Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-10-16 22:32:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,012
Phosphoric Acid
Phosphoric Acid
Gist
Phosphoric acid (H3PO4) is a weak mineral acid used in food, agriculture, and industry, and it is found as a clear liquid or white crystalline solid. In foods, it's used as an acidulant and preservative, while in fertilizers, it promotes plant growth. Due to its corrosive nature, concentrated solutions require protective gear to avoid skin, eye, and respiratory irritation.
Phosphoric acid has numerous uses, most notably in the production of phosphate fertilizers. It is also used in the food and beverage industry as an acidic flavoring agent and preservative, particularly in soft drinks. Other common applications include metal treatment, cleaning products, water treatment, detergents, and in the manufacturing of some pharmaceuticals and personal care products.
Summary
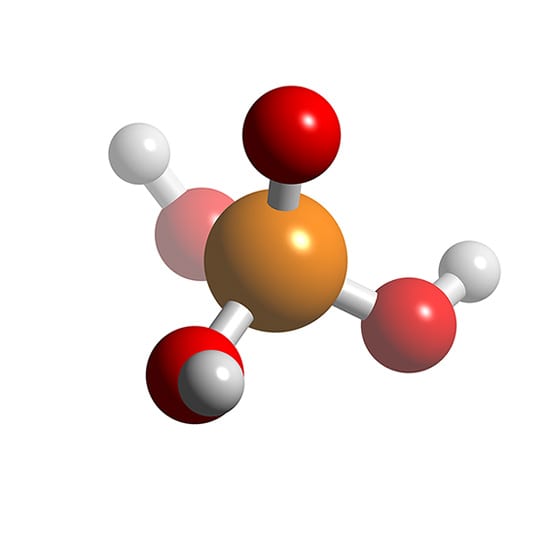
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H3PO4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.
The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature.
Purification
Phosphoric acid produced from phosphate rock or thermal processes often requires purification. A common purification method is liquid–liquid extraction, which involves the separation of phosphoric acid from water and other impurities using organic solvents, such as tributyl phosphate (TBP), methyl isobutyl ketone (MIBK), or n-octanol. Nanofiltration involves the use of a premodified nanofiltration membrane, which is functionalized by a deposit of a high molecular weight polycationic polymer of polyethyleneimines. Nanofiltration has been shown to significantly reduce the concentrations of various impurities, including cadmium, aluminum, iron, and rare earth elements. The laboratory and industrial pilot scale results showed that this process allows the production of food-grade phosphoric acid.
Fractional crystallization can achieve higher purities typically used for semiconductor applications. Usually a static crystallizer is used. A static crystallizer uses vertical plates, which are suspended in the molten feed and which are alternatingly cooled and heated by a heat transfer medium. The process begins with the slow cooling of the heat transfer medium below the freezing point of the stagnant melt. This cooling causes a layer of crystals to grow on the plates. Impurities are rejected from the growing crystals and are concentrated in the remaining melt. After the desired fraction has been crystallized, the remaining melt is drained from the crystallizer. The purer crystalline layer remains adhered to the plates. In a subsequent step, the plates are heated again to liquify the crystals and the purified phosphoric acid drained into the product vessel. The crystallizer is filled with feed again and the next cooling cycle is started.
Details
Phosphoric acid, (H3PO4) is the most important oxygen acid of phosphorus, used to make phosphate salts for fertilizers. It is also used in dental cements, in the preparation of albumin derivatives, and in the sugar and textile industries. It serves as an acidic, fruitlike flavouring in food products.
Pure phosphoric acid is a crystalline solid (melting point 42.35° C, or 108.2° F); in less concentrated form it is a colourless syrupy liquid. The crude acid is prepared from phosphate rock, while acid of higher purity is made from white phosphorus.
Phosphoric acid forms three classes of salts corresponding to replacement of one, two, or three hydrogen atoms. Among the important phosphate salts are: sodium dihydrogen phosphate (NaH2PO4), used for control of hydrogen ion concentration (acidity) of solutions; disodium hydrogen phosphate (Na2HPO4), used in water treatment as a precipitant for highly charged metal cations; trisodium phosphate (Na3PO4), used in soaps and detergents; calcium dihydrogen phosphate or calcium superphosphate (Ca[H2PO4]2), a major fertilizer ingredient; calcium monohydrogen phosphate (CaHPO4), used as a conditioning agent for salts and sugars.
Phosphoric acid molecules interact under suitable conditions, often at high temperatures, to form larger molecules (usually with loss of water). Thus, diphosphoric, or pyrophosphoric, acid (H4P2O7) is formed from two molecules of phosphoric acid, less one molecule of water. It is the simplest of a homologous series of long chain molecules called polyphosphoric acids, with the general formula H(HPO3)nOH, in which n = 2, 3, 4, . . . . Metaphosphoric acids, (HPO3)n, in which n = 3, 4, 5, . . ., are another class of polymeric phosphoric acids. The known metaphosphoric acids are characterized by cyclic molecular structures. The term metaphosphoric acid is used also to refer to a viscous, sticky substance that is a mixture of both long chain and ring forms of (HPO3)n. The various polymeric forms of phosphoric acid are also prepared by hydration of phosphorus oxides.
Additional Information
Phosphoric acid, also known as orthophosphoric acid, is a chemical compound. It is also an acid. Its chemical formula is H3PO4. It contains hydrogen and phosphate ions. Its official IUPAC name is trihydroxidooxidophosphorus.
Properties
Phosphoric acid is a white solid. It melts easily to make a viscous liquid. It tastes sour when diluted (mixed with a lot of water). It can be deprotonated three times. It is very strong, although not as much as the other acids like hydrochloric acid. It does not have any odor. It is corrosive when concentrated. Salts of phosphoric acid are called phosphates.
Preparation
Phosphoric acid can be made by dissolving phosphorus(V) oxide in water. This makes a very pure phosphoric acid that is good for food. A less pure form is made by reacting sulfuric acid with phosphate rock. This can be purified to make food-grade phosphoric acid if needed.
Uses
It is used to make sodas sour. It is also used when a nonreactive acid is needed. It can be used to make hydrogen halides, such as hydrogen chloride. Phosphoric acid is heated with a sodium halide to make the hydrogen halide and sodium phosphate. It is used to react with rust to make black iron(III) phosphate, which can be scraped off, leaving pure iron. It can be used to clean teeth.
There are many minor uses of phosphoric acid. Phosphoric acid with a certain isotope of phosphorus is used for nuclear magnetic resonance. It is also used as an electrolyte in some fuel cells. It can be used as a flux. It can etch certain things in semiconductor making.
Safety
Phosphoric acid is one of the least toxic acids. When it is diluted, it just has a sour taste. When it is concentrated, it can corrode metals.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1