Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-09-30 21:36:14
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,726
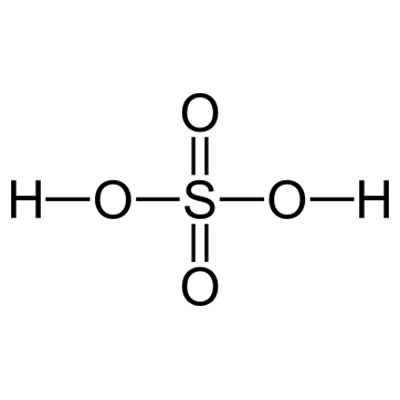
Sulfuric Acid
Sulfuric Acid
Gist
Sulfuric acid (H2SO4) is a highly corrosive, colorless, strong mineral acid used in major industrial applications like fertilizer, battery, and chemical manufacturing. It's known for its dehydrating and oxidizing properties, especially at high concentrations. Due to its dangerous nature, it can cause severe burns and irritation upon contact with skin, eyes, and respiratory systems, so it must be handled with extreme caution.
Sulfuric acid's primary use, accounting for a large portion of its production, is in the manufacturing of fertilizers to enrich soil. It is also crucial for producing other chemicals, including acids, detergents, dyes, and pigments, and serves as a vital component in lead-acid batteries. Other significant industrial applications involve petroleum refining, metal processing (like steel pickling), rayon production, and as a powerful drain cleaner for home and industrial use.
Summary
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.
Pure sulfuric acid does not occur naturally due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus, the reverse procedure of adding water to the acid is generally avoided since the heat released may boil the solution, spraying droplets of hot acid during the process. Upon contact with body tissue, sulfuric acid can cause severe acidic chemical burns and secondary thermal burns due to dehydration. Dilute sulfuric acid is substantially less hazardous without the oxidative and dehydrating properties; though, it is handled with care for its acidity.
Many methods for its production are known, including the contact process, the wet sulfuric acid process, and the lead chamber process. Sulfuric acid is also a key substance in the chemical industry. It is most commonly used in fertilizer manufacture but is also important in mineral processing, oil refining, wastewater treating, and chemical synthesis. It has a wide range of end applications, including in domestic acidic drain cleaners, as an electrolyte in lead-acid batteries, as a dehydrating compound, and in various cleaning agents. Sulfuric acid can be obtained by dissolving sulfur trioxide in water.
Details
Sulfuric acid is a dense, colourless, oily, corrosive liquid; one of the most commercially important of all chemicals. Sulfuric acid is prepared industrially by the reaction of water with sulfur trioxide (see sulfur oxide), which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. In one of its most familiar applications, sulfuric acid serves as the electrolyte in lead–acid storage batteries.
Pure sulfuric acid has a specific gravity of 1.830 at 25 °C (77 °F); it freezes at 10.37 °C (50.7 °F). When heated, the pure acid partially decomposes into water and sulfur trioxide; the latter escapes as a vapour until the concentration of the acid falls to 98.3 percent. This mixture of sulfuric acid and water boils at a constant temperature of 338 °C (640 °F) at one atmosphere pressure. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent.
Due to its affinity for water, pure anhydrous sulfuric acid does not exist in nature. Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. These aerosols can then reform into sulfur dioxide (SO2), a constituent of acid rain, though volcanic activity is a relatively minor contributor to acid rainfall.
Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4−). In dilute solutions the hydrogen sulfate ions also dissociate, forming more hydronium ions and sulfate ions. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue.
The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals.
Additional Information
Sulfuric acid is one of the most important compounds made by the chemical industry. It is used to make, literally, hundreds of compounds needed by almost every industry.
Uses of sulfuric acid
By far the largest amount of sulfuric acid is used to make phosphoric acid, used, in turn, to make the phosphate fertilizers, calcium dihydrogenphosphate and the ammonium phosphates. It is also used to make ammonium sulfate, which is a particularly important fertilizer in sulfur-deficient.
It is widely used in metal processing for example in the manufacture of copper and the manufacture of zinc and in cleaning the surface of steel sheet, known as 'pickling', prior to it being covered in a thin layer of tin, used to make cans for food.
It is also used to make caprolactam, which is converted into polyamide 6 and in the manufacture of titanium dioxide, used, for example, as a pigment.
Amongst its many other uses is in the manufacture of hydrofluoric acid and phenol with propanone all of which are used in many industries.
Manufacture of sulfuric acid
The process for producing sulfuric acid has four stages:
a) extraction of sulfur
b) conversion of sulfur to sulfur dioxide
c) conversion of sulfur dioxide to sulfur trioxide
d) conversion of sulfur trioxide to sulfuric acid
(a) Extraction of sulfur
Easily the most important source of sulfur is its recovery from natural gas and oil. These contain sulfur compounds, both organic and hydrogen sulfide both of which must be removed before they are used as fuels or chemical feedstock.
Another important source of sulfur is as sulfur dioxide from metal refining. Many metal ores occur as sulfides and are roasted to form an oxide and sulfur dioxide, for example, in the manufacture of lead:
Other metals manufactured from their sulfide ores include copper, nickel and zinc.
Worldwide about 35% of the sulfur is obtained as sulfur dioxide from sulfide ore roasting and this is increasing, as plants which traditionally passed the sulfur dioxide to atmosphere are recovering it as sulfuric acid. In particular, China makes most of its sulfuric acid from pyrites, an iron sulfide ore.
Sulfuric acid is also obtained from ammonium sulfate, a by-product in the manufacture of poly(methyl 2-methylpropenoate) and also recovered from 'spent' (i.e. used) sulfuric acid.
(b) Conversion of sulfur to sulfur dioxide
If sulfur is the feedstock, it must first be converted to sulfur dioxide. Molten sulfur is sprayed into a furnace and burnt in a blast of dry air at about 1300 K. The sulfur burns with a characteristic blue flame.
As excess air is used the emerging gas contains about 10-12% sulfur dioxide and 10% oxygen, by volume. The gases are very hot and so are passed through heat exchangers (waste heat boilers).
The gases are cooled to about 700 K and the water in the surrounding boiler pipes is converted into steam. In manufacturing one tonne of sulfuric acid, one tonne of high pressure steam is also produced.
(c) Conversion of sulfur dioxide to sulfur trioxide (The Contact Process)
A typical plant contains one cylindrical vessel which acts as a fixed bed reactor with four separate beds of catalyst, known as a converter, heated to 700 K, through which the sulfur dioxide and air pass.
The catalyst, vanadium(V) oxide on silica, is generally in the form of small pellets, to which caesium sulfate has been added as a promoter (Figure 2). The function of the promoter is to lower the melting point of vanadium(V) oxide so that it is molten at 700 K.
(d) Conversion of sulfur trioxide to sulfuric acid
The sulfur trioxide formed from the third bed (and the small amount from the fourth bed) are now converted to sulfuric acid.
However, water itself cannot be used for absorption as there is a large temperature rise, and a sulfuric acid mist is formed, which is difficult to handle. Instead, sulfuric acid of about 98% concentration is used. This is kept at this concentration by addition of water and removal of acid at that concentration.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1