing a threat to safety through the possibility of splashing. The resulting solution is usually colorless and odorless. As with other alkaline solutions, it feels slippery with skin contact due to the process of saponification that occurs between NaOH and natural skin oils.
Viscosity
Concentrated (50%) aqueous solutions of sodium hydroxide have a characteristic viscosity, 78 mPa·s, that is much greater than that of water (1.0 mPa·s) and near that of olive oil (85 mPa·s) at room temperature. The viscosity of aqueous NaOH, as with any liquid chemical, is inversely related to its temperature, i.e., its viscosity decreases as temperature increases, and vice versa. The viscosity of sodium hydroxide solutions plays a direct role in its application as well as its storage.
Hydrates
Sodium hydroxide can form several hydrates NaOH·nH2O, which result in a complex solubility diagram that was described in detail by Spencer Umfreville Pickering in 1893. The known hydrates and the approximate ranges of temperature and concentration (mass percent of NaOH) of their saturated water solutions are:
* Heptahydrate, NaOH·7H2O: from −28 °C (18.8%) to −24 °C (22.2%).
* Pentahydrate, NaOH·5H2O: from −24 °C (22.2%) to −17.7 °C (24.8%).
* Tetrahydrate, NaOH·4H2O, α form: from −17.7 °C (24.8%) to 5.4 °C (32.5%).
* Tetrahydrate, NaOH·4H2O, β form: metastable.
* Trihemihydrate, NaOH·3.5H2O: from 5.4 °C (32.5%) to 15.38 °C (38.8%) and then to 5.0 °C (45.7%).
* Trihydrate, NaOH·3H2O: metastable.
* Dihydrate, NaOH·2H2O: from 5.0 °C (45.7%) to 12.3 °C (51%).
* Monohydrate, NaOH·H2O: from 12.3 °C (51%) to 65.10 °C (69%) then to 62.63 °C (73.1%).
Early reports refer to hydrates with n = 0.5 or n = 2/3, but later careful investigations failed to confirm their existence.
The only hydrates with stable melting points are NaOH·H2O (65.10 °C) and NaOH·3.5H2O (15.38 °C). The other hydrates, except the metastable ones NaOH·3H2O and NaOH·4H2O (β) can be crystallized from solutions of the proper composition, as listed above. However, solutions of NaOH can be easily supercooled by many degrees, which allows the formation of hydrates (including the metastable ones) from solutions with different concentrations.
For example, when a solution of NaOH and water with 1:2 mole ratio (52.6% NaOH by mass) is cooled, the monohydrate normally starts to crystallize (at about 22 °C) before the dihydrate. However, the solution can easily be supercooled down to −15 °C, at which point it may quickly crystallize as the dihydrate. When heated, the solid dihydrate might melt directly into a solution at 13.35 °C; however, once the temperature exceeds 12.58 °C it often decomposes into solid monohydrate and a liquid solution. Even the n = 3.5 hydrate is difficult to crystallize, because the solution supercools so much that other hydrates become more stable.
A hot water solution containing 73.1% (mass) of NaOH is a eutectic that solidifies at about 62.63 °C as an intimate mix of anhydrous and monohydrate crystals.
A second stable eutectic composition is 45.4% (mass) of NaOH, that solidifies at about 4.9 °C into a mixture of crystals of the dihydrate and of the 3.5-hydrate.
The third stable eutectic has 18.4% (mass) of NaOH. It solidifies at about −28.7 °C as a mixture of water ice and the heptahydrate NaOH·7H2O.
When solutions with less than 18.4% NaOH are cooled, water ice crystallizes first, leaving the NaOH in solution.
The α form of the tetrahydrate has density 1.33 g/{cm}^3. It melts congruously at 7.55 °C into a liquid with 35.7% NaOH and density 1.392 g/{cm}^3, and therefore floats on it like ice on water. However, at about 4.9 °C it may instead melt incongruously into a mixture of solid NaOH·3.5H2O and a liquid solution.
The β form of the tetrahydrate is metastable, and often transforms spontaneously to the α form when cooled below −20 °C. Once initiated, the exothermic transformation is complete in a few minutes, with a 6.5% increase in volume of the solid. The β form can be crystallized from supercooled solutions at −26 °C, and melts partially at −1.83 °C.
The "sodium hydroxide" of commerce is often the monohydrate (density 1.829 g/cm3). Physical data in technical literature may refer to this form, rather than the anhydrous compound.
Crystal structure
NaOH and its monohydrate form orthorhombic crystals with the space groups Cmcm (oS8) and Pbca (oP24), respectively. The monohydrate cell dimensions are a = 1.1825, b = 0.6213, c = 0.6069 nm. The atoms are arranged in a hydrargillite-like layer structure, with each sodium atom surrounded by six oxygen atoms, three each from hydroxide ions and three from water molecules. The hydrogen atoms of the hydroxyls form strong bonds with oxygen atoms within each O layer. Adjacent O layers are held together by hydrogen bonds between water molecules
Additional Information
Caustic soda is the chemical compound sodium hydroxide (NaOH). This compound is an alkali – a type of base that can neutralize acids and is soluble in water. Today caustic soda can be manufactured in the form of pellets, flakes, powders, solutions and more.
What is caustic soda used for?
Caustic soda has become a common ingredient in the production of many everyday items. Commonly known as lye, it has been used to make soap for centuries, and its ability to dissolve grease makes it a common ingredient in oven cleaners and products used to unclog drains.
Caustic soda is often used to manufacture cleaning products like soaps and detergents.
Sodium hydroxide also plays a key role in processing wood pulp to create paper and cardboard boxes, which have become increasingly essential over the course of the global COVID-19 pandemic as medical supplies are shipped long distances.
The chemical compound is also used to break down the sedimentary rock that aluminum is extracted from. The mineral then goes on to be used in a number of items like construction materials, automobiles and consumer goods like food packaging and soda cans.
One perhaps unexpected use for caustic soda is in the manufacturing of pharmaceuticals like blood thinners and cholesterol medication.
A versatile water treatment product, sodium hydroxide is often used to maintain the safety and cleanliness of pools by removing harmful metals like lead and copper. As a base, sodium hydroxide lowers acidity, regulating water's pH. Additionally, the compound can be used to create sodium hypochlorite, which further disinfects water.
A co-product of the chlorine manufacturing process, caustic soda has been used for decades to create products that enhance our lives every day.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1