Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2201 2024-07-04 14:02:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2203) LDL
Gist
LDL , the "bad" cholesterol, transports cholesterol particles throughout your body. LDL cholesterol builds up in the walls of your arteries, making them hard and narrow. High-density lipoprotein (HDL). HDL , the "good" cholesterol, picks up excess cholesterol and takes it back to your liver.
Summary
Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons (aka ULDL by the overall density naming convention), very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to cells. LDL has been associated with the progression of atherosclerosis.
Overview
Lipoproteins transfer lipids (fats) around the body in the extracellular fluid, making fats available to body cells for receptor-mediated endocytosis. Lipoproteins are complex particles composed of multiple proteins, typically 80–100 proteins per particle (organized by a single apolipoprotein B for LDL and the larger particles). A single LDL particle is about 220–275 angstroms in diameter, typically transporting 3,000 to 6,000 fat molecules per particle, and varying in size according to the number and mix of fat molecules contained within. The lipids carried include all fat molecules with cholesterol, phospholipids, and triglycerides dominant; amounts of each vary considerably.
A good clinical interpretation of blood lipid levels is that high LDL, in combination with a high amount of triglycerides, which indicates a high likelihood of the LDL being oxidised, is associated with increased risk of cardiovascular diseases.
Biochemistry
Structure
Each native LDL particle enables emulsification, i.e. surrounding the fatty acids being carried, enabling these fats to move around the body within the water outside cells. Each particle contains a single apolipoprotein B-100 molecule (Apo B-100, a protein that has 4536 amino acid residues and a mass of 514 kDa), along with 80 to 100 additional ancillary proteins. Each LDL has a highly hydrophobic core consisting of polyunsaturated fatty acid known as linoleate and hundreds to thousands (about 1500 commonly cited as an average) of esterified and unesterified cholesterol molecules. This core also carries varying numbers of triglycerides and other fats and is surrounded by a shell of phospholipids and unesterified cholesterol, as well as the single copy of Apo B-100. LDL particles are approximately 22 nm (0.00000087 in.) to 27.5 nm in diameter and have a mass of about 3 million daltons. Since LDL particles contain a variable and changing number of fatty acid molecules, there is a distribution of LDL particle mass and size. Determining the structure of LDL has been a tough task because of its heterogeneous structure. However, the structure of LDL at human body temperature in native condition, with a resolution of about 16 Angstroms using cryogenic electron microscopy, has been described in 2011.
Details
Cholesterol travels in the blood via “bad” LDL (low-density lipoprotein) and “good” HDL (high-density lipoprotein). Excess LDL cholesterol can form plaque on blood vessels, narrowing them and making it hard for blood to reach organs like the heart.
Blood cholesterol, a waxy, fat-like substance, is made by your liver. Cholesterol is essential for full-body health. It’s needed for actions such as hormone creation and digesting fatty foods.
While our bodies make all the cholesterol we need, dietary cholesterol is found in most animal foods: meat, poultry, eggs, seafood, and dairy products.
What is LDL cholesterol?
Cholesterol is carried through the blood on two types of proteins called lipoproteins. These lipoproteins include LDL (low-density lipoprotein), which is sometimes referred to as “bad” cholesterol, and HDL (high-density lipoprotein), or what is typically referred to as “good” cholesterol.
The science over “good” and “bad” cholesterol has shifted quite a bit recently, so how can you be sure that you’re not putting your health in danger? Read on for everything you need to know about LDL — backed by the most recent science.
LDL vs. HDL, good vs. bad
If cholesterol is essential for overall health, why would one type be bad?
In simple terms, if there is too much LDL cholesterol running through your blood vessels, it can, over time, start to build up on the sides of those blood vessels. This buildup is typically referred to as “plaque.”
Plaque buildup in your blood vessels can eventually cause those vessels to become narrower. The more narrow your blood vessels are, the harder it is for blood to reach your heart and other organs.
When blood flow becomes very blocked, it can cause chest pain (angina) and even a heart attack.
HDL cholesterol, on the other hand, returns cholesterol to the liver so it can be flushed from the body.
What should your LDL level be?
In general, most adults want to keep their LDL cholesterol levels in a certain rangeTrusted Source. Because a lot of other personal factors play into these numbers, it’s important to have a healthcare professional check your levels to help them create specific recommendations for you to go by.
LDL Cholesterol Level : Category
Less than 100mg/dL : Recommended
100-129mg/dL : Slightly above recommended
130-159 mg/dL : Borderline high
160-189 mg/dL : High
190 mg/dL and above Very high
Dangers of high cholesterol
If you have high LDL (bad) cholesterol, you may not even know it, because there are typically no symptoms associated with this issue. This is why routine blood work is so important.
If you have extremely high LDL levels, you may notice little bumps on your skin called xanthomas or gray-white rings around the corneas of your eye called corneal arcus.
High LDL complications
Besides heart attack, there are other serious complications of not treating “bad” cholesterol.
* atherosclerosis, which is plaque buildup throughout the body
* carotid artery disease
* coronary heart disease
* peripheral artery disease
* stroke
* sudden cardiac arrest
Certain individuals may need medication or surgery due to complications of long-term high cholesterol.
LDL diagnosis
The best way to find out if you have too much LDL cholesterol is having your doctor order a blood test that checks your levels. Your doctor will also request and review your family history, as high cholesterol can sometimes be hereditary.
The test your doctor will likely order is called a lipid panel. This panel shows your LDL, HDL, and other types of non-HDL cholesterol that can raise your risk of complications.
You will be diagnosed with “high cholesterol” if your non-HDL cholesterol level is higher than what your doctor thinks is ideal for you. Your doctor will also review your lab tests to see if your HDL, the healthy cholesterol, is too low.
There may be follow-up tests and visits if your doctor is concerned that you may need medication or further intervention.
How common is high cholesterol?
According to the Centers for Disease Control and Prevention (CDC), between 2015 and 2016, more than 12 percent of adults ages 20 and older had total cholesterol levels higher than 240 mg/dL, which is quite high. About 7 percent of U.S. children and adolescents ages 6 to 19 were also found to have high cholesterol.
While it’s known that individuals living with high cholesterol are at an elevated risk of developing heart disease, new research suggests that individuals living with moderately high cholesterol for a long time, who also have higher blood pressure, may have the same risk of heart disease as those who have high cholesterol for only a short period of time.
Who needs to get checked?
Everyone should get their cholesterol checked, starting at age 20 and then every 4 to 6 years after that if their risk remains low.
After age 40, your doctor may want to check your levels more often. Typically, people assigned male at birth who are ages 45 to 65, along with people assigned female at birth who are ages 55 to 65, should have their cholesterol checked every 1 to 2 years.
Risk factors for high cholesterol
Everyone’s risk for high cholesterol goes up with age. This is because the older we get, the harder it becomes for our bodies to filter out cholesterol.
A family history of high cholesterol can also increase risk.
While it’s impossible to control aging and family history, there are some behaviors that increase the risk of developing high cholesterol that can be changed
Individuals living with obesity and type 2 diabetes are more at risk for an increase in bad cholesterol and a dip in good cholesterol.
It’s important to work with your doctor, who can provide support and resources, to help you adhere to their recommendations on how to lower your risk. Recommendations may include losing excess weight and focusing on finding what works best for you in managing your diabetes.
Other behaviors that may put you at a higher risk include:
* smoking, which can damage blood vessels and may lower good cholesterol
* eating a diet high in saturated and trans fat, which includes foods like fatty meats and dairy-based desserts
* not getting enough physical movement throughout the week (2 hours and 30 minutes of moderate-intensity exercise per week is recommended)
* drinking an excess of alcohol
The composition of LDL cholesterol: Why it matters
While it was traditionally thought that high LDL cholesterol as a whole was “bad” and a predictor of heart disease complications, new research, including a 2019 study from Ohio University, suggests that the real predictor of complications may be a particular subclass of LDL.
LDL is comprised of three subclasses of low-density lipoproteins, A, B, and I. According to researchers, one subclass — subclass B — was found to be the most damaging and a much better predictor of potential heart attacks than the total measurement of LDL.
While this type of research is new and evolving, if you are concerned about your LDL numbers and the possibility of complications, talk with your doctor.
How to lower LDL cholesterol
If you’ve been diagnosed with high LDL, the good news is that there are ways to lower it to a healthier range.
If your doctor is concerned about your LDL levels, they may prescribe medication, such as:
* Statins. Statins are the most commonly prescribed medication for high cholesterol. They have been shown to lower the risk of heart attack and stroke in individuals with high LDL
* Ezetimibe. These medications are sometimes prescribed if statins are not effective.
* Bile acid sequestrants. These medications are prescribed if an individual cannot take statins, or if their cholesterol levels need to be lowered more than statins alone can do.
* PCSK9 inhibitors. PCSK9 inhibitors are injected into the skin every couple of weeks and are prescribed when someone is at an unusually high risk for complications
* Lomitapide and Mipomersen. These drugs are typically prescribed for individuals who have a family history of high cholesterol.
Each drug has its own side effects, so it’s important to talk with your doctor about why they’re prescribing a specific medication and what the possible side effects might be.
Your doctor will also likely recommend specific lifestyle changes regardless of whether you’re prescribed medication.
Lifestyle changes
If your lipid test shows high or borderline-high LDL levels, your doctor will most likely recommend some lifestyle changes that can make a positive impact on your cholesterol as a whole based on your specific situation.
Increase physical activity
Regular physical activity can help lower both your cholesterol and blood pressure levels, and may even help you lose excess weight (if that’s something your doctor has advised or it’s simply a personal goal). Moderate exercise, which can be anything from brisk walking to riding a bike, for a few hours a week is helpful.
Eat a heart-healthy diet
Focusing on the things you can eat on a heart-healthy diet, instead of focusing on things you should not eat, can make this lifestyle change seem less daunting. When you’re eating for heart health and to lower cholesterol, it’s a great idea to focus on:
* lean meats
* seafood
* fat-free or low fat milk, cheese, and yogurt
* whole grains
* fruits and vegetables
Eating foods that are naturally high in fiber, like oatmeal and beans, as well as unsaturated fats, like olive oil, avocados, and nuts, are also good choices when you’re eating for heart health.
Talking with a dietician is a great way to make sure your new diet includes all the essential nutrients and vitamins you need to stay healthy and energized.
Limit alcohol
Drinking too much alcohol can raise triglycerides. When you combine elevated triglycerides with high LDL cholesterol levels, it can increase your risk for heart attack and stroke. Limiting your alcohol intake, or cutting it out entirely, can help your body recover.
Quit smoking
Smoking is difficult on your body in a number of ways, including aiding LDLs in creating narrower blood vessels. If you smoke, consider quitting. Talk with your doctor about cessation programs and other supportive resources that can help you begin the process of quitting smoking.
Outlook
Getting your cholesterol levels checked, especially if you have a family history of high cholesterol, is an essential part of staying informed about your health. If you’re younger than age 40, you may only need to get it checked every few years, but your doctor will help you decide what’s best.
If you see high LDL levels on your lipid test, remember you’re not alone. Over 93 million U.S. adults ages 20 and older have what would be considered high cholesterol. And there are many ways to treat elevated “bad” cholesterol levels, from medication to lifestyle changes.
Taking a proactive approach to lowering your cholesterol is also a positive step toward increasing your overall health — so it’s a win-win situation.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2202 2024-07-05 14:09:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2204) HDL
Gist
High-density lipoprotein (HDL) cholesterol is known as the "good" cholesterol because it helps remove other forms of cholesterol from your bloodstream. Higher levels of HDL cholesterol are associated with a lower risk of heart disease.
Summary
High-density lipoprotein (HDL) is one of the five major groups of lipoproteins. Lipoproteins are complex particles composed of multiple proteins which transport all fat molecules (lipids) around the body within the water outside cells. They are typically composed of 80–100 proteins per particle (organized by one, two or three ApoA). HDL particles enlarge while circulating in the blood, aggregating more fat molecules and transporting up to hundreds of fat molecules per particle.
Overview
Lipoproteins are divided into five subgroups, by density/size (an inverse relationship), which also correlates with function and incidence of cardiovascular events. Unlike the larger lipoprotein particles, which deliver fat molecules to cells, HDL particles remove fat molecules from cells. The lipids carried include cholesterol, phospholipids, and triglycerides, amounts of each are variable.
Increasing concentrations of HDL particles are associated with decreasing accumulation of atherosclerosis within the walls of arteries, reducing the risk of sudden plaque ruptures, cardiovascular disease, stroke and other vascular diseases. HDL particles are commonly referred to as "good cholesterol", because they transport fat molecules out of artery walls, reduce macrophage accumulation, and thus help prevent or even regress atherosclerosis. Higher HDL-C may not necessarily be protective against cardiovascular disease and may even be harmful in extremely high quantities, with an increased cardiovascular risk, especially in hypertensive patients.
Testing
Because of the high cost of directly measuring HDL and LDL (low-density lipoprotein) protein particles, blood tests are commonly performed for the surrogate value, HDL-C, i.e. the cholesterol associated with ApoA-1/HDL particles. In healthy individuals, about 30% of blood cholesterol, along with other fats, is carried by HDL. This is often contrasted with the amount of cholesterol estimated to be carried within low-density lipoprotein particles, LDL, and called LDL-C. HDL particles remove fats and cholesterol from cells, including within artery wall atheroma, and transport it back to the liver for excretion or re-utilization; thus the cholesterol carried within HDL particles (HDL-C) is sometimes called "good cholesterol" (despite being the same as cholesterol in LDL particles). Those with higher levels of HDL-C tend to have fewer problems with cardiovascular diseases, while those with low HDL-C cholesterol levels (especially less than 40 mg/dL or about 1 mmol/L) have increased rates for heart disease. Higher native HDL levels are correlated with lowered risk of cardiovascular disease in healthy people.
The remainder of the serum cholesterol after subtracting the HDL is the non-HDL cholesterol. The concentration of these other components, which may cause atheroma, is known as the non-HDL-C. This is now preferred to LDL-C as a secondary marker as it has been shown to be a better predictor and it is more easily calculated.
Details
In the blood, cholesterol is transported by lipoproteins known as high-density lipoprotein (HDL) and low-density lipoprotein (LDL). HDL takes cholesterol to the liver for release, while LDL brings it to the arteries. You want to aim for high HDL and low LDL levels.
Your body needs cholesterol to function properly, including making hormones and vitamin D, and supporting digestion.
Your liver generates enough cholesterol to handle these tasks, but your body doesn’t just get cholesterol this way.
Food is the main source of cholesterol, especially meat and dairy. If you eat a lot of these foods and have risk factors, your cholesterol levels may become elevated over time.
Lipoproteins are made of fat and proteins. They serve as carriers for cholesterol to move through your body.
HDL is popularly known as “good cholesterol” because it collects other types of cholesterol from the body and transports them to the liver to be released from the body.
LDL transports large amounts of cholesterol to the arteries for cell repair. Often called “bad cholesterol” because when it occurs in excess, it can build up in artery walls.
Too much cholesterol in the arteries may lead to a buildup of plaque known as atherosclerosis, which can increase the risk of blood clots.
If a blood clot breaks away and blocks an artery in your heart or brain, you may have a stroke or heart attack.
Plaque buildup may also reduce blood flow and oxygen to major organs. Oxygen deprivation to your organs or arteries may lead to other complications, like kidney disease or peripheral arterial disease.
Optimal levels of HDL can protect your body from LDL. HDL helps rid the body of excess LDL cholesterol, making it less likely to end up in the arteries.
Lifestyle factors are the main factor in cholesterol levels. You may have higher LDL levels and lower HDL levels if you:
* have obesity
* follow a diet high in red meat, full-fat dairy products, saturated fats, trans fats, and processed foods
* have a large waist circumference (over 40 inches for males or over 35 inches for females)
* do not engage in regular physical activity and exercise
* use tobacco
In some cases, high LDL is inherited. This condition is called familial hypercholesterolemia (FH). FH is caused by a genetic mutation that affects the ability of a person’s liver to get rid of extra LDL cholesterol.
This may lead to high LDL levels and an increased risk of heart attack and stroke at a young age.
Know your numbers
You may not even know if you have high cholesterol because it doesn’t cause noticeable symptoms.
The only way to find out your cholesterol levels is through a blood test that measures cholesterol in milligrams per deciliter of blood (mg/dL).
When you get your cholesterol numbers checked, you may receive results for:
* Triglycerides: This number may vary per laboratory, but it should usually be below 150 mg/dL. Triglycerides are a common type of fat. If your triglycerides are high, your LDL is also high, or your HDL is low, your risk of developing atherosclerosis may be elevated.
* HDL: The higher this number, the better. It should be at least higher than 50 mg/dL for females and 40 mg/dL for males.
* LDL: The lower this number, the better. Experts recommend LDL to be no more than 130 mg/dL if you don’t have a history of heart disease, blood vessel disease, or diabetes. If you do have a history of these conditions, LDL should be no more than 70 mg/dL or 55 mg/dL if a doctor believes you are at an increased risk.
* Total blood cholesterol: This includes your HDL, LDL, and 20% of your total triglycerides, and it should be within the normal range your laboratory sets.
How to manage suboptimal cholesterol levels
Experts often recommend lifestyle changes to manage high LDL and total cholesterol levels, including:
* eating a balanced, nutritious diet
* exercising regularly and moving more throughout the day
* managing stress
* maintaining the recommended weight for your age and height
* ceasing to use tobacco, if you smoke
Sometimes lifestyle changes aren’t enough, especially if you have FH. You may need ongoing management with one or more cholesterol-lowering medications, such as:
* statins to help your liver get rid of cholesterol
* bile-acid binding medications to help your body use extra cholesterol to produce bile
* cholesterol absorption inhibitors to prevent your small intestines from absorbing cholesterol and releasing it into your bloodstream
* injectable medications that cause your liver to absorb more LDL cholesterol
Medications and supplements to reduce triglyceride levels may also be used, such as omega-3 fatty acids and fibrates.
The takeaway
LDL refers to a low-density protein that carries cholesterol to the arteries. If there’s too much LDL cholesterol, you may have a higher risk of heart disease and stroke.
HDL is a high-density protein that collects cholesterol from the body and takes it to the liver for removal. Optimal levels of HDL help the body get rid of LDL cholesterol, so the higher your HDL, the better.
A blood test can let you know how much LDL and HDL you have, and a doctor can advise on the next steps if you have high cholesterol.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2203 2024-07-06 14:05:26
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2205) Spectrometer
Gist
A spectrometer is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed.
Summary
Spectrometer, Device for detecting and analyzing wavelengths of electromagnetic radiation, commonly used for molecular spectroscopy; more broadly, any of various instruments in which an emission (as of electromagnetic radiation or particles) is spread out according to some property (as energy or mass) into a spectrum and measurements are made at points or regions along the spectrum. As used in traditional laboratory analysis, a spectrometer includes a radiation source and detection and analysis equipment. Emission spectrometers excite molecules of a sample to higher energy states and analyze the radiation emitted when they decay to the original energy state. Absorption spectrometers pass radiation of known wavelength through a sample, varying the wavelengths to produce a spectrum of results; the detector system reveals to what extent each wavelength is absorbed. Fourier-transform spectrometers resemble absorption spectrometers but use a broad band of radiation; a computer analyzes the output to find the absorption spectrum. Different designs allow study of various kinds of samples over many frequencies, at different temperatures or pressures, or in an electric or magnetic field. Mass spectrometers spread out the atomic or molecular components in a sample according to their masses and then detect the sorted components.
Details
A spectrometer is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in astronomy to analyze the chemical composition of stars and planets, and spectrometers gather data on the origin of the universe.
Examples of spectrometers are devices that separate particles, atoms, and molecules by their mass, momentum, or energy. These types of spectrometers are used in chemical analysis and particle physics.
Types of spectrometer
Optical spectrometers or optical emission spectrometer
Optical absorption spectrometers
Optical spectrometers (often simply called "spectrometers"), in particular, show the intensity of light as a function of wavelength or of frequency. The different wavelengths of light are separated by refraction in a prism or by diffraction by a diffraction grating. Ultraviolet–visible spectroscopy is an example.
These spectrometers utilize the phenomenon of optical dispersion. The light from a source can consist of a continuous spectrum, an emission spectrum (bright lines), or an absorption spectrum (dark lines). Because each element leaves its spectral signature in the pattern of lines observed, a spectral analysis can reveal the composition of the object being analyzed.
A spectrometer that is calibrated for measurement of the incident optical power is called a spectroradiometer. [2]
Optical emission spectrometers
Optical emission spectrometers (often called "OES or spark discharge spectrometers"), is used to evaluate metals to determine the chemical composition with very high accuracy. A spark is applied through a high voltage on the surface which vaporizes particles into a plasma. The particles and ions then emit radiation that is measured by detectors (photomultiplier tubes) at different characteristic wavelengths.
Electron spectroscopy
Some forms of spectroscopy involve analysis of electron energy rather than photon energy. X-ray photoelectron spectroscopy is an example.
Mass spectrometer
A mass spectrometer is an analytical instrument that is used to identify the amount and type of chemicals present in a sample by measuring the mass-to-charge ratio and abundance of gas-phase ions.
Time-of-flight spectrometer
The energy spectrum of particles of known mass can also be measured by determining the time of flight between two detectors (and hence, the velocity) in a time-of-flight spectrometer. Alternatively, if the particle-energy is known, masses can be determined in a time-of-flight mass spectrometer.
Magnetic spectrometer
When a fast charged particle (charge q, mass m) enters a constant magnetic field B at right angles, it is deflected into a circular path of radius r, due to the Lorentz force. The momentum p of the particle is then given by
,where m and v are mass and velocity of the particle. The focusing principle of the oldest and simplest magnetic spectrometer, the semicircular spectrometer, invented by J. K. Danisz, is shown on the left. A constant magnetic field is perpendicular to the page. Charged particles of momentum p that pass the slit are deflected into circular paths of radius r = p/qB. It turns out that they all hit the horizontal line at nearly the same place, the focus; here a particle counter should be placed. Varying B, this makes possible to measure the energy spectrum of alpha particles in an alpha particle spectrometer, of beta particles in a beta particle spectrometer, of particles (e.g., fast ions) in a particle spectrometer, or to measure the relative content of the various masses in a mass spectrometer.
Since Danysz' time, many types of magnetic spectrometers more complicated than the semicircular type have been devised.
Resolution
Generally, the resolution of an instrument tells us how well two close-lying energies (or wavelengths, or frequencies, or masses) can be resolved. Generally, for an instrument with mechanical slits, higher resolution will mean lower intensity.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2204 2024-07-07 14:20:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2206) Spirit lamp
Gist
An alcohol burner or spirit lamp is a piece of laboratory equipment used to produce an open flame. It can be made from brass, glass, stainless steel or aluminium.
Spirit lamps are often used for heating small test tubes, performing flame tests, and for simple chemistry experiments. They are also commonly used in medical settings for heating surgical instruments and for sterilizing small equipment.
Details
An alcohol burner or spirit lamp is a piece of laboratory equipment used to produce an open flame. It can be made from brass, glass, stainless steel or aluminium.
Uses
Alcohol burners are preferred for some uses over Bunsen burners for safety purposes, and in laboratories where natural gas is not available. Their flame is limited to approximately 5 centimeters (two inches) in height, with a comparatively lower temperature than the gas flame of the Bunsen burner.
While they do not produce flames as hot as other types of burners, they are sufficiently hot for performing some chemistries, standard microbiology laboratory procedures, and can be used for flame sterilization of other laboratory equipment.
A small alcohol burner is also preferred for camping when the need for fire is modest. It burns the alcohol vapor that rises due to the heat from the flame through the holes on the top perimeter of the container.
Operation
Typical fuel is denatured alcohol, methanol, or isopropanol. A cap is used as a snuffer for extinguishing the flame.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2205 2024-07-07 22:11:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2207) Rib cage
Gist
The rib cage consists of 24 ribs (2 sets of 12), which are attached to a long, flat bone in the centre of the chest called the sternum. The ribs are connected to the sternum with a strong, somewhat flexible material called cartilage.
Summary
Rib cage, in vertebrate anatomy, basketlike skeletal structure that forms the chest, or thorax, and is made up of the ribs and their corresponding attachments to the sternum (breastbone) and the vertebral column. The rib cage surrounds the lungs and the heart, serving as an important means of bony protection for these vital organs.In total, the rib cage consists of the 12 thoracic vertebrae and the 24 ribs, in addition to the sternum. With each succeeding rib, from the first, or uppermost, the curvature of the rib cage becomes more open. The rib cage is semirigid but expansile, able to increase in size. The small joints between the ribs and the vertebrae permit a gliding motion of the ribs on the vertebrae during breathing and other activities.
The first seven ribs in the rib cage are attached to the sternum by pliable cartilages called costal cartilages; these ribs are called true ribs. Of the remaining five ribs, which are called false, the first three have their costal cartilages connected to the cartilage above them. The last two, the floating ribs, have their cartilages ending in the muscle in the abdominal wall. The configuration of the lower five ribs gives freedom for the expansion of the lower part of the rib cage and for the movements of the diaphragm, which has an extensive origin from the rib cage and the vertebral column. The motion is limited by the ligamentous attachments between ribs and vertebrae.
Details
The rib cage or thoracic cage is an endoskeletal enclosure in the thorax of most vertebrates that comprises the ribs, vertebral column and sternum, which protect the vital organs of the thoracic cavity, such as the heart, lungs and great vessels and support the shoulder girdle to form the core part of the axial skeleton.
A typical human thoracic cage consists of 12 pairs of ribs and the adjoining costal cartilages, the sternum (along with the manubrium and xiphoid process), and the 12 thoracic vertebrae articulating with the ribs. The thoracic cage also provides attachments for extrinsic skeletal muscles of the neck, upper limbs, upper abdomen and back, and together with the overlying skin and associated fascia and muscles, makes up the thoracic wall.
In tetrapods, the rib cage intrinsically holds the muscles of respiration (diaphragm, intercostal muscles, etc.) that are crucial for active inhalation and forced exhalation, and therefore has a major ventilatory function in the respiratory system.
Structure
There are thirty-three vertebrae in the human vertebral column. The rib cage is associated with TH1−TH12. Ribs are described based on their location and connection with the sternum. All ribs are attached posteriorly to the thoracic vertebrae and are numbered accordingly one to twelve. Ribs that articulate directly with the sternum are called true ribs, whereas those that do not articulate directly are termed false ribs. The false ribs include the floating ribs (eleven and twelve) that are not attached to the sternum at all.
Attachment
The terms true ribs and false ribs describe rib pairs that are directly or indirectly attached to the sternum respectively. The first seven rib pairs known as the fixed or vertebrosternal ribs are the true ribs (Latin: costae verae) as they connect directly to the sternum via their own individual costal cartilages. The next five pairs (eighth to twelfth) are the false ribs (Latin: costae spuriae) or vertebrochondral ribs, which do not connect directly to the sternum. The first three pairs of vertebrochondral ribs (eighth to tenth) connect indirectly to the sternum via the costal cartilages of the ribs above them, and the overall elasticity of their articulations allows the bucket handle movements of the rib cage essential for respiratory activity.
The phrase floating rib (Latin: costae fluctuantes) or vertebral rib refers to the two lowermost (the eleventh and twelfth) rib pairs; so-called because they are attached only to the vertebrae and not to the sternum or any of the costal cartilages. These ribs are relatively small and delicate, and include a cartilaginous tip.
The spaces between the ribs are known as intercostal spaces; they contain the instrinsic intercostal muscles and the neurovascular bundles containing intercostal nerves, arteries and veins. The superficial surface of the rib cage is covered by the thoracolumbar fascia, which provides external attachments for the neck, back, pectoral and abdominal muscles.
Parts of rib
Each rib consists of a head, neck, and a shaft. All ribs are attached posteriorly to the thoracic vertebrae. They are numbered to match the vertebrae they attach to – one to twelve, from top (T1) to bottom. The head of the rib is the end part closest to the vertebra with which it articulates. It is marked by a kidney-shaped articular surface which is divided by a horizontal crest into two articulating regions. The upper region articulates with the inferior costal facet on the vertebra above, and the larger region articulates with the superior costal facet on the vertebra with the same number. The transverse process of a thoracic vertebra also articulates at the transverse costal facet with the tubercle of the rib of the same number. The crest gives attachment to the intra-articular ligament.
The neck of the rib is the flattened part that extends laterally from the head. The neck is about 3 cm long. Its anterior surface is flat and smooth, whilst its posterior is perforated by numerous foramina and its surface rough, to give attachment to the ligament of the neck. Its upper border presents a rough crest (crista colli costae) for the attachment of the anterior costotransverse ligament; its lower border is rounded.
On the posterior surface at the neck, is an eminence—the tubercle that consists of an articular and a non-articular portion. The articular portion is the lower and more medial of the two and presents a small, oval surface for articulation with the transverse costal facet on the end of the transverse process of the lower of the two vertebrae to which the head is connected. The non-articular portion is a rough elevation and affords attachment to the ligament of the tubercle. The tubercle is much more prominent in the upper ribs than in the lower ribs.
The angle of a rib (costal angle) may both refer to the bending part of it, and a prominent line in this area, a little in front of the tubercle. This line is directed downward and laterally; this gives attachment to a tendon of the iliocostalis muscle. At this point, the rib is bent in two directions, and at the same time twisted on its long axis.
The distance between the angle and the tubercle is progressively greater from the second to the tenth ribs. The area between the angle and the tubercle is rounded, rough, and irregular, and serves for the attachment of the longissimus dorsi muscle.
Bones:
Ribs and vertebrae
The first rib (the topmost one) is the most curved and usually the shortest of all the ribs; it is broad and flat, its surfaces looking upward and downward, and its borders inward and outward.
The head is small and rounded, and possesses only a single articular facet, for articulation with the body of the first thoracic vertebra. The neck is narrow and rounded. The tubercle, thick and prominent, is placed on the outer border. It bears a small facet for articulation with the transverse costal facet on the transverse process of T1. There is no angle, but at the tubercle, the rib is slightly bent, with the convexity upward, so that the head of the bone is directed downward. The upper surface of the body is marked by two shallow grooves, separated from each other by a slight ridge prolonged internally into a tubercle, the scalene tubercle, for the attachment of the anterior scalene; the anterior groove transmits the subclavian vein, the posterior the subclavian artery and the lowest trunk of the brachial plexus. Behind the posterior groove is a rough area for the attachment of the medial scalene. The under surface is smooth and without a costal groove. The outer border is convex, thick, and rounded, and at its posterior part gives attachment to the first digitation of the serratus anterior. The inner border is concave, thin, and sharp, and marked about its center by the scalene tubercle. The anterior extremity is larger and thicker than that of any of the other ribs.
The second rib is the second uppermost rib in humans or second most frontal in animals that walk on four limbs. In humans, the second rib is defined as a true rib since it connects with the sternum through the intervention of the costal cartilage anteriorly (at the front). Posteriorly, the second rib is connected with the vertebral column by the second thoracic vertebra. The second rib is much longer than the first rib, but has a very similar curvature. The non-articular portion of the tubercle is occasionally only feebly marked. The angle is slight and situated close to the tubercle. The body is not twisted so that both ends touch any plane surface upon which it may be laid; but there is a bend, with its convexity upward, similar to, though smaller than that found in the first rib. The body is not flattened horizontally like that of the first rib. Its external surface is convex, and looks upward and a little outward; near the middle of it is a rough eminence for the origin of the lower part of the first and the whole of the second digitation of the serratus anterior; behind and above this is attached the posterior scalene. The internal surface, smooth, and concave, is directed downward and a little inward: on its posterior part there is a short costal groove between the ridge of the internal surface of the rib and the inferior border. It protects the intercostal space containing the intercostal veins, intercostal arteries, and intercostal nerves.
The ninth rib has a frontal part at the same level as the first lumbar vertebra. This level is called the transpyloric plane, since the pylorus is also at this level.
The tenth rib attaches directly to the body of vertebra T10 instead of between vertebrae like the second through ninth ribs. Due to this direct attachment, vertebra T10 has a complete costal facet on its body.
The eleventh and twelfth ribs, the floating ribs, have a single articular facet on the head, which is of rather large size. They have no necks or tubercles, and are pointed at their anterior ends. The eleventh has a slight angle and a shallow costal groove, whereas the twelfth does not. The twelfth rib is much shorter than the eleventh rib, and only has a one articular facet.
Sternum
The sternum is a long, flat bone that forms the front of the rib cage. The cartilages of the top seven ribs (the true ribs) join with the sternum at the sternocostal joints. The costal cartilage of the second rib articulates with the sternum at the sternal angle making it easy to locate.
The manubrium is the wider, superior portion of the sternum. The top of the manubrium has a shallow, U-shaped border called the jugular (suprasternal) notch. The clavicular notch is the shallow depression located on either side at the superior-lateral margins of the manubrium. This is the site of the sternoclavicular joint, between the sternum and clavicle. The first ribs also attach to the manubrium.
The transversus thoracis muscle is innervated by one of the intercostal nerves and superiorly attaches at the posterior surface of the lower sternum. Its inferior attachment is the internal surface of costal cartilages two through six and works to depress the ribs.
Development
Expansion of the rib cage in males is caused by the effects of testosterone during puberty. Thus, males generally have broad shoulders and expanded chests, allowing them to inhale more air to supply their muscles with oxygen.
Variation
Variations in the number of ribs occur. About 1 in 200–500 people have an additional cervical rib, and there is a female predominance. Intrathoracic supernumerary ribs are extremely rare. The rib remnant of the 7th cervical vertebra on one or both sides is occasionally replaced by a free extra rib called a cervical rib, which can mechanically interfere with the nerves (brachial plexus) going to the arm.
In several ethnic groups, most significantly the Japanese, the tenth rib is sometimes a floating rib, as it lacks a cartilaginous connection to the seventh rib.
Function
The human rib cage is a component of the human respiratory system. It encloses the thoracic cavity, which contains the lungs. An inhalation is accomplished when the muscular diaphragm, at the floor of the thoracic cavity, contracts and flattens, while the contraction of intercostal muscles lift the rib cage up and out.
Expansion of the thoracic cavity is driven in three planes; the vertical, the anteroposterior and the transverse. The vertical plane is extended by the help of the diaphragm contracting and the abdominal muscles relaxing to accommodate the downward pressure that is supplied to the abdominal viscera by the diaphragm contracting. A greater extension can be achieved by the diaphragm itself moving down, rather than simply the domes flattening. The second plane is the anteroposterior and this is expanded by a movement known as the 'pump handle'. The downward sloping nature of the upper ribs are as such because they enable this to occur. When the external intercostal muscles contract and lift the ribs, the upper ribs are able also to push the sternum up and out. This movement increases the anteroposterior diameter of the thoracic cavity, and hence aids breathing further. The third, transverse, plane is primarily expanded by the lower ribs (some say it is the 7th to 10th ribs in particular), with the diaphragm's central tendon acting as a fixed point. When the diaphragm contracts, the ribs are able to evert (meaning turn outwards or inside out) and produce what is known as the bucket handle movement, facilitated by gliding at the costovertebral joints. In this way, the transverse diameter is expanded and the lungs can fill.
The circumference of the normal adult human rib cage expands by 3 to 5 cm during inhalation.
Clinical significance
Rib fractures are the most common injury to the rib cage. These most frequently affect the middle ribs. When several adjacent ribs incur two or more fractures each, this can result in a flail chest which is a life-threatening condition.
A dislocated rib can be painful and can be caused simply by coughing, or for example by trauma or lifting heavy weights.
One or more costal cartilages can become inflamed – a condition known as costochondritis; the resulting pain is similar to that of a heart attack.
Abnormalities of the rib cage include pectus excavatum ("sunken chest") and pectus carinatum ("pigeon chest"). A bifid rib is a bifurcated rib, split towards the sternal end, and usually just affecting one of the ribs of a pair. It is a congenital defect affecting about 1.2% of the population. It is often without symptoms though respiratory difficulties and other problems can arise.
Rib removal is the surgical removal of one or more ribs for therapeutic or cosmetic reasons.
Rib resection is the removal of part of a rib.
Regeneration
The ability of the human rib to regenerate itself has been appreciated for some time. However, the repair has only been described in a few case reports. The phenomenon is has been appreciated particularly by craniofacial surgeons, who use both cartilage and bone material from the rib for ear, jaw, face, and skull reconstruction.
The perichondrium and periosteum are fibrous sheaths of vascular connective tissue surrounding the rib cartilage and bone respectively. These tissues containing a source of progenitor stem cells that drive regeneration..
Additional Information
The rib cage is also known as the thoracic cage, and the primary rib cage function is to protect the organs inside the chest. These organs include the heart and lungs, which are two of our most important organs.
The thoracic cage bones include more than just the ribs, though. They also include the sternum and the thoracic vertebrae, where the ribs form.
Unfortunately, while the ribs protect the heart and lungs, they can become damaged for various reasons.
What Is the Rib Cage?
The rib cage is part of the axial skeleton. The average human is born with the same number of ribs regardless of gender. The ribs articulate with the thoracic vertebra. For example, the first rib, or rib 1, is the most significant and corresponds to the T1 thoracic vertebrae. Rib 2 corresponds to the T2 thoracic vertebra, rib 3 corresponds to the T3 thoracic vertebrae, and so on.
Where Is the Rib Cage?
The chest is where the rib cage is located. It surrounds the heart and lungs and is positioned posteriorly to the thoracic vertebrae. Each rib has two ends, one with various components and bumps, while the other is rounded and smooth.
How Many Ribs Do We Have?
The average person is born with 24 ribs—12 on each side. The ribs are located in the thoracic cage and thorax, along with their costal cartilages and the sternum. Each rib is made up of a few different components: the head, the neck, the tubercle, the angle, and the body.
Rib Cage Injuries and Conditions
Rib deformities occur in some babies during childbirth or due to genetic mutations inherited from one or both parents. In some cases, these deformities may happen spontaneously. This is known as de novo gene mutations. These deformities can range in severity from mild to life-threatening.
Some deformities can cause the lungs to constrict, which can cause difficulty breathing. Other deformities include:
* Extra ribs
* Missing ribs
* Short ribs
* Abnormally shaped ribs
* Ribs that have been fused
One condition relating to the ribs is called thoracic insufficiency syndrome. This occurs when the ribs are deformed, creating a small chest where healthy lungs cannot develop correctly.
Most of the time, these deformities happen due to genetic mutations. Sometimes these mutations happen as a result of genes passed down from the parents. Other times, these mutations occur on their own.
Rib deformities can happen in isolated incidents or alongside other issues. For example, patients with Down syndrome are often born with extra ribs. Sometimes, patients with Down syndrome are also born with a missing pair of ribs. In these cases, it is rare for health issues to occur.
There are also other conditions in which rib deformities appear. These include:
* Juene syndrome: This condition occurs when the chest and rib cage are abnormally small. As a result, severe breathing difficulties occur.
* Spondylocostal dysplasia: This condition is rare and occurs when abnormalities in the development of the spine and ribs occur. It is common for patients with this condition to have fused or missing ribs and an abnormally curved spine.
* Spondylothoracic dysplasia: This condition occurs when ribs are fused near the spine. In addition, vertebrae are misshapen or fused. Babies born with spondylothoracic dysplasia have small chests and severe breathing difficulties.
If rib deformities are minor, they are unlikely to cause symptoms. These deformities are usually only detected during x-rays. Children with minor deformities, such as an extra or missing rib, are unlikely to have health issues.
Symptoms of rib deformities that are more severe include:
* A chest that is narrow or smaller than normal
* A crooked chest
* Trouble breathing
* A lower abdomen that expands abnormally during inhalation
Additionally, other symptoms can happen when deformities occur alongside other conditions, such as:
* Short height
* Abnormally short legs and arms
* Shortened torso
* Rigid neck
* Scoliosis
* Extra toes or fingers
Rib deformities can be detected during pregnancy through ultrasound imaging. If ultrasounds do not detect deformities, though, x-rays may be necessary when the child is born, mainly if symptoms such as a small chest and breathing problems occur.
Genetic testing can also be done in cases where parents are concerned about inherited conditions.
Treating Rib Deformities
Treatment will vary depending on the severity and type of deformity. No health issues are present in minor cases, and treatment isn’t needed. However, if the deformity causes significant health issues such as difficulty breathing or harms the development of the lungs, your child may require breathing support. This could include intubation or a tracheotomy.
Vertical expandable prosthetic titanium rib (VEPTR) surgery may be recommended. This surgery allows your child’s rib, spine, and lungs to grow correctly and expand by implanting titanium ribs into your child’s body. This treatment will require surgical adjustment until your child’s skeletal muscles reach full maturity. Once maturity is reached, an additional surgery known as spinal fusion may be necessary.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2206 2024-07-08 14:06:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2208) Murphy bed
Gist
Murphy bed is a bed with a frame that can be folded into a space in a wall.
Murphy beds are used for space-saving purposes, much like trundle beds, and are popular where floor space is limited, such as small houses, apartments, hotels, mobile homes and college dormitories. In recent years, Murphy bed units have included options such as lighting, storage cabinets, and office components.
Details
A Murphy bed (also known as a pull-down bed, fold-down bed, or wall bed) is a bed that is hinged at one end to store vertically against the wall, or inside a closet or cabinet. Since they often can be used as both a bed or a closet, Murphy beds are multifunctional furniture.
History
The Murphy bed is named after William Lawrence Murphy (1876–1957), president of the Murphy Bed and Door Company.
Pre-Murphy folding beds
Under the name "bureau bedstead" the fold-up bed appeared in the 18th century, but never gained popularity. When closed, the bed looked like a bureau with fake drawers, hence the name. Gloag points to three 18th century pieces: one manufactured by Gillows of Lancaster and London in 1788, another one advertised by John Taylor in 1769, and the third one with a description published in the Prices for Cabinet Work in 1797.
Foldup beds were offered in the US through the Sears, Roebuck & Co. catalog, before Murphy's inventions.
Murphy
Murphy applied for his first patents around 1900. According to legend, he was wooing an opera singer, but living in a one-room apartment in San Francisco, and the moral code of the time frowned upon a woman entering a man's bedroom. Murphy's invention converted his bedroom into a parlor, enabling him to entertain.
Murphy introduced pivot and counterbalanced designs for which he received a series of patents, including one for a "Disappearing Bed" on June 18, 1912, and another for a "Design for a Bed" on June 27, 1916.
Murphy beds are used for space-saving purposes, much like trundle beds, and are popular where floor space is limited, such as small houses, apartments, hotels, mobile homes and college dormitories. In recent years, Murphy bed units have included options such as lighting, storage cabinets, and office components. They saw a resurgence in popularity in the early 2010s due to the weak economy, with children moving back in with their parents and families choosing to renovate homes rather than purchasing larger ones.
In 1989, the United States Court of Appeals for the Second Circuit ruled that the term "Murphy Bed" had entered common usage so thoroughly that it was no longer eligible for trademark protection.
Designs and models
Few Murphy beds have box springs. Instead, the mattress usually lies on a wood platform or wire mesh and is held in place so as not to sag when in a closed position. The mattress is attached to the bed frame, often with elastic straps to hold the mattress in position when the unit is folded upright. Pistons-lifts or torsion springs make modern Murphy beds easy to lower and raise.
Since the first model several other variations and designs have been created, including: sideways-mounted Murphy beds, Murphy bunk beds, and solutions that include other functions. Murphy beds exist with tables or desks that fold down when the bed is folded up, and there are also models with sofas and shelving solutions.
Risks
If not secured or used properly, a Murphy bed could collapse on the operator. A 1945 court case in Illinois found that a tenant assumed the risk of injury from a wall bed installed in a rented inn room. In 1982, a drunk man suffocated inside a closed Murphy bed, and two women were entrapped and suffocated by an improperly installed wall bed in 2005. A 2014 lawsuit alleged that a defective Murphy bed led to the death of a Staten Island man. In April 2022, Bestar Wall Beds of Quebec, Canada, recalled 129,000 beds in the United States and 53,000 beds in Canada after a 79-year-old woman was killed and 60 others injured by falling beds. Later that year, Cyme Tech, also of Quebec, Canada, recalled 8,200 beds after 146 reports of falling beds resulting in 62 injuries.
In popular culture
Murphy beds were a common setup for comic scenes in early cinema, including in silent films. The earliest known film to feature a Murphy bed is the lost 1900 Biograph Company film A Bulletproof Bed, which was remade in 1903 by Edison Pictures as the extant film Subub Surprises the Burglar. It was a recurrent slapstick element in many Keystone Studios productions of the 1910s, including Cursed by His Beauty (1914), Fatty's Reckless Fling (1915), He Wouldn't Stay Down (1915), and Bath Tub Perils (1916). Charlie Chaplin's 1916 One AM also features an exaggerated encounter with a Murphy bed.
Later films which use Murphy beds as comic props (often to cause injury or frustration, or to hide a clandestine guest) include Laurel and Hardy's Be Big (1930), Jimmy Stewart and Ginger Rogers's Vivacious Lady (1938), Buster Keaton's Spite Marriage (1929) and Nothing But Pleasure (1940), Abbott and Costello's Hit the Ice (1943), several Three Stooges shorts (including 1952's Corny Casanovas), It's a Mad, Mad, Mad, Mad World, the Popeye cartoon Shuteye Popeye, Bob Hope's Boy, Did I Get a Wrong Number (1966), the James Bond film You Only Live Twice, The Night They Raided Minsky's, Mel Brooks's Silent Movie, The Pink Panther Strikes Again, The Great Muppet Caper, Police Academy 2, Who Framed Roger Rabbit, Spy Hard, and Freddy vs. Jason. Murphy beds have also been used in television series; for example, an episode of Laverne and Shirley (re-creates scenes from Chaplin's One A.M.) and Love American Style ("Love and the Murphy's Bed"), Australian prime time soap opera, Number 96, and Caroline Channing's Murphy bed in 2 Broke Girls. Murphy beds were a routine enough feature of comic film to invite commentary from retailers; one store based in Vancouver, British Columbia remarked in an advertisement, "Gone are the days of Laurel and Hardy where the beds were portrayed as a fold away trap for your worst enemies."
In comics, the Murphy bed is depicted in the Tintin book Red Rackham's Treasure
as being an invention of Professor Calculus.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2207 2024-07-09 13:28:36
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2209) Bunsen burner
Gist
A Bunsen burner is a type of gas burner that is used in many chemistry procedures in a laboratory setting. It is used to heat substances, to combust substances, and to sterilize objects on high heat. Many different types of gases can be used in a burner such as methane, butane, propane, or a mixture of them.
Summary
Bunsen burner, device for combining a flammable gas with controlled amounts of air before ignition; it produces a hotter flame than would be possible using the ambient air and gas alone. Named for Robert Bunsen, the German chemist who introduced it in 1855 (from a design by Peter Desdega, who likely modified an earlier design by Michael Faraday), the Bunsen burner was the forerunner of the gas-stove burner and the gas furnace. The Bunsen burner consists of a metal tube on a base with a gas inlet at the lower end of the tube, which may have an adjusting valve; openings in the sides of the tube can be regulated by a collar to admit as much air as desired. The mixture of air and gas (optimally about 1 part gas to 3 parts air) is forced by gas pressure to the top of the tube, where it is ignited with a match. It burns with a pale blue flame, the primary flame, seen as a small inner cone, and a secondary, almost colourless flame, seen as a larger, outer cone, which results when the remaining gas is completely oxidized by the surrounding air.
The hottest part of the Bunsen flame, which is found just above the tip of the primary flame, reaches about 1,500 °C (2,700 °F). With too little air, the gas mixture will not burn completely and will form tiny carbon particles that are heated to glowing, making the flame luminous. With too much air, the flame may burn inside the burner tube; that is, it may strike back. The Meker and Fisher burners, variations of the original Bunsen burner, have metallic grids to increase the turbulence of the mixture and keep the flame at the top of the tube. The Fisher burner uses forced air. There is no secondary flame dependent on surrounding air, because these improvements introduce sufficient air for complete combustion, and the heat of the primary flame is augmented.
Details
A Bunsen burner, named after Robert Bunsen, is a kind of ambient air gas burner used as laboratory equipment; it produces a single open gas flame, and is used for heating, sterilization, and combustion.
The gas can be natural gas (which is mainly methane) or a liquefied petroleum gas, such as propane, butane, or a mixture. Combustion temperature achieved depends in part on the adiabatic flame temperature of the chosen fuel mixture.
History
In 1852, the University of Heidelberg hired Bunsen and promised him a new laboratory building. The city of Heidelberg had begun to install coal-gas street lighting, and the university laid gas lines to the new laboratory.
The designers of the building intended to use the gas not just for lighting, but also as fuel for burners for laboratory operations. For any burner lamp, it was desirable to maximize the temperature of its flame, and minimize its luminosity (which represented lost heating energy). Bunsen sought to improve existing laboratory burner lamps as regards economy, simplicity, and flame temperature, and adapt them to coal-gas fuel.
While the building was under construction in late 1854, Bunsen suggested certain design principles to the university's mechanic, Peter Desaga, and asked him to construct a prototype. Similar principles had been used in an earlier burner design by Michael Faraday, and in a device patented in 1856 by gas engineer R. W. Elsner. The Bunsen/Desaga design generated a hot, sootless, non-luminous flame by mixing the gas with air in a controlled fashion before combustion. Desaga created adjustable slits for air at the bottom of the cylindrical burner, with the flame issuing at the top. When the building opened early in 1855, Desaga had made 50 burners for Bunsen's students. Two years later Bunsen published a description, and many of his colleagues soon adopted the design. Bunsen burners are now used in laboratories around the world.
Operation
The device in use today safely burns a continuous stream of a flammable gas such as natural gas (which is principally methane) or a liquefied petroleum gas such as propane, butane, or a mixture of both.
The hose barb is connected to a gas nozzle on the laboratory bench with rubber tubing. Most laboratory benches are equipped with multiple gas nozzles connected to a central gas source, as well as vacuum, nitrogen, and steam nozzles. The gas then flows up through the base through a small hole at the bottom of the barrel and is directed upward. There are open slots in the side of the tube bottom to admit air into the stream using the Venturi effect, and the gas burns at the top of the tube once ignited by a flame or spark. The most common methods of lighting the burner are using a match or a spark lighter.
The amount of air mixed with the gas stream affects the completeness of the combustion reaction. Less air yields an incomplete and thus cooler reaction, while a gas stream well mixed with air provides oxygen in a stoichiometric amount and thus a complete and hotter reaction. The air flow can be controlled by opening or closing the slot openings at the base of the barrel, similar in function to the choke in a carburettor.
If the collar at the bottom of the tube is adjusted so more air can mix with the gas before combustion, the flame will burn hotter, appearing blue as a result. If the holes are closed, the gas will only mix with ambient air at the point of combustion, that is, only after it has exited the tube at the top. This reduced mixing produces an incomplete reaction, producing a cooler but brighter yellow, which is often called the "safety flame" or "luminous flame". The yellow flame is luminous due to small soot particles in the flame, which are heated to incandescence. The yellow flame is considered "dirty" because it leaves a layer of carbon on whatever it is heating. When the burner is regulated to produce a hot, blue flame, it can be nearly invisible against some backgrounds. The hottest part of the flame is the tip of the inner flame, while the coolest is the whole inner flame. Increasing the amount of fuel gas flow through the tube by opening the needle valve will increase the size of the flame. However, unless the airflow is adjusted as well, the flame temperature will decrease because an increased amount of gas is now mixed with the same amount of air, starving the flame of oxygen.
Generally, the burner is placed underneath a laboratory tripod, which supports a beaker or other container. The burner will often be placed on a suitable heatproof mat to protect the laboratory bench surface.
A Bunsen burner is also used in microbiology laboratories to sterilise pieces of equipment and to produce an updraft that forces airborne contaminants away from the working area.
Variants
Other burners based on the same principle exist. The most important alternatives to the Bunsen burner are:
Teclu burner – The lower part of its tube is conical, with a round screw nut below its base. The gap, set by the distance between the nut and the end of the tube, regulates the influx of the air in a way similar to the open slots of the Bunsen burner. The Teclu burner provides better mixing of air and fuel and can achieve higher flame temperatures than the Bunsen burner.
Meker burner – The lower part of its tube has more openings with larger total cross-section, admitting more air and facilitating better mixing of air and gas. The tube is wider and its top is covered with a wire grid. The grid separates the flame into an array of smaller flames with a common external envelope, and also prevents flashback to the bottom of the tube, which is a risk at high air-to-fuel ratios and limits the maximum rate of air intake in a conventional Bunsen burner. Flame temperatures of up to 1,100–1,200 °C (2,000–2,200 °F) are achievable if properly used. The flame also burns without noise, unlike the Bunsen or Teclu burners.
Tirrill burner – The base of the burner has a needle valve which allows the regulation of gas intake directly from the burner, rather than from the gas source. Maximum temperature of flame can reach 1560 °C.
Additional information
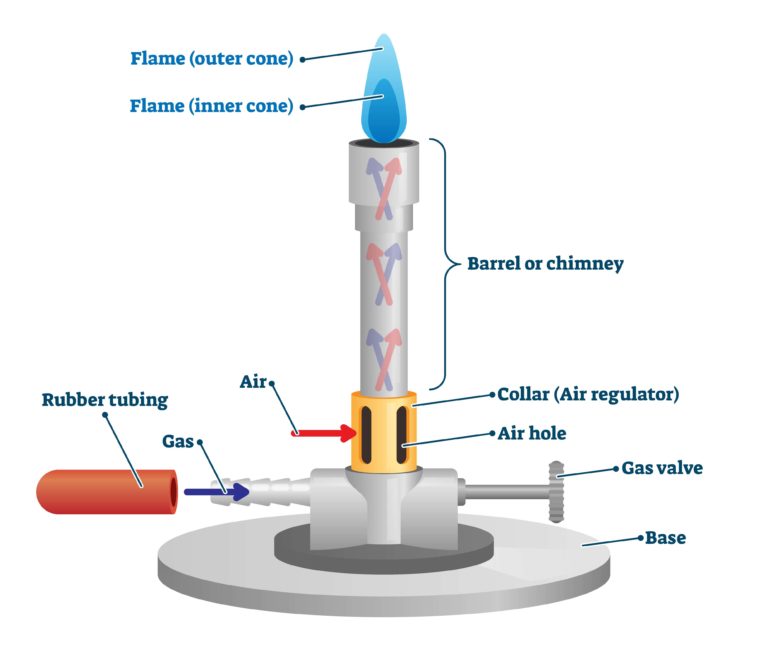
A Bunsen burner is a type of gas burner commonly used as a heat source in laboratory experiments. The burner consists of a flat base with a straight tube extending vertically, known as the barrel or chimney. Natural gas (predominantly methane) or a liquified petroleum gas such as propane or butane is supplied at the bottom of the chimney.
Bunsen burners are normally fitted with a hose barb at the base of the chimney to allow rubber tubing to supply the gas from a gas nozzle on the laboratory bench. There may also be a gas value on the Bunsen burner. The other critical component of a Bunsen burner is the air hole. This is located near the bottom of the chimney, just above the gas inlet. The air hole allows pre-mixing of air and gas before combustion occurs at the top of the chimney. A collar around the base of the chimney, with a hole that aligns with the air hole, acts as an air regulator, allowing the air in the pre-mixture to be adjusted.
Air is drawn into the air hole due to the Venturi effect. A fluid flow transfers energy in three ways, potential energy, pressure and kinetic energy. Bernoulli’s principle states that, due to conservation of energy, a change in velocity must result in either a change in the potential energy or a change in the fluid’s pressure. When a fluid flow increases in velocity, normally it is the pressure which decreases. Because the gas in a Bunsen burner is flowing through the chimney, it has a lower pressure than the static air surrounding it. This difference in pressure causes air to be drawn into the air hole as the gas flows past it, a phenomenon known as the Venturi effect.
As the air hole is opened the flame progresses from an unsteady orange flame to a more steady orange, a steady purple and finally a roaring blue flame. This progression results in increasing flame temperature. The unsteady orange flame produced when the air hole is completely closed is highly visible and of lower temperature. This safety flame is, therefore, used for lighting and as the default position when the Bunsen burner is not in use.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2208 2024-07-10 15:01:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2210) Cuticle
Gist
1. an area of hard skin at the base of the nails on your fingers and toes.
2. a hard outer layer that covers and protects a plant.
Summary
Cuticle, the outer layer or part of an organism that comes in contact with the environment. In many invertebrates the dead, noncellular cuticle is secreted by the epidermis. This layer may, as in the arthropods, contain pigments and chitin; in humans the cuticle is the epidermis.
In some higher plants, the cuticle is a water-impervious protective layer covering the epidermal cells of leaves and other parts and limiting water loss. It consists of cutin, a waxy, water-repellent substance allied to suberin, which is found in the cell walls of corky tissue. Cutin is especially noticeable on many fruits—e.g., apple, nectarine, and cherry, which can be buffed to a high gloss. Carnauba wax is derived from the cuticles of the leaves of Copernicia cerifera, a Brazilian palm.
Details
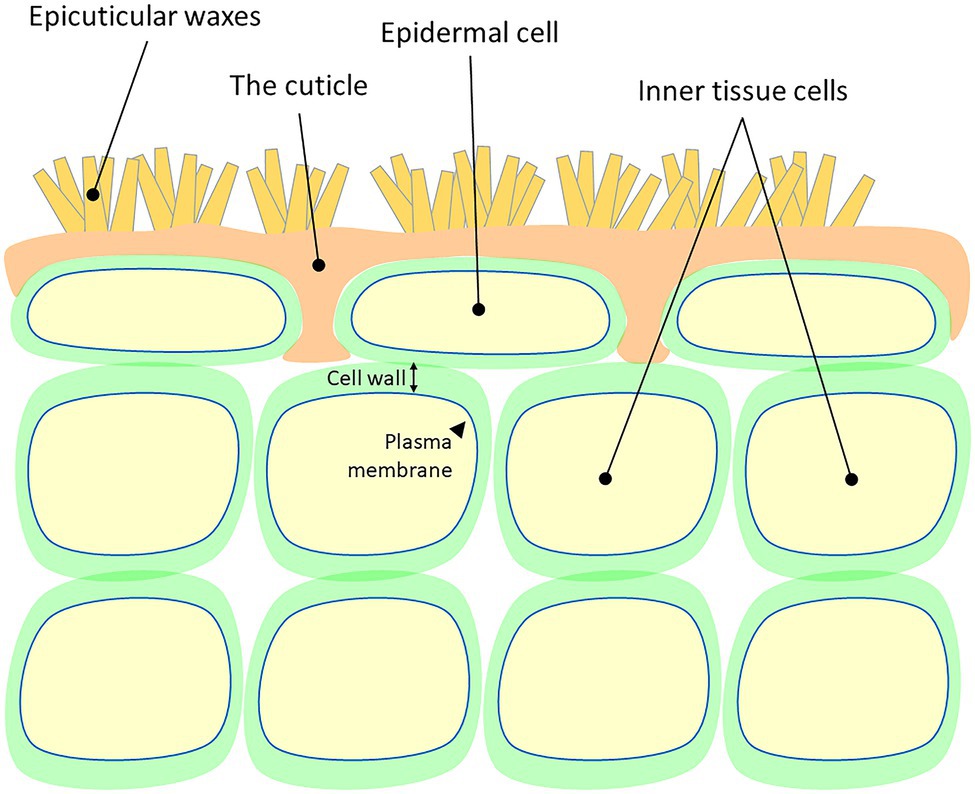
A cuticle, or cuticula, is any of a variety of tough but flexible, non-mineral outer coverings of an organism, or parts of an organism, that provide protection. Various types of "cuticle" are non-homologous, differing in their origin, structure, function, and chemical composition.
Human anatomy
In human anatomy, "cuticle" can refer to several structures, but it is used in general parlance, and even by medical professionals, to refer to the thickened layer of skin surrounding fingernails and toenails (the eponychium), and to refer to the superficial layer of overlapping cells covering the hair shaft (cuticula pili), consisting of dead cells, that locks the hair into its follicle. It can also be used as a synonym for the epidermis, the outer layer of skin.
Cuticle of invertebrates
In zoology, the invertebrate cuticle or cuticula is a multi-layered structure outside the epidermis of many invertebrates, notably arthropods and roundworms, in which it forms an exoskeleton.
The main structural components of the nematode cuticle are proteins, highly cross-linked collagens and specialised insoluble proteins known as "cuticlins", together with glycoproteins and lipids.
The main structural component of arthropod cuticle is chitin, a polysaccharide composed of N-acetylglucosamine units, together with proteins and lipids. The proteins and chitin are cross-linked. The rigidity is a function of the types of proteins and the quantity of chitin. It is believed that the epidermal cells produce protein and also monitors the timing and amount of protein to be incorporated into the cuticle.
Often, in the cuticle of arthropods, structural coloration is observed, produced by nanostructures. In the mealworm beetle, Tenebrio molitor, cuticular color may suggest pathogen resistance in that darker individuals are more resistant to pathogens compared to more tan individuals.
Botany
In botany, plant cuticles are protective, hydrophobic, waxy coverings produced by the epidermal cells of leaves, young shoots and all other aerial plant organs. Cuticles minimize water loss and effectively reduce pathogen entry due to their waxy secretion. The main structural components of plant cuticles are the unique polymers cutin or cutan, impregnated with wax. Plant cuticles function as permeability barriers for water and water-soluble materials. They prevent plant surfaces from becoming wet and also help to prevent plants from drying out. Xerophytic plants such as cacti have very thick cuticles to help them survive in their arid climates. Plants that live in range of sea's spray also may have thicker cuticles that protect them from the toxic effects of salt.
Some plants, particularly those adapted to life in damp or aquatic environments, have an extreme resistance to wetting. A well-known example is the sacred lotus. This adaptation is not purely the physical and chemical effect of a waxy coating but depends largely on the microscopic shape of the surface. When a hydrophobic surface is sculpted into microscopic, regular, elevated areas, sometimes in fractal patterns, too high and too closely spaced for the surface tension of the liquid to permit any flow into the space between the plateaus, then the area of contact between liquid and solid surfaces may be reduced to a small fraction of what a smooth surface might permit. The effect is to reduce wetting of the surface substantially.
Structural coloration is also observed in the cuticles of plants.
Mycology
"Cuticle" is one term used for the outer layer of tissue of a mushroom's basidiocarp, or "fruit body". The alternative term "pileipellis", Latin for "skin" of a "cap" (meaning "mushroom") might be technically preferable, but is perhaps too cumbersome for popular use. It is the part removed in "peeling" mushrooms. On the other hand, some morphological terminology in mycology makes finer distinctions, such as described in the article on the "pileipellis". Be that as it may, the pileipellis (or "peel") is distinct from the trama, the inner fleshy tissue of a mushroom or similar fruiting body, and also from the spore-bearing tissue layer, the hymenium.
Additional information
The cuticle is a layer of clear skin located along the bottom edge of your finger or toe, which is called the nail bed. The cuticle protects new nails from bacteria when they grow out from the nail root.
The area around the cuticle is delicate. It can get dry, damaged, and infected. It’s important to care for the entire nail area and keep it clean so that your nails stay healthy.
Cuticle vs. nail lunula
The cuticle is the transparent skin located above and around the nail base. The lunula is the half-moon shape seen at the base of the nail. The lunula is located above the cuticle.
Hair cuticles
Human hair also contains cuticles. These are different from nail cuticles but have a similar function. Hair cuticles serve as a protective layer for the hair. They’re composed of dead, overlapping cells.
When healthy, these cuticles give your hair shine and protect its inner layers from damage.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2209 2024-07-11 14:10:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2211) Sleep Apnea
Gist
Sleep Apnea is a potentially serious sleep disorder in which breathing repeatedly stops and starts. If you snore loudly and feel tired even after a full night's sleep, you might have sleep apnea.
Summary
Sleep Apnea is a common condition in which your breathing stops and restarts many times while you sleep. This can prevent your body from getting enough oxygen. You may want to talk to your healthcare provider about sleep apnea if someone tells you that you snore or gasp during sleep, or if you experience other symptoms of poor-quality sleep, such as excessive daytime sleepiness.
There are two types of sleep apnea.
* Obstructive sleep apnea happens when your upper airway becomes blocked many times while you sleep, reducing or completely stopping airflow. This is the most common type of sleep apnea. Anything that could narrow your airway such as obesity, large tonsils, or changes in your hormone levels can increase your risk for obstructive sleep apnea.
* Central sleep apnea happens when your brain does not send the signals needed to breathe. Health conditions that affect how your brain controls your airway and chest muscles can cause central sleep apnea.
To diagnose sleep apnea, your provider may have you do a sleep study. Breathing devices such as continuous positive air pressure (CPAP) machines and lifestyle changes are common sleep apnea treatments. If these treatments do not work, surgery may be recommended to correct the problem that is causing your sleep apnea. If your sleep apnea is not diagnosed or treated, you may not get enough good quality sleep. This can lead to trouble concentrating, making decisions, remembering things, or controlling your behavior. Sleep apnea is also linked to serious health problems.
Details
Sleep apnea is a serious sleep disorder that happens when your breathing stops and starts while you're asleep. If it goes untreated, it can cause loud snoring, daytime tiredness, or more serious problems like heart trouble or high blood pressure.
This condition is different from regular, or primary, snoring. Primary snoring may be caused by nose or throat conditions, your sleep style (especially back sleeping), being overweight or older, or using alcohol or other depressants. While both types of snoring happen when tissues in the back of your throat vibrate, people with sleep apnea tend to:
* Snore much more loudly than those with regular snoring
* Pause for over 10 seconds while they breathe
* Take shallow breaths, gasp, or choke
* Be restless during sleep
Is sleep apnea dangerous?
Sleep apnea itself isn't thought to be fatal. But research has found that people who have the condition are twice as likely to die suddenly within a given time period than those who don't--especially if it's not treated. That's because of its links to serious conditions like high blood pressure, heart disease, stroke, and diabetes.
Sleep Apnea Types
There are three types of sleep apnea:
* Obstructive sleep apnea. This is the most common type. It results when your airways repeatedly get completely or partially blocked as you sleep . This usually happens because the soft tissue at the back of your throat collapses when the muscles in your face and neck relax while you sleep. During these episodes, your diaphragm and chest muscles must work harder than normal to open your airways. You may start to breathe with loud gasps, and your body may jerk. This can affect your sleep, lower the flow of oxygen to your vital organs, and lead to abnormal heart rhythms.
* Central sleep apnea. With this type, your airway doesn't get blocked. Instead, your brain fails to tell your muscles to breathe because of issues in your respiratory control center. It's related to the function of your central nervous system. This type most often affects people with neuromuscular disease such as amyotrophic lateral sclerosis (ALS, or Lou Gehrig's disease), those who've had a stroke, or those who have heart failure or other forms of heart, kidney, or lung disease.
* Complex sleep apnea syndrome. With this condition, you have a combination of obstructive and central sleep apnea. When you have the obstructive type but it turns into the central type after you get treatment, that's called treatment-emergent central sleep apnea.
Effects of Sleep Apnea
When you briefly stop breathing because of sleep apnea, the oxygen levels in your blood drop. This triggers a brain reflex that wakes you up long enough to start breathing again.
These repeated awakenings keep you from spending enough time in the deep stages of sleep. The more serious your condition is, the more often your sleep will be interrupted.
Losing sleep makes you feel tired during the day. You may be less productive at work or school, and feel irritable, moody, or sad. You might be forgetful or find it hard to concentrate. And you're more likely to have accidents at work or while driving.
Sleep Apnea Causes
What causes sleep apnea depends on what type you have:
* Obstructive sleep apnea. Things that narrow your airway or interfere with your airflow, including obesity, enlarged tonsils or adenoids, or a thick neck, can cause this type.
* Central sleep apnea. Anything that affects your brain's control of your breathing and chest muscles can cause this type. This includes hormone levels as well as certain health conditions. Opioid use can have this effect, as can changes that come with aging.
Some research has indicated that apnea might run in families.
Sleep Apnea Risk Factors
This condition can affect anyone, but some things put you more at risk:
* Being overweight
* A large neck circumference that could make your airways more narrow
* A narrowed airway that you inherited or developed from large tonsils or adenoids
* Being male (or having been identified as male at birth)
* Older age
* A family history of sleep apnea
* Smoking
* Use of alcohol, sedatives, or tranquilizers
* Nasal congestion
* Medical conditions such as type 2 diabetes, congestive heart failure, high blood pressure, Parkinson's disease, PCOS, hormonal disorders, a previous stroke, or chronic lung diseases like asthma
Sleep Apnea Symptoms
You probably won't notice your first symptoms of obstructive sleep apnea, but your bed partner may make you aware of them. The most common signs are:
* Snoring
* Fatigue or sleepiness during the day
* Restlessness while sleeping, or often waking up at night
* Dry mouth or sore throat when you wake up
* Waking up suddenly after gasping or choking
* Trouble concentrating, forgetfulness, or crankiness
* Depression or anxiety
* Frequent need to get up to pee at night
* Night sweats
* Sexual dysfunction
* Headaches
People with central sleep apnea usually say they wake up a lot or have insomnia. They also might have a choking or gasping sensation when they awaken.
Sleep apnea symptoms in women
Women (and those who were identified as female at birth) who have the condition may be less less likely than men to snore. For them, signs of sleep apnea may include:
* Fatigue
* Daytime drowsiness
* Anxiety or depression
* Headaches, often in the morning
* Trouble sleeping, including often waking up during the night
Sleep apnea symptoms in children
The symptoms might not be as obvious in children. Warning signs include:
* Sluggishness or sleepiness, which could be mistaken for laziness in the classroom
* Hyperactivity or problems focusing at school
* Poor academic performance
* Trouble swallowing
* Daytime mouth breathing
* An inward movement of the rib cage when inhaling
* Sweating a lot at night
* Heartburn
* Unusual sleeping positions, like sleeping on their hands and knees, or with the neck extended
* Often moving their arms or legs during sleep
* Loud snoring
* Bedwetting.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2210 2024-07-12 14:33:26
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2212) Coffee bean
A coffee bean is a seed from the Coffea plant and the source for coffee. It is the pit inside the red or purple fruit. This fruit is often referred to as a coffee cherry, and like the cherry, it is a fruit with a pit. Even though the coffee beans are not technically beans, they are referred to as such because of their resemblance to true beans. The fruits most commonly contain two stones with their flat sides together. A small percentage of cherries contain a single seed, called a "peaberry". Peaberries make up only around 10% to 15% of all coffee beans. It is a fairly common belief that they have more flavour than normal coffee beans. Like Brazil nuts (a seed) and white rice, coffee beans consist mostly of endosperm.
The two most economically important varieties of coffee plants are the Arabica and the Robusta; approximately 60% of the coffee produced worldwide is Arabica and ~40% is Robusta. Arabica beans consist of 0.8–1.4% caffeine and Robusta beans consist of 1.7–4.0% caffeine. As coffee is one of the world's most widely consumed beverages, coffee beans are a major cash crop and an important export product, accounting for over 50% of some developing nations' foreign exchange earnings. This has made coffee very important in culture and food around the world. In 2017, 70% of total coffee production was exported, worth US$19.9 billion. The global coffee industry is massive and valued at $495.50 billion as of 2023, the biggest producer of coffee and coffee beans is Brazil. Other main exporters of coffee beans are Colombia, Vietnam and Ethiopia.
History
Significant dates
* According to legend, the coffee plant was discovered in Ethiopia by a goat herder named Kaldi around 850 AD, who observed increased physical activity in his goats after they consumed coffee beans.
* The coffee plant was first found in the mountains of Yemen. Then by 1500, it was exported to the rest of the world through the port of Mokha, Yemen.
* First cultivation in India (Chikmagalur) – 1600
* First cultivation in Europe – 1616
* First cultivation in Java – 1699
* First cultivation in Caribbean (Cuba, Hispaniola, Jamaica, Puerto Rico) – 1715–1730
* First cultivation in South America – 1730
* First cultivation in Dutch East Indies – 1720
* Roasted beans first sold on retail market (Pittsburgh) – 1865
* Important spray-drying techniques developed in 1950s, which along with freeze drying are a method to create instant coffee
Distribution
Brazil produces about 45% of the world's total coffee exports. The United States imports more coffee than any other nation. As of 2015, Americans consumed approximately 400 million cups of coffee per day, making the United States the leading consumer of coffee in the world.
Coffee plants grow within a defined area between the tropics of Cancer and Capricorn, termed the bean belt or coffee belt.
Etymology
The Oxford English Dictionary suggests that the European languages generally appear to have gotten the name from Turkish kahveh, about 1600, perhaps through Italian caffè. Arab qahwah, in Turkish pronounced kahveh, the name of the infusion or beverage; said by Arab lexicographers to have originally meant "wine" or some type of wine, and to be a derivative of a verb-root qahiya "to have no appetite". Another common theory is that the name derives from Kaffa Province, Ethiopia, where the species may have originated.
Coffee plant
The coffee tree averages from 5–10 m (16–33 ft) in height. As the tree gets older, it produces less fruit and slowly loses any pest- and disease-resistance. The coffee beans come from the seeds which contained in fruits from trees and shrubs naturally grown in African forests. Humans produce coffee by roasting, grinding and brewing the green coffee beans.
Coffee plants are often grown in rows spaced apart depending on the desired density chosen by the farmer. Some farmers plant other trees, such as shade trees or other cash-crop trees, such as orange trees around them or plant the coffee on the sides of hills, because they need specific conditions to flourish. Ideally, Arabica coffee beans are grown at temperatures between 15 and 24 °C (59 and 75 °F) and Robusta between 24 and 30 °C (75 and 86 °F) and receive between 500 and 3,000 mm (20 and 118 in) of rainfall per year. More rain is needed at the beginning of the season when the fruit is developing and less later in the season as it ripens.
Two lesser known species grown for consumption are Coffea liberica and Coffea racemosa.
Processing
When the fruit is ripe, it is almost always handpicked, using either "selective picking", where only the ripe fruit is removed, or "strip-picking", where all of the fruit is removed from a limb all at once. Selective picking is often used to produce higher quality coffee because the cherries are picked at their ripest. Strip-picking is indiscriminate and will harvest unripe, ripe, and over-ripe fruit. To improve quality after strip-picking, the harvest must be sorted.
Two methods are primarily used to process coffee berries. The first, "wet" or "washed" process, has historically usually been carried out in Central America and areas of Africa. The flesh of the cherries is separated from the seeds and then the seeds are fermented – soaked in water for about two days. This softens the mucilage, which is a sticky pulp residue that is still attached to the seeds. Then this mucilage is washed off with water.
The "dry processing" method, cheaper and simpler, was historically used for lower-quality beans in Brazil and much of Africa, but now brings a premium when done well. Twigs and other foreign objects are separated from the berries and the fruit is then spread out in the sun on concrete, bricks or raised beds for 2–3 weeks, turned regularly for even drying.
In Asia a third type of processing exists, where the Asian palm civet eats coffee berries and excretes the beans. Because the civet prefers the taste of the ripest cherries, the civet selectively harvests the cherries. Its digestive system then processes the beans by breaking down the mucilage and pulp surrounding the seed. Once the seeds are excreted by the civet, they can be harvested, processed and sold as a niche product. Once they are finally processed, these beans are called kopi luwak, and are often marketed as a rare and expensive coffee.
Composition
The term "green coffee bean" refers to unroasted mature or immature coffee beans. These have been processed by wet or dry methods to remove the outer pulp and mucilage and have an intact wax layer on the outer surface. When immature, they are green. When mature, they have a brown to yellow or reddish color and typically weigh 300 to 330 mg per dried coffee bean. Nonvolatile and volatile compounds in green coffee beans, such as caffeine, deter many insects and animals from eating them. Further, both nonvolatile and volatile compounds contribute to the flavor of the coffee bean when it is roasted. Nonvolatile nitrogenous compounds (including alkaloids, trigonelline, proteins, and free amino acids) and carbohydrates are of major importance in producing the full aroma of roasted coffee and for its biological action. Since the mid-2000s, green coffee extract has been sold as a nutritional supplement and has been clinically studied for its chlorogenic acid content and for its lipolytic and weight-loss properties.
Nonvolatile alkaloids
Caffeine (1,3,7-trimethylxanthine) is the alkaloid most present in green and roasted coffee beans. The content of caffeine is between 1.0% and 2.5% by weight of dry green coffee beans. The content of caffeine does not change during maturation of green coffee beans, but higher caffeine content is found in plants grown at higher altitudes. Lower concentrations of theophylline, theobromine, paraxanthine, liberine, and methylliberine can be found. The concentration of theophylline, an alkaloid noted for its presence in green tea, is reduced during the roasting process, usually about 15 minutes at 230 °C (446 °F), whereas the concentrations of most other alkaloids are not changed. The solubility of caffeine in water increases with temperature and with the addition of chlorogenic acids, citric acid, or tartaric acid, all of which are present in green coffee beans. For example, 1 g (0.035 oz) of caffeine dissolves in 46 mL (1.6 US fl oz) of water at room temperature, and 5.5 mL (0.19 US fl oz) at 80 °C (176 °F). The xanthine alkaloids are odorless, but have a bitter taste in water, which is masked by organic acids present in green coffee.
Trigonelline (N-methyl-nicotinate) is a derivative of vitamin B3 that is not as bitter as caffeine. In green coffee beans, the content is between 0.6% and 1.0%. At a roasting temperature of 230 °C (446 °F), 85% of the trigonelline is degraded to nicotinic acid, leaving small amounts of the unchanged molecule in the roasted beans.
Proteins and amino acids
Proteins account for 8% to 12% of dried green coffee beans. A majority of the proteins are of the 11-S storage kind (alpha – component of 32 kDa, beta – component of 22 kDa), most of which are degraded to free amino acids during maturation of green coffee beans. Further, 11-S storage proteins are degraded to their individual amino acids under roasting temperature, thus are an additional source of bitter components due to generation of Maillard reaction products. High temperature and oxygen concentration and low pH degrade 11-S storage proteins of green coffee beans to low-molecular-weight peptides and amino acids. The degradation is accelerated in the presence of organic acids such as chlorogenic acids and their derivatives. Other proteins include enzymes, such as catalase and polyphenol oxidase, which are important for the maturation of green coffee beans. Mature coffee contains free amino acids (4.0 mg amino acid/g robusta coffee and up to 4.5 mg amino acid/g arabica coffee). In Coffea arabica, alanine is the amino acid with the highest concentration, i.e. 1.2 mg/g, followed by asparagine of 0.66 mg/g, whereas in C. robusta, alanine is present at a concentration of 0.8 mg/g and asparagine at 0.36 mg/g. The free hydrophobic amino acids in fresh green coffee beans contribute to the unpleasant taste, making it impossible to prepare a desirable beverage with such compounds. In fresh green coffee from Peru, these concentrations have been determined as: isoleucine 81 mg/kg, leucine 100 mg/kg, valine 93 mg/kg, tyrosine 81 mg/kg, phenylalanine 133 mg/kg. The concentration of gamma-aminobutyric acid (a neurotransmitter) has been determined between 143 mg/kg and 703 mg/kg in green coffee beans from Tanzania. Roasted coffee beans do not contain any free amino acids; the amino acids in green coffee beans are degraded under roasting temperature to Maillard products (reaction products between the aldehyde group of sugar and the alpha-amino group of the amino acids). Further, diketopiperazines, e.g. cyclo(proline-proline), cyclo(proline-leucine), and cyclo(proline-isoleucine), are generated from the corresponding amino acids, and are the major source of the bitter taste of roasted coffee. The bitter flavor of diketopiperazines is perceptible at around 20 mg/liter of water. The content of diketopiperazines in espresso is about 20 to 30 mg, which is responsible for its bitterness.
Carbohydrates
Carbohydrates make up about 50% of the dry weight of green coffee beans. The carbohydrate fraction of green coffee is dominated by polysaccharides, such as arabinogalactan, galactomannan, and cellulose, contributing to the tasteless flavor of green coffee. Arabinogalactan makes up to 17% of dry weight of green coffee beans, with a molecular weight of 90 kDa to 200 kDa. It is composed of beta-1-3-linked galactan main chains, with frequent members of arabinose (pentose) and galactose (hexose) residues at the side chains comprising immunomodulating properties by stimulating the cellular defense system (Th-1 response) of the body. Mature brown to yellow coffee beans contain fewer residues of galactose and arabinose at the side chain of the polysaccharides, making the green coffee bean more resistant to physical breakdown and less soluble in water. The molecular weight of the arabinogalactan in coffee is higher than in most other plants, improving the cellular defense system of the digestive tract compared to arabinogalactan with lower molecular weight. Free monosaccharides are present in mature brown to yellow-green coffee beans. The free part of monosaccharides contains sucrose (gluco-fructose) up to 9000 mg/100 g of arabica green coffee bean, a lower amount in robustas, i.e. 4500 mg/100 g. In arabica green coffee beans, the content of free glucose was 30 to 38 mg/100 g, free fructose 23 to 30 mg/100 g; free galactose 35 mg/100 g and mannitol 50 mg/100 g dried coffee beans, respectively. Mannitol is a powerful scavenger for hydroxyl radicals, which are generated during the peroxidation of lipids in biological membranes.
Lipids
The lipids found in green coffee include: linoleic acid, palmitic acid, oleic acid, stearic acid, arachidic acid, diterpenes, triglycerides, unsaturated long-chain fatty acids, esters, and amides. The total content of lipids in dried green coffee is 11.7–14 g/100 g. Lipids are present on the surface and in the interior matrix of green coffee beans. On the surface, they include derivatives of carboxylic acid-5-hydroxytryptamides with an amide bond to fatty acids (unsaturated C6 to C24) making up to 3% of total lipid content or 1200 to 1400 microgram/g dried green coffee bean. Such compounds form a wax-like cover on the surface of the coffee bean (200–300 mg lipids/100 g dried green coffee bean) protecting the interior matrix against oxidation and insects. Further, such molecules have antioxidative activity due to their chemical structure. Lipids of the interior tissue are triglycerides, linoleic acid (46% of total free lipids), palmitic acid (30% to 35% of total free lipids), and esters. Arabica beans have a higher content of lipids (13.5–17.4 g lipids/100 g dried green coffee beans) than robustas (9.8–10.7 g lipids/100 g dried green coffee beans). The content of diterpenes is about 20% of the lipid fraction. The diterpenes found in green coffee include cafestol, kahweol and 16-O-methylcafestol. Some of these diterpenes have been shown in in vitro experiments to protect liver tissue against chemical oxidation. In coffee oil from green coffee beans the diterpenes are esterified with saturated long chain fatty acids.
Nonvolatile chlorogenic acids
Chlorogenic acids belong to a group of compounds known as phenolic acids, which are antioxidants. The content of chlorogenic acids in dried green coffee beans of arabica is 65 mg/g and of robusta 140 mg/g, depending on the timing of harvesting. At roasting temperature, more than 70% of chlorogenic acids are destroyed, leaving a residue less than 30 mg/g in the roasted coffee bean. In contrast to green coffee, green tea contains an average of 85 mg/g polyphenols. These chlorogenic acids could be a valuable, inexpensive source of antioxidants. Chlorogenic acids are homologous compounds comprising caffeic acid, ferulic acid and 3,4-dimethoxycinnamic acid, which are connected by an ester bond to the hydroxyl groups of quinic acid. The antioxidant capacity of chlorogenic acid is more potent than of ascorbic acid (vitamin C) or mannitol, which is a selective hydroxy-radical scavenger. Chlorogenic acids have a bitter taste in low concentrations such as 50 mg/L water. At higher concentrations of 1 g/L water, they have a sour taste. Chlorogenic acids increase the solubility of caffeine and are important modulators of taste.
Volatile compounds
Volatile compounds of green coffee beans include short-chain fatty acids, aldehydes, and nitrogen-containing aromatic molecules, such as derivatives of pyrazines (green-herbaceous-earthy odor). Briefly, such volatile compounds are responsible for the less pleasing odor and taste of green coffee versus roasted coffee. Commercial success was realized by Starbucks in creating Green Bean Refreshers using a process that primarily isolates the caffeine from the green beans but does not actually use steeped liquid from the beans. Many consumers experiment with creating green bean "extract" by steeping green coffee beans in hot water. Often, the recommended times of steeping (20 minutes to 1 hour) extract too much caffeine to provide a pleasant taste. A steeping time of 12 minutes or under provides a more palatable liquid that can be used as a base for a drink containing more of the nutrients and less caffeine that using just isolated caffeine extract. The alkaline stock base that results can be paired with acidic or fruity extracts, with or without sweetener, to mask the vegetable-like taste of the extract.
When green coffee beans are roasted, other molecules with the typical pleasant aroma of coffee are generated, which are not present in fresh green coffee. During roasting, the major part of the unpleasant-tasting volatile compounds are neutralised. Unfortunately, other important molecules such as antioxidants and vitamins present in green coffee are destroyed. Volatile compounds with nauseating odor for humans have been identified, including acetic acid (pungent, unpleasant odor), propionic acid (odor of sour milk, or butter), butanoic acid (odor of rancid butter, present in green coffee with 2 mg/100 g coffee beans), pentanoic acid (unpleasant fruity flavor, present in green coffee at 40 mg/100 g in coffee beans), hexanoic acid (fatty-rancid odor), heptanoic acid (fatty odor), octanoic acid (repulsive oily rancid odor); nonanoic acid (mild nut-like fatty odor); decanoic acid (sour repulsive odor), and derivatives of such fatty acids – 3-methyl-valeric acid (sour, green-herbaceous, unpleasant odor), acetaldehyde (pungent-nauseating odor, even when highly diluted, present in dried green coffee beans at concentrations of about 5 mg/kg), propanal (choking effect on respiratory system, penetrating-nauseating), butanal (nauseating effect, present in dried green coffee beans at 2–7 mg/kg), or pentanal (very repulsive nauseating effect).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2211 2024-07-13 14:44:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2213) Electron Shell
Gist
An electron shell is the outside part of an atom around the atomic nucleus. It is a group of atomic orbitals with the same value of the principal quantum number n. Electron shells have one or more electron subshells, or sublevels.
Details
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond to the principal quantum numbers (n = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ...). A useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements represents an electron shell.
Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n^2) electrons. For an explanation of why electrons exist in these shells, see electron configuration.
Each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals.
History
In 1913, Niels Bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. At that time, Bohr allowed the capacity of the inner orbit of the atom to increase to eight electrons as the atoms got larger, and "in the scheme given below the number of electrons in this [outer] ring is arbitrary put equal to the normal valency of the corresponding element". Using these and other constraints, he proposed configurations that are in accord with those now known only for the first six elements.
The shell terminology comes from Arnold Sommerfeld's modification of the 1913 Bohr model. During this period Bohr was working with Walther Kossel, whose papers in 1914 and in 1916 called the orbits "shells". Sommerfeld retained Bohr's planetary model, but added mildly elliptical orbits (characterized by additional quantum numbers ℓ and m) to explain the fine spectroscopic structure of some elements. The multiple electrons with the same principal quantum number (n) had close orbits that formed a "shell" of positive thickness instead of the circular orbit of Bohr's model which orbits called "rings" were described by a plane.
The existence of electron shells was first observed experimentally in Charles Barkla's and Henry Moseley's X-ray absorption studies. Moseley's work did not directly concern the study of electron shells, because he was trying to prove that the periodic table was not arranged by weight, but by the charge of the protons in the nucleus. However, because the number of electrons in an electrically neutral atom equals the number of protons, this work was extremely important to Niels Bohr who mentioned Moseley's work several times in his 1962 interview. Moseley was part of Rutherford's group, as was Niels Bohr. Moseley measured the frequencies of X-rays emitted by every element between calcium and zinc and found that the frequencies became greater as the elements got heavier. This led to the theory that electrons were emitting X-rays when they were shifted to lower shells. This led to the conclusion that the electrons were in Kossel's shells with a definite limit per shell, labeling the shells with the letters K, L, M, N, O, P, and Q. The origin of this terminology was alphabetic. Barkla, who worked independently from Moseley as an X-ray spectrometry experimentalist, first noticed two distinct types of scattering from shooting X-rays at elements in 1909 and named them "A" and "B". Barkla described these two types of X-ray diffraction: the first was unconnected with the type of material used in the experiment and could be polarized. The second diffraction beam he called "fluorescent" because it depended on the irradiated material. It was not known what these lines meant at the time, but in 1911 Barkla decided there might be scattering lines previous to "A", so he began at "K". However, later experiments indicated that the K absorption lines are produced by the innermost electrons. These letters were later found to correspond to the n values 1, 2, 3, etc. that were used in the Bohr model. They are used in the spectroscopic Siegbahn notation.
The work of assigning electrons to shells was continued from 1913 to 1925 by many chemists and a few physicists. Niels Bohr was one of the few physicists who followed the chemist's work of defining the periodic table, while Arnold Sommerfeld worked more on trying to make a relativistic working model of the atom that would explain the fine structure of the spectra from a classical orbital physics standpoint through the Atombau approach. Einstein and Rutherford, who did not follow chemistry, were unaware of the chemists who were developing electron shell theories of the periodic table from a chemistry point of view, such as Irving Langmuir, Charles Bury, J.J. Thomson, and Gilbert Lewis, who all introduced corrections to Bohr's model such as a maximum of two electrons in the first shell, eight in the next and so on, and were responsible for explaining valency in the outer electron shells, and the building up of atoms by adding electrons to the outer shells. So when Bohr outlined his electron shell atomic theory in 1922, there was no mathematical formula for the theory. So Rutherford said he was hard put "to form an idea of how you arrive at your conclusions". Einstein said of Bohr's 1922 paper that his "electron-shells of the atoms together with their significance for chemistry appeared to me like a miracle – and appears to me as a miracle even today". Arnold Sommerfeld, who had followed the Atombau structure of electrons instead of Bohr who was familiar with the chemists' views of electron structure, spoke of Bohr's 1921 lecture and 1922 article on the shell model as "the greatest advance in atomic structure since 1913". However, the electron shell development of Niels Bohr was basically the same theory as that of the chemist Charles Rugeley Bury in his 1921 paper.
As work continued on the electron shell structure of the Sommerfeld-Bohr Model, Sommerfeld had introduced three "quantum numbers n, k, and m, that described the size of the orbit, the shape of the orbit, and the direction in which the orbit was pointing." Because we use k for the Boltzmann constant, the azimuthal quantum number was changed to ℓ. When the modern quantum mechanics theory was put forward based on Heisenberg's matrix mechanics and Schrödinger's wave equation, these quantum numbers were kept in the current quantum theory but were changed to n being the principal quantum number, and m being the magnetic quantum number.
However, the final form of the electron shell model still in use today for the number of electrons in shells was discovered in 1923 by Edmund Stoner, who introduced the principle that the nth shell was described by 2(n^2). Seeing this in 1925, Wolfgang Pauli added a fourth quantum number, "spin", during the old quantum theory period of the Sommerfeld-Bohr Solar System atom to complete the modern electron shell theory.
Subshells
Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals. For example, the first (K) shell has one subshell, called 1s; the second (L) shell has two subshells, called 2s and 2p; the third shell has 3s, 3p, and 3d; the fourth shell has 4s, 4p, 4d and 4f; the fifth shell has 5s, 5p, 5d, and 5f and can theoretically hold more in the 5g subshell that is not occupied in the ground-state electron configuration of any known element.
* The first column is the "subshell label", a lowercase-letter label for the type of subshell. For example, the "4s subshell" is a subshell of the fourth (N) shell, with the type (s) described in the first row.
* The second column is the azimuthal quantum number (ℓ) of the subshell. The precise definition involves quantum mechanics, but it is a number that characterizes the subshell.
* The third column is the maximum number of electrons that can be put into a subshell of that type. For example, the top row says that each s-type subshell (1s, 2s, etc.) can have at most two electrons in it. Each of the following subshells (p, d, f, g) can have 4 more electrons than the one preceding it.
* The fourth column says which shells have a subshell of that type. For example, looking at the top two rows, every shell has an s subshell, while only the second shell and higher have a p subshell (i.e., there is no "1p" subshell).
* The final column gives the historical origin of the labels s, p, d, and f. They come from early studies of atomic spectral lines. The other labels, namely g, h, and i, are an alphabetic continuation following the last historically originated label of f.
Number of electrons in each shell
Each subshell is constrained to hold 4ℓ + 2 electrons at most, namely:
* Each s subshell holds at most 2 electrons
* Each p subshell holds at most 6 electrons
* Each d subshell holds at most 10 electrons
* Each f subshell holds at most 14 electrons
* Each g subshell holds at most 18 electrons
Therefore, the K shell, which contains only an s subshell, can hold up to 2 electrons; the L shell, which contains an s and a p, can hold up to 2 + 6 = 8 electrons, and so forth; in general, the nth shell can hold up to 2n^2 electrons.
Although that formula gives the maximum in principle, in fact that maximum is only achieved (in known elements) for the first four shells (K, L, M, N). No known element has more than 32 electrons in any one shell. This is because the subshells are filled according to the Aufbau principle. The first elements to have more than 32 electrons in one shell would belong to the g-block of period 8 of the periodic table. These elements would have some electrons in their 5g subshell and thus have more than 32 electrons in the O shell (fifth principal shell).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2212 2024-07-14 00:03:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2214) Shopping Mall
A shopping mall (or simply mall) is a large indoor shopping center, usually anchored by department stores. The term mall originally meant a pedestrian promenade with shops along it, but in the late 1960s, it began to be used as a generic term for the large enclosed shopping centers that were becoming increasingly commonplace. In the United Kingdom and other countries, shopping malls may be called shopping centers.
In recent decades, malls have declined considerably in North America, particularly in subprime locations, and some have closed and become so-called "dead malls". Successful exceptions have added entertainment and experiential features, added big-box stores as anchors, or converted to other specialized shopping center formats such as power centers, lifestyle centers, factory outlet centers, and festival marketplaces. In Canada, shopping centres have frequently been replaced with mixed-use high-rise communities. In many European countries and Asian countries, shopping malls continue to grow and thrive.
Terminology
In the United States, Persian Gulf countries, and India, the term shopping mall is usually applied to enclosed retail structures (and is generally abbreviated to simply mall), while shopping center usually refers to open-air retail complexes; both types of facilities usually have large parking lots, face major traffic arterials, and have few pedestrian connections to surrounding neighborhoods. Outside of North America, the terms shopping precinct and shopping arcade are also used.
In the U.K., such complexes are considered shopping centers (Commonwealth English: shopping centre), though shopping center covers many more sizes and types of centers than the North American mall. Other countries follow U.K. usage. In Canadian English, and often in Australia and New Zealand, the term mall may be used informally but shopping center or merely center will feature in the name of the complex (such as Toronto Eaton Centre). The term mall is less-commonly a part of the name of the complex.
Types
The International Council of Shopping Centers, based in New York City, classifies two types of shopping centers as malls: regional malls and superregional malls. A regional mall, per the International Council of Shopping Centers, is a shopping mall with 400,000 sq ft (37,000 m^2) to 800,000 sq ft (74,000 m^2) gross leasable area with at least two anchor stores. A super-regional mall, per the International Council of Shopping Centers, is a shopping mall with over 800,000 sq ft (74,000 m^2) of gross leasable area, three or more anchors, mass merchant, more variety, fashion apparel, and serves as the dominant shopping venue for the region (25 miles or 40 km) in which it is located. Not classified as malls are smaller formats such as strip malls and neighborhood shopping centers, and specialized formats such as power centers, festival marketplaces, and outlet centers.
History:
Forerunners to the shopping mall
In 1798, the first covered shopping passage was built in Paris, the Passage du Caire. The Burlington Arcade in London was opened in 1819. The Arcade in Providence, Rhode Island, built in 1828, claims to be the first shopping arcade in the United States. Western European cities in particular built many arcade-style shopping centers. The Galleria Vittorio Emanuele II in Milan, which opened in 1877, was larger than its predecessors, and inspired the use of the term "galleria" for many other shopping arcades and malls.
In the mid-20th century, with the rise of the suburb and automobile culture in the United States, a new style of shopping center was created away from downtowns. Early shopping centers designed for the automobile include Market Square, Lake Forest, Illinois (1916), and Country Club Plaza, Kansas City, Missouri (1924).
The suburban shopping center concept evolved further in the United States after World War II, with larger open-air shopping centers anchored by major department stores, such as the 550,000-square-foot (51,000 m^2) Broadway-Crenshaw Center in Los Angeles, built in 1947 and anchored by a five-story Broadway and a May Company California.
Downtown pedestrian malls and use of term mall
In the late 1950s and into the 1960s, the term "shopping mall" was first used, but in the original sense of the word "mall", meaning a pedestrian promenade in the U.S., or in U.K. usage, a "shopping precinct". Early downtown pedestrianized malls included the Kalamazoo Mall (the first, in 1959), "Shoppers' See-Way" in Toledo, Lincoln Road Mall in Miami Beach, Santa Monica Mall (1965).
Although Bergen Mall opened in 1957 using the name "mall" and inspired other suburban shopping centers to rebrand themselves as malls, these types of properties were still referred to as "shopping centers" until the late 1960s.
Enclosed malls
The enclosed shopping center, which would eventually be known as the shopping mall, did not appear in mainstream until the mid-1950s. One of the earliest examples was the Valley Fair Shopping Center in Appleton, Wisconsin, which opened on March 10, 1955. Valley Fair featured a number of modern features including central heating and cooling, a large outdoor parking area, semi-detached anchor stores, and restaurants. Later that year the world's first fully enclosed shopping mall was opened in Luleå, in northern Sweden (architect: Ralph Erskine) and was named Shopping; the region now claims the highest shopping center density in Europe.
The idea of a regionally-sized, fully enclosed shopping complex was pioneered in 1956 by the Austrian-born architect and American immigrant Victor Gruen. This new generation of regional-size shopping centers began with the Gruen-designed Southdale Center, which opened in the Twin Cities suburb of Edina, Minnesota, United States in October 1956. For pioneering the soon-to-be enormously popular mall concept in this form, Gruen has been called the "most influential architect of the twentieth century" by Malcolm Gladwell.
The first retail complex to be promoted as a "mall" was Paramus, New Jersey's Bergen Mall, which opened with an open-air format on November 14, 1957, and was later enclosed in 1973. Aside from Southdale Center, significant early enclosed shopping malls were Harundale Mall (1958) in Glen Burnie, Maryland, Big Town Mall (1959) in Mesquite, Texas, Chris-Town Mall (1961) in Phoenix, Arizona, and Randhurst Center (1962) in Mount Prospect, Illinois.
Other early malls moved retailing away from the dense, commercial downtowns into the largely residential suburbs. This formula (enclosed space with stores attached, away from downtown, and accessible only by automobile) became a popular way to build retail across the world. Gruen himself came to abhor this effect of his new design; he decried the creation of enormous "land wasting seas of parking" and the spread of suburban sprawl.
Even though malls mostly appeared in suburban areas in the U.S., some U.S. cities facilitated the construction of enclosed malls downtown as an effort to revive city centers and allow them to compete effectively with suburban malls. Examples included Main Place Mall in Buffalo (1969) and The Gallery (1977, now Fashion District Philadelphia) in Philadelphia. Other cities created open-air pedestrian malls.
In the United States, developers such as A. Alfred Taubman of Taubman Centers extended the concept further in 1980, with terrazzo tiles at the Mall at Short Hills in New Jersey, indoor fountains, and two levels allowing a shopper to make a circuit of all the stores. Taubman believed carpeting increased friction, slowing down customers, so it was removed. Fading daylight through glass panels was supplemented by gradually increased electric lighting, making it seem like the afternoon was lasting longer, which encouraged shoppers to linger.
Decline of shopping malls in the United States
In the United States, in the mid-1990s, malls were still being constructed at a rate of 140 a year. But in 2001, a PricewaterhouseCoopers study found that underperforming and vacant malls, known as "greyfield" and "dead mall" estates, were an emerging problem. In 2007, a year before the Great Recession, no new malls were built in America, for the first time in 50 years. City Creek Center Mall in Salt Lake City, which opened in March 2012, was the first to be built since the recession.
Malls began to lose consumers to open-air power centers and lifestyle centers during the 1990s, as consumers preferred to park right in front of and walk directly into big-box stores with lower prices and without the overhead of traditional malls (i.e., long enclosed corridors).
Another issue was that the growth-crazed American commercial real estate industry had simply built too many nice places to shop—far more than could be reasonably justified by the actual growth of the American population, retail sales, or any other economic indicator. The number of American shopping centers exploded from 4,500 in 1960 to 70,000 by 1986 to just under 108,000 by 2010.
Thus, the number of dead malls increased significantly in the early 21st century. The economic health of malls across the United States has been in decline, as revealed by high vacancy rates. From 2006 to 2010, the percentage of malls that are considered to be "dying" by real estate experts (have a vacancy rate of at least 40%), unhealthy (20–40%), or in trouble (10–20%) all increased greatly, and these high vacancy rates only partially decreased from 2010 to 2014. In 2014, nearly 3% of all malls in the United States were considered to be "dying" (40% or higher vacancy rates) and nearly one-fifth of all malls had vacancy rates considered "troubling" (10% or higher). Some real estate experts say the "fundamental problem" is a glut of malls in many parts of the country creating a market that is "extremely over-retailed". By the time shopping mall operator Unibail-Rodamco-Westfield decided to exit the American market in 2022, the United States had an average of 24.5 square feet of retail space per capita (in contrast to 4.5 square feet per capita in Europe).
In 2019, The Shops & Restaurants at Hudson Yards opened as an upscale mall in New York City with "a 'Fifth Avenue' mix of shops", such as H&M, Zara, and Sephora below them. This is one of the first two malls built recently, along with American Dream in which both opened in 2019 since City Creek Center.
Online shopping has also emerged as a major competitor to shopping malls. In the United States, online shopping has accounted for an increasing share of total retail sales. In 2013, roughly 200 out of 1,300 malls across the United States were going out of business. To combat this trend, developers have converted malls into other uses including attractions such as parks, movie theaters, gyms, and even fishing lakes. In the United States, the 600,000 square foot Highland Mall will be a campus for Austin Community College. In France, the So Ouest mall outside of Paris was designed to resemble elegant, Louis XV-style apartments and includes 17,000 square metres (180,000 sq ft) of green space. The Australian mall company Westfield launched an online mall (and later a mobile app) with 150 stores, 3,000 brands and over 1 million products.
The COVID-19 pandemic also significantly impacted the retail industry. Government regulations temporarily closed malls, increased entrance controls, and imposed strict public sanitation requirements.
Design:
Vertical malls
High land prices in populous cities have led to the concept of the "vertical mall", in which space allocated to retail is configured over a number of stories accessible by elevators and/or escalators (usually both) linking the different levels of the mall. The challenge of this type of mall is to overcome the natural tendency of shoppers to move horizontally and encourage shoppers to move upwards and downwards. The concept of a vertical mall was originally conceived in the late 1960s by the Mafco Company, former shopping center development division of Marshall Field & Co. The Water Tower Place skyscraper in Chicago, Illinois was built in 1975 by Urban Retail Properties. It contains a hotel, luxury condominiums, and office space and sits atop a block-long base containing an eight-level atrium-style retail mall that fronts on the Magnificent Mile.
Vertical malls are common in densely populated conurbations in East and Southeast Asia. Hong Kong in particular has numerous examples such as Times Square, Dragon Centre, Apm, Langham Place, ISQUARE, Hysan Place and The One.
A vertical mall may also be built where the geography prevents building outward or there are other restrictions on construction, such as historic buildings or significant archeology. The Darwin Shopping Centre and associated malls in Shrewsbury, UK, are built on the side of a steep hill, around the former town walls; consequently the shopping center is split over seven floors vertically – two locations horizontally – connected by elevators, escalators and bridge walkways. Some establishments incorporate such designs into their layout, such as Shrewsbury's former McDonald's, split into four stories with multiple mezzanines which featured medieval castle vaults – complete with arrowslits – in the basement dining rooms.
Components:
Food court
A common feature of shopping malls is a food court: this typically consists of a number of fast food vendors of various types, surrounding a shared seating area.
Department stores
When the shopping mall format was developed by Victor Gruen in the mid-1950s, signing larger department stores was necessary for the financial stability of the projects, and to draw retail traffic that would result in visits to the smaller stores in the mall as well. These larger stores are termed anchor stores or draw tenants. In physical configuration, anchor stores are normally located as far from each other as possible to maximize the amount of traffic from one anchor to another.
Regional differences:
Europe
There are a reported 222 malls in Europe. In 2014, these malls had combined sales of US$12.47 billion. This represented a 10% bump in revenues from the prior year.
U.K. and Ireland
In the United Kingdom and Ireland, both open-air and enclosed centers are commonly referred to as shopping centres. Mall primarily refers to either a shopping mall – a place where a collection of shops all adjoin a pedestrian area – or an exclusively pedestrianized street that allows shoppers to walk without interference from vehicle traffic.
The majority of British enclosed shopping centres, the equivalent of a U.S. mall, are located in city centres, usually found in old and historic shopping districts and surrounded by subsidiary open air shopping streets. Large examples include West Quay in Southampton; Manchester Arndale; Bullring Birmingham; Liverpool One; Trinity Leeds; Buchanan Galleries in Glasgow; St James Quarter in Edinburgh; and Eldon Square in Newcastle upon Tyne. In addition to the inner city shopping centres, large UK conurbations will also have large out-of-town "regional malls" such as the Metrocentre in Gateshead; Meadowhall Centre, Sheffield serving South Yorkshire; the Trafford Centre in Greater Manchester; White Rose Centre in Leeds; the Merry Hill Centre near Dudley; and Bluewater in Kent. These centres were built in the 1980s and 1990s, but planning regulations prohibit the construction of any more. Out-of-town shopping developments in the UK are now focused on retail parks, which consist of groups of warehouse style shops with individual entrances from outdoors. Planning policy prioritizes the development of existing town centres, although with patchy success. Westfield London (White City) is the largest shopping centre in Europe.
Russia
In Russia, on the other hand, as of 2013 a large number of new malls had been built near major cities, notably the MEGA malls such as Mega Belaya Dacha mall near Moscow. In large part they were financed by international investors and were popular with shoppers from the emerging middle class.
Management and legal issues:
Shopping property management firms
A shopping property management firm is a company that specializes in owning and managing shopping malls. Most shopping property management firms own at least 20 malls. Some firms use a similar naming scheme for most of their malls; for example, Mills Corporation puts "Mills" in most of its mall names and SM Prime Holdings of the Philippines puts "SM" in all of its malls, as well as anchor stores such as The SM Store, SM Appliance Center, SM Hypermarket, SM Cinema, and SM Supermarket. In the UK, The Mall Fund changes the name of any center it buys to "The Mall (location)", using its pink-M logo; when it sells a mall the center reverts to its own name and branding, such as the Ashley Centre in Epsom. Similarly, following its rebranding from Capital Shopping Centres, intu Properties renamed many of its centres to "intu (name/location)" (such as intu Lakeside); again, malls removed from the network revert to their own brand (see for instance The Glades in Bromley).
Legal issues
One controversial aspect of malls has been their effective displacement of traditional main streets or high streets. Some consumers prefer malls, with their parking garages, controlled environments, and private security guards, over central business districts (CBD) or downtowns, which frequently have limited parking, poor maintenance, outdoor weather, and limited police coverage.
In response, a few jurisdictions, notably California, have expanded the right of freedom of speech to ensure that speakers will be able to reach consumers who prefer to shop, eat, and socialize within the boundaries of privately owned malls. The Supreme Court decision Pruneyard Shopping Center v. Robins was issued on 9 June 1980 which affirmed the decision of the California Supreme Court in a case that arose out of a free speech dispute between the Pruneyard Shopping Center in Campbell, California, and several local high school students.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2213 2024-07-14 17:52:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2215) Cherry Juice
Details
Cherry juice is a fruit juice consisting of the juice of cherries. It is consumed as a beverage and used as an ingredient in various foods, processed foods and beverages. It is also marketed as a health supplement. It is produced by hot- or cold-pressing cherries, collecting the juice, and then filtering and pasteurizing it.
Usage:
As a food
Cherry juice is a mass-produced food product that is consumed as a beverage and used as an ingredient in various foods, processed foods and beverages. It is sometimes used as an ingredient in cherry ice cream and in cherry pie filling. It is also used as an ingredient in cherry brandy and cherry bounce. Cherry jelly has also been produced using the juice. Cherry juice concentrate is used by food manufacturers in the production of fruit juice blends. Cherry juice from the Montmorency cherry is used to produce cherry essence, which is used as a flavor concentrate by food manufacturers.
In alcoholic beverages
Kirsch fruit brandy is sometimes produced via the distillation of fermented cherry juice. Cherry juice is also used as an ingredient in beer. For example, Samuel Smith Old Brewery's cherry beer contains 17% of organic cherry juice, and Three Floyds Brewing produces its Battle of Charro II Imperial Brett IPA using cherry juice as an ingredient. Cherry cider has also been brewed by some companies using cherry juice. Sweetened cherry juice is sometimes used in the production of kriek lambic, a distinctively sour, cherry beer style from Belgium.
As a dietary supplement
Montmorency cherry juice is produced as a dietary supplement, and is manufactured as a concentrate and in capsules as a freeze-dried powder.
Claims have been made that cherry juice can be helpful for improving sleep for people with insomnia, but there is no good evidence to support these claims.
Commercial production
Large-scale commercial cherry juice production is typically produced using a hot extraction or a cold extraction method.
Hot extraction involves heating the cherries, pressing them, and then straining and filtering to remove solids. Hot pressed cherry juice typically has a deeper coloration compared to that produced using cold extraction. The heating of the fruit also serves to prevent the juice from browning, because the heating stops natural enzymic actions that occur when the fruit is macerated.
Cold extraction involves first removing the pits from fresh cherries and then pressing them and collecting the juice. The juice is then heated to kill microorganisms, stop enzyme activity and to solidify particulate matter prior to filtering. As with hot-extracted juice, the cold-extracted juice is also typically strained and filtered. Cold-extracted cherry juice has a greater likeness to the flavor of fresh cherries, and its coloration is lighter compared to that of hot-extracted juice.
Frozen cherries are sometimes used, which enables the creation of a juice that has the cherry-like flavor of cold-extracted juice and a deeper coloration such as that produced by hot extraction.
Ascorbic acid is sometimes added as a color stabilizer prior to the cherries being pressed. The juice is typically filtered and clarified prior to being packaged, and pasteurization or flash pasteurization is typically utilized. It is sometimes processed as a frozen concentrate. Commercial cherry juice concentrate is shipped in bulk containers to food manufacturers and in smaller, consumer-sized containers for retail sales.
In the United States, cherry juice is produced mostly in the state of Wisconsin. More minute amounts are produced in the U.S. states of New York, Pennsylvania and Colorado.
Beverage production
Pure cherry juice has a strong flavor and can have high acidity, so when produced commercially as a beverage product it is sometimes diluted with water to make it more palatable. Sugar syrup or dry sugar is sometimes added to the product when produced as a beverage. Mixtures of both hot-pressed and cold-pressed juices are sometimes used in the production of cherry juice beverages, which allows for a product that has a desirable coloration and flavor for consumers. Cherry juice is also produced as a carbonated beverage product.
History
Herodotus notes that cherry juice was consumed by the Argippaeans, either fresh or mixed with milk. Cherry juice was also drunk by ancient Romans.
In the late 19th century, cherry juice was not produced in the United States, and was imported from Germany. The imported juice was used by wholesale liquor and drug companies, as well as soda producers. Drug companies typically used the juice to produce syrups for soda water, and liquor companies used it to produce cherry brandy, cherry bounce and liqueurs. German-imported cherry juice was fortified with alcohol to prevent the juice from fermenting, which would spoil it. During this time, juice produced in Magdeburg, Germany from black cherries grown in the area was typically exported to the U.S.
Additional Information
Cherry juice is not only refreshingly delicious, but it provides some solid health benefits, too. With about 120 calories per 1-cup serving, it’s rich in nutrients like potassium and iron.
There are many different varieties of cherry juice. Look for juices that use 100-percent cherry juice with no added sweeteners. Cherry juice “math” typically add sugar and preservatives.
You will also see juice “from concentrate” and “not from concentrate.” Both options are nutritionally similar.
“Not from concentrate” means they put the fresh juice directly into the bottle. “From concentrate” means they squeezed and then filtered the juice, extracting water. It is then rehydrated and packaged.
There are also different types of cherries used to produce juice. Tart cherry juice is sour to taste and provides a higher amount of anthocyanins compared to black cherry juice, which is sweeter in taste and has less anthocyanins. Anthocyanins promote anti-inflammatory processes in the body. Both are great, nutritious options.
Read on for seven reasons to sip and savor cherry juice.
1. Helps post-workout recovery
Cherry juice may help recovery post-exercise. It is naturally high in potassium, which conducts electrical impulses throughout the body.
This mineral also helps maintain blood pressure, hydration, muscle recovery, nerve impulses, digestion, heart rate, and pH balance. Cherries contain about 330 milligrams (mg) of potassium per cup, which is almost 10 percent of your daily recommended value.
2. Fights inflammation and arthritis pain
Research shows that the antioxidants in tart cherry juice can reduce pain and inflammation from osteoarthritis (OA).
A 2012 study showed that drinking cherry juice twice a day for 21 days reduced the pain felt by people with OA. Blood tests also showed that they experienced significantly less inflammation.
3. Reduces swelling
When people experience pain from swelling, they often turn to nonsteroidal anti-inflammatory drugs (NSAIDs). However, the effects of these drugs can be harmful, especially when you take them too often or have allergies.
A 2004 study found that cherry juice supplements can reduce inflammation and pain-related behavior in animals, showing promise as a treatment for swelling in humans.
4. Boosts immunity
Like all fruits and vegetables, cherries pack a powerful antioxidant and antiviral punch. Flavonoids, a type of antioxidant in cherry juice, are made by plants to fight infection. Research shows that these chemicals can have a significant impact on immune system function.
5. Regulates metabolism and fights fat
There is some evidence in animals that tart cherries can help adjust your body’s metabolism and your ability to lose abdominal body fat. One study showed that anthocyanins, a type of flavonoid responsible for cherries’ red color, act against the development of obesity.
Another study in rats found that tart cherries can help reduce inflammation and abdominal fat, and lower the risk of metabolic syndrome.
6. Helps you sleep
The anti-inflammatory properties of cherry juice combined with a dash of sleep-regulating melatonin may help you sleep better, according to a small 2010 study. The results suggest that tart cherry juice has similar effects as insomnia medications like valerian or melatonin on older adults.
7. Blocks cancer growth
In a 2003 study, researchers pitted cherry juice against the NSAID sulindac, which is the most common preventive anti-inflammatory treatment for colon tumors. Although an animal study, it is notable that cherry juice — unlike the NSAID — reduced the growth of cancer cells.
Even without its antioxidants and nutrients, cherry juice is deliciously tart and refreshing. Try replacing sodas and sports drinks with something that can really make a difference to your health.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2214 2024-07-15 14:21:24
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2216) Coconut water
Details
Coconut water (also coconut juice) is the clear liquid inside young coconuts (fruits of the coconut palm). In early development, it serves as a suspension for the endosperm of the coconut during the nuclear phase of development. As development continues, the endosperm matures into its cellular phase and deposits into the rind of the coconut pulp. The liquid inside young coconuts is sometimes preferred to the liquid of a ripened coconut. Coconut water from young green coconuts is also known specifically as buko juice in Philippine English.
Harvesting
Fresh coconuts are typically harvested from the tree while they are green. A hole may be bored into the coconut to provide access to the "meat" (liquid and pulp). In young coconuts, the liquid and air may be under some pressure and may spray slightly when the inner husk is first penetrated. Coconuts that have fallen to the ground are susceptible to rot and damage from insects or other animals.
Products
Plain coconut water has long been a popular drink in tropical countries, where it is available fresh, canned, or bottled.
Coconuts for drinking are served chilled, fresh, or packaged. They are often sold by street vendors who cut them open with machetes or similar implements in front of customers. Coconut water for retail can be found in ordinary aluminum cans, Tetra Paks, glass bottles or plastic bottles, sometimes with coconut pulp or coconut jelly included.
Coconut water can be fermented to produce coconut vinegar (though coconut sap is used more often). It is also used to make nata de coco, a jelly-like food.
Nutritional value
Nutritional value per 100 g (3.5 oz)
Energy : 79 kJ (19 kcal)
Carbohydrates : 3.71 g
Sugars : 2.61 g
Dietary fiber : 1.1 g
Fat : 0.20 g
Saturated : 0.176 g
Monounsaturated : 0.008 g
Polyunsaturated : 0.002 g
Protein : 0.72 g
Tryptophan : 0.008 g
Threonine : 0.026 g
Isoleucine : 0.028 g
Leucine : 0.053 g
Lysine : 0.032 g
Methionine : 0.013 g
Cystine : 0.014 g
Phenylalanine : 0.037 g
Tyrosine : 0.022 g
Valine : 0.044 g
Arginine : 0.118 g
Histidine : 0.017 g
Alanine : 0.037 g
Aspartic acid : 0.070 g
Glutamic acid : 0.165 g
Glycine : 0.034 g
Proline : 0.030 g
Serine : 0.037 g
Vitamins : Quantity%DV
Thiamine (B1) : 3%0.030 mg
Riboflavin (B2) : 4%0.057 mg
Niacin (B3) : 1%0.080 mg
Pantothenic acid (B5) : 1%0.043 mg
Vitamin B6 : 2%0.032 mg
Folate (B9) : 1%3 μg
Choline : 0%1.1 mg
Vitamin C : 3%2.4 mg
Minerals : Quantity%DV
Calcium : 2%24 mg
Copper : 4%0.04 mg
Iron : 2%0.29 mg
Magnesium : 6%25 mg
Manganese : 6%0.142 mg
Phosphorus : 2%20 mg
Potassium : 8%250 mg
Selenium : 2%1 μg
Sodium : 5%105 mg
Zinc : 1%0.10 mg
Other constituents : Quantity 95 g
Percentages estimated using US recommendations for adults, except for potassium, which is estimated based on expert recommendation from the National Academies.
Providing 79 kilojoules (19 kilocalories) of food energy in a 100-millilitre (3+1⁄2-US-fluid-ounce) amount, coconut water is 95% water and 4% carbohydrates, with negligible protein and fat content (table). Coconut water contains small amounts of vitamins and dietary minerals, all under 10% of the Daily Value (DV).
Risks
The Food and Drug Administration has identified a risk of bacterial contamination in coconut water sold as "raw".
Anecdotal sources describe coconut water being used in the southern part of India for senicide, the killing of elderly people, a procedure known as thalaikoothal. In this custom, the elderly person is made to drink an excessive amount of coconut water, eventually resulting in fever and death, the exact causes of which have not been determined.
Commercialization
Since the early 21st century, coconut water has been marketed in Western countries as a natural energy or sports drink having low levels of fat, carbohydrates, and calories, and significant electrolyte content.
False advertising
Marketing claims attributing health benefits to coconut water are not based on science and are disallowed by certain regulatory agencies like the United States Food and Drug Administration which warned producers about misleading marketing claims that coconut water is antiviral, can lower cholesterol, can regulate blood glucose levels, and other false claims, as inappropriate for the product.
Some companies have faced class-action lawsuits over false advertising claims that the product was "super-hydrating", "nutrient-packed", and "mega-electrolyte". The plaintiffs also alleged that one company, Vita Coco, falsely claimed that its product had "15 times the electrolytes found in sports drinks" and misrepresented the levels of sodium and magnesium as advertised. The company denied any wrongdoing and settled the lawsuit for US$10 million in April 2012.
Medical use in Cambodia
Although substituting coconut water for saline is not recommended by physicians today, it was a common practice during the Khmer Rouge regime in Cambodia from 1975 to 1979. The Documentation Center of Cambodia cited the practice of allowing untrained nurses to administer green coconut water during the Pol Pot regime as a crime against humanity.
Additional Information
Coconut water can be a nutrient-rich choice for hydrating. It may benefit your health, including the heart and kidneys.
In recent years, coconut water has become quite the trendy beverage.
In addition to being naturally sweet and hydrating, coconut water is loaded with several important nutrients, including minerals that many people don’t get enough of.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2215 2024-07-16 13:53:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2217) Coconut milk
Summary
Coconut milk can provide essential vitamins and nutrients, including vitamin C and iron. It may also contains a lot of calories and saturated fats.
Coconut milk has recently become very popular.
It’s a tasty alternative to cow’s milk that may also provide a number of health benefits.
What Is Coconut Milk?
Coconut milk comes from the white flesh of mature brown coconuts, which are the fruit of the coconut tree.
The milk has a thick consistency and a rich, creamy texture.
Thai and other Southeast Asian cuisines commonly include this milk. It’s also popular in Hawaii, India and certain South American and Caribbean countries.
Coconut milk should not be confused with coconut water, which is found naturally in immature green coconuts.
Unlike coconut water, the milk does not occur naturally. Instead, solid coconut flesh is mixed with water to make coconut milk, which is about 50% water.
By contrast, coconut water is about 94% water. It contains much less fat and far fewer nutrients than coconut milk.
Coconut milk comes from the flesh of mature brown coconuts. It is used in many traditional cuisines around the world.
Details
Coconut milk is an opaque, milky-white liquid extracted from the grated pulp of mature coconuts. The opacity and rich taste of coconut milk are due to its high oil content, most of which is saturated fat. Coconut milk is a traditional food ingredient used in Southeast Asia, Oceania, South Asia, and East Africa. It is also used for cooking in the Caribbean, tropical Latin America, and West Africa, where coconuts were introduced during the colonial era.
Coconut milk is differentiated into subtypes based on fat content. They can be generalized into coconut cream (or thick coconut milk) with the highest amount of fat; coconut milk (or thin coconut milk) with a maximum of around 20% fat; and coconut skim milk with negligible amounts of fat. This terminology is not always followed in commercial coconut milk sold in Western countries.
Coconut milk can also be used to produce milk substitutes (differentiated as "coconut milk beverages"). These products are not the same as regular coconut milk products which are meant for cooking, not drinking. A sweetened, processed, coconut milk product from Puerto Rico is also known as cream of coconut. It is used in many desserts and beverages like the piña colada, though it should not be confused with coconut cream.
Nutrition
In a 100 milliliter (ml) portion, coconut milk contains 230 kilocalories and is 68% water, 24% total fat, 6% carbohydrates, and 2% protein. The fat composition includes 21 grams of saturated fat, half of which is lauric acid.
Coconut milk is a rich source (20% or more of the Daily Value, DV) of manganese (44% DV per 100 g) and an adequate source (10–19% DV per 100 g) of phosphorus, iron, and magnesium, with no other nutrients in significant content .
Definition and terminology
Coconut milk is a relatively stable oil-in-water emulsion with proteins that act as emulsifiers and thickening agents. It is opaque and milky white in color and ranges in consistency from watery to creamy. Based on fat content, coconut milk is divided into different subtypes generally simplified into "coconut cream", "coconut milk", and "coconut skim milk", from highest to lowest respectively. Coconut milk and coconut cream (also called "thin coconut milk" and "thick coconut milk", respectively) are traditionally differentiated in countries where coconuts are native based on the stages of extraction. They are also differentiated in modern standards set by the Asian and Pacific Coconut Community (APCC) and the Food and Agriculture Organization of the United Nations (FAO). However, the terminologies are not always followed in commercial coconut milk (especially in western countries) because these standards are not mandatory. This can cause confusion among consumers.
Coconut milk can also sometimes be confused with coconut water. Coconut water is the clear fluid found within the coconut seed, while coconut milk is the extracted liquid derived from the manual or mechanical crushing of the white inner flesh of mature coconuts. Coconut cream should also not be confused with creamed coconut, which is a semi-solid paste made from finely ground coconut pulp, and cream of coconut, which is a processed product made from heavily sweetened coconut cream.
Traditional preparation:
Coconut milk is traditionally made by grating the white inner flesh of mature coconuts and mixing the shredded coconut pulp with a small amount of hot water in order to suspend the fat present in the grated pulp. The grating process can be carried out manually or by machine.
Coconut milk is also traditionally divided into two grades: coconut cream (or thick coconut milk) and thin coconut milk. Coconut cream contains around 20% to 50% fat; while thin coconut milk contains 5% to 20% fat. Coconut cream is extracted from the first pressings of grated coconut pulp directly through cheesecloth. Sometimes a small amount of hot water may also be added, but generally coconut cream is extracted with no added water. Thin coconut milk, on the other hand, is produced by the subsequent pressings after soaking the squeezed coconut pulp with hot water.
Gravity separation can also be used to derive a top layer of coconut cream and a bottom layer of coconut skim milk. This is achieved by simply allowing the extracted liquid to stand for an hour. Conversely, coconut cream can be diluted into thinner coconut milk by simply adding water.
Traditionally prepared coconut milk is utilized immediately after being freshly extracted because it spoils easily when exposed to air. It becomes rancid after a few hours at room temperatures 28 to 30 °C (82 to 86 °F) due to lipid oxidation and lipolysis. Rancid coconut milk gives off a strong unpleasant smell and has a distinctive soapy taste.
As coconut cream contains a higher amount of soluble, suspended solids, it works well as a good ingredient for desserts and rich and dry sauces. On the other hand, thin milk contains less amount of these soluble solids. Thus, it is mainly used in general cooking. The distinction between coconut cream and thin coconut milk is not usually made in western nations due to the fact that fresh coconut milk is uncommon in these countries and most consumers buy coconut milk in cartons or cans.
Coconut milk is also an intermediate step in the traditional wet process methods of producing virgin coconut oil by gradual heating, churning, or fermentation. These methods, however, are less efficient than coconut oil production from copra.
Coconut graters
Coconut graters (also called "coconut scrapers"), a necessary tool for traditionally extracting coconut milk, were part of the material culture of the Austronesian peoples. From Island Southeast Asia, it was carried along with the sea voyages of the Austronesian expansion both for colonization and trade, reaching as far as Polynesia in the east, and Madagascar and the Comoros in the west in prehistoric times. The technology also spread to non-Austronesian cultures in coastal East Africa by proximity. Manual coconut graters remain a standard kitchen equipment in households in the tropical Asia-Pacific and Eastern Africa, underscoring the importance of coconut milk and coconut oil extraction in the Indo-Pacific.
The basic design of coconut graters consists of a low bench or stool with a horizontal serrated disk (made of metal in Asia and Africa, and stone or shell in Oceania) attached on one end. A person sits on the bench and repeatedly scrapes the inner surface of halved coconut shells with both hands over the metal disk. The scrapings are gathered by a container placed below.
More modern mechanical coconut graters dating back to the mid-1800s consist of serrated blades with a hand crank. This version is believed to be a British invention.
Additional Information
Coconut milk is one of the best substitutes for bovine milk around. You can drink it straight, use it as creamer in your coffee or tea, mix it with your chai, or even just cook with it. There is really nothing that you can’t use coconut milk for. And with this recipe you don’t have to buy your coconut milk out of a box, you can make it yourself so that its always fresh from the coconut.
Health Benefits
Coconut milk is essentially made from two separate coconut products: the coconut water and the coconut cream. It is made by extracting these products directly from the coconut. Thanks to being a coconut extract it contains all the vitamins and minerals found in coconuts like, magnesium, iron, copper, manganese, vitamin C, selenium, and folate. These help your body to improve digestion, reduce inflammation, boost blood circulation, and stimulate weight loss. Coconut milk also contains a decent amount of electrolytes which helps with water retention.
:max_bytes(150000):strip_icc():format(webp)/20231201-SEA-CoconutMilk-KarinaMatalon-06-feb006003a26400b9d639490025bf80a.jpg)
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2216 2024-07-17 13:56:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2218) Grapefruit juice
Details
Grapefruit juice is the juice from grapefruits. It is rich in vitamin C and ranges from sweet-tart to very sour. Variations include white grapefruit, pink grapefruit and ruby red grapefruit juice.
Grapefruit juice is important in medicine because of its interactions with many common drugs including caffeine and medications, which can alter how they behave in the body.
Grapefruit juice is a common breakfast beverage in the United States.
Drug interactions
Grapefruit and grapefruit juice have been found to interact with numerous drugs, in many cases resulting in adverse effects. This happens in two ways: one is that grapefruit can block an enzyme which metabolizes medication, and if the drug is not metabolized, then the level of the drug in the blood can become too high, leading to an adverse effect. The other effect is that grapefruit can block the absorption of drugs in the intestine, and if the drug is not absorbed, then not enough of it is in the blood to have a therapeutic effect.
One whole grapefruit or a glass of 200 mL (6.8 US fl oz) of grapefruit juice can cause drug overdose toxicity. Drugs which are incompatible with grapefruit are typically labeled on the container or package insert. People taking drugs can ask their health care provider or pharmacist questions about grapefruit/drug interactions.
Use in certain drinks
Grapefruit juice is used in several drinks, such as the sea breeze (which consists of grapefruit juice, vodka, and cranberry juice); the salty dog, the grapefruit mimosa, and a grapefruit radler.
Canadian regulations
Canadian regulations on commercially produced and sold grapefruit juice are that it must be made from clean, mature grapefruit and may contain sugar, invert sugar, dextrose, glucose solids and class II preservatives such as benzoic acid, amylase, cellulase and pectinase. According to Canadian standards, grapefruit juice should contain more than 1.15 milliequivalents of free amino acid per 100 millilitres (3.5 imp fl oz; 3.4 US fl oz); more than 70 milligrams of potassium per 100 ml; and have an absorbance value for total polyphenolics of no less than 0.310. During the production process, the sugar content in the juice, before the addition of sugar, invert sugar, dextrose or glucose solids, should have a Brix reading of no less than 9.3. It must contain 0.7% to 2.1% of acid by weight as anhydrous citric acid.
Additional Information
This low calorie, nutrient dense fruit comes with a host of health benefits. It’s high in vitamin C and antioxidants, can benefit your heart and immune system, and more.
Grapefruit is a tropical citrus fruit known for its sweet yet tart taste. It is rich in nutrients, antioxidants, and fiber. This makes it one of the healthiest citrus fruits you can eat.
Plus, research shows that grapefruit may have some powerful health benefits. These include weight loss and a reduced risk of heart disease.
Here are 10 evidence-based health benefits of grapefruit.
1. It’s low in calories, yet high in nutrients
Grapefruit is a great food to include in a balanced diet. That’s because it’s high in nutrients but low in calories. In fact, it’s one of the lowest-calorie fruits.
It provides a decent amount of fiber, in addition to more than 15 beneficial vitamins and minerals.
Here are some of the major nutrients found in half of a medium-sized grapefruitTrusted Source:
Calories: 52
Carbs: 13 grams
Protein: 1 gram
Fiber: 2 grams
Vitamin C: 43% of the daily value (DV)
Vitamin A: 8% of the DV
Potassium: 4% of the DV
Thiamine: 4% of the DV
Folate: 4% of the DV
Magnesium: 3% of the DV
Additionally, it is a rich source of some powerful antioxidant plant compounds, which are likely responsible for many of its health benefits.
2. It may benefit your immune system
Eating grapefruit regularly may be beneficial for your immune system.
It’s prized for its high vitamin C content. Vitamin C has antioxidant properties known to protect your cells from harmful bacteria and viruses.
Additionally, studies have shown vitamin C may be beneficial for helping people recover more quickly from the common cold.
Many other vitamins and minerals found in grapefruit are known to benefit immunity, including vitamin A. Vitamin A has been shown to help protect against inflammation and several infectious diseases.
Grapefruit also provides small amounts of B vitamins, zinc, copper, and iron. These all work together in the body to promote immune system function. They also help maintain the integrity of your skin, which acts as a protective barrier to infection.
3. Grapefruit has weight loss benefits
Grapefruit is a weight loss–friendly food.
It has several properties linked to weight loss, especially its fiber content. This helps promote fullness and reduce calorie intake.
Grapefruit contains a decent amount of fiber — 2 grams in half of a medium-sized fruit.
Additionally, grapefruit contains few calories but lots of water, which is another characteristic that may help with weight loss.
Several studies have found weight-reducing effects associated with consuming grapefruit. For instance, one older study found that participants experienced a reduced waist size when they consumed grapefruit daily with their meals.
However, there were no significant differences in the reduction of waist size between the study participants who drank water, those who ate grapefruit, and those who drank grapefruit juice.
This isn’t to say that grapefruit will produce weight loss on its own, but adding it to an already balanced, nutritious diet may prove to be beneficial.
4. Grapefruit may help prevent insulin resistance and diabetes
Eating grapefruit regularly may have the potential to prevent insulin resistance, reducing the risk of diabetes.
Insulin resistance occurs when your cells stop responding to insulin.
Insulin is a hormone that regulates many processes in your body. It’s involved in many aspects of your metabolism, but it’s most commonly known for its role in blood sugar control.
Insulin resistance ultimately leads to higher insulin and blood sugar levels, two primary risk factors for type 2 diabetes.
Eating grapefruit may help control insulin levels, meaning it may have the ability to reduce your likelihood of becoming insulin resistant.
In one 2006 study, subjects who ate half of a fresh grapefruit three times a day before meals experienced a significant reduction in both insulin levels and insulin resistance, compared with the group of people who didn’t eat grapefruit.
Plus, eating fruit as a whole, instead of fruit juice, is generally associated with better blood sugar control and a reduced risk of type 2 diabetes.
5. Eating grapefruit may improve heart health
Regularly consuming grapefruit is thought to improve heart health by reducing risk factors for heart disease, such as high blood pressure and cholesterol.
In a 2017 meta-analysis of 3 clinical trials, people who ate grapefruit experienced significant reductions in systolic blood pressure.
These effects are likely due to the important nutrients that grapefruit contains, which play a role in keeping your heart functioning properly.
First, grapefruit is fairly high in potassium, a mineral responsible for many aspects of heart health. Half a grapefruit provides about 5% of your daily potassium needs.
Adequate potassium intake is associated with a reduced risk of high blood pressure. Additionally, it has been shown to lower the risk of death from heart disease.
Second, the fiber in grapefruit may also boost heart health, given that a high fiber intake is associated with lower blood pressure and cholesterol levels.
Overall, researchers claim that including fiber and antioxidant-rich fruits like grapefruit as part of a healthy diet helps protect against conditions like heart disease and stroke.
6. It’s high in powerful antioxidants
Grapefruit contains a few different antioxidants that provide various health benefits, including a reduced risk of several diseases.
Antioxidants protect your cells from damage caused by free radicals, which are unstable molecules that may cause harmful reactions in your body.
Here’s an overview of the most important antioxidants in grapefruit:
* Vitamin C. This is a powerful, water-soluble antioxidant that is present in high amounts in grapefruit. It may protect cells from damage that often leads to heart disease and cancer.
* Beta-carotene. It’s converted into vitamin A in the body and is thought to help reduce the risk of some chronic conditions, including heart disease, cancer, and eye-related disorders like macular degeneration.
* Lycopene. This is known for its potential ability to prevent the development of certain types of cancer, especially prostate cancer. It may also help slow the growth of tumors and decrease the side effects of common cancer treatments.
* Flavanones. Their anti-inflammatory properties have been shown to reduce blood pressure and cholesterol levels, reducing the risk of heart disease.
7. It may reduce the risk of kidney stones
Consuming grapefruit may reduce your risk of developing kidney stones, which result from a buildup of waste materials in the kidneys.
These waste materials are products of metabolism that are typically filtered through the kidneys and removed from the body in urine.
However, when they crystallize in the kidneys, they become stones. Larger kidney stones may cause a blockage in the urinary system, which can be incredibly painful.
The most common type of kidney stone is calcium oxalate stones. Citric acid, an organic acid found in grapefruit, may be effective at preventing them by binding with calcium in your kidneys and flushing it out of your body, though evidence is mixed.
Also, citric acid has the ability to increase the volume and pH of your urine, producing an environment that is less favorable to the formation of kidney stones.
8. Grapefruit has hydration benefits
Grapefruit contains a lot of water and is, therefore, very hydrating. In fact, water makes up most of the fruit’s weight.
There are almost 4 ounces (118 ml) of water in half of a medium-sized grapefruit, which accounts for about 88% of its total weight.
While drinking lots of water is the best way to stay hydrated, eating water-rich foods can also help.
9. It’s easy to add to your diet
Grapefruit requires little-to-no preparation, so it’s fairly easy to add to your diet.
Even if you live a busy, on-the-go lifestyle, you can still enjoy grapefruit on a regular basis without worrying about it taking up too much of your time.
Here are some ways you can enjoy grapefruit:
* Snack on grapefruit slices alone.
* Eat it as an alternative to dessert foods that are less nutritious.
* Try this salad, which combines grapefruit with arugula and pecans.
* Blend it into a smoothie with other fruits and veggies.
* Include it in a breakfast parfait with yogurt and honey.
10. Grapefruit has benefits for skin
Grapefruit contains vitamin C, which helps protect the skin against sun damage, aging, and inflammation.
Vitamin C is often used in serums to heal the skin, brighten dark spots, and smooth the skin surface. However, studies also show that an increased intake of vitamin C through foods like grapefruit may help with hyperpigmentation, discoloration, and signs of aging.
Vitamin C helps the body produce more collagen, which has been shown to benefit skin hydration and wrinkles.
Grapefruit also contains citric acid, malic acid, and tartaric acid. These are all different types of alpha-hydroxy acids (AHAs). AHAs are often used in skin care products due to their variety of benefits, including improved skin texture and elasticity.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2217 2024-07-18 14:13:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2219) Blackcurrant
Details
The blackcurrant (Ribes nigrum), also known as black currant or cassis, is a deciduous shrub in the family Grossulariaceae grown for its edible berries. It is native to temperate parts of central and northern Europe and northern Asia, where it prefers damp fertile soils. It is widely cultivated both commercially and domestically.
It is winter hardy, but cold weather at flowering time during the spring may reduce the size of the crop. Bunches of small, glossy black fruit develop along the stems in the summer and can be harvested by hand or by machine.
The raw fruit is particularly rich in vitamin C and polyphenols. Blackcurrants can be eaten raw but are usually cooked in sweet or savoury dishes. They are used to make jams, preserves, and syrups and are grown commercially for the juice market. The fruit is also used to make alcoholic beverages and dyes.
Description
Ribes nigrum is a medium-sized shrub, growing to 1.5 by 1.5 metres (5 by 5 feet). The leaves are alternate, simple, 3 to 5 centimetres (1+1⁄4 to 2 inches) broad and long with five palmate lobes and a serrated margin. All parts of the plant are strongly aromatic. The flowers are produced in racemes known as "strigs" up to 8 cm (3 in) long containing 10–20 flowers, each about 8 millimetres (3⁄8 in) in diameter. Each flower has a hairy calyx with yellow glands, the five lobes of which are longer than the inconspicuous petals. There are five stamens surrounding the stigma and style and two fused carpels. The flowers open in succession from the base of the strig and are mostly insect pollinated, but some pollen is distributed by the wind. A pollen grain landing on a stigma will germinate and send a slender pollen tube down the style to the ovule. In warm weather this takes about 48 hours but in cold weather it may take a week, and by that time, the ovule may have passed the stage where it is receptive. If fewer than about 35 ovules are fertilised, the fruit may not be able to develop and will fall prematurely. Frost can damage both unopened and open flowers when the temperature falls below −1.9 °C (28.6 °F). The flowers at the base of the strig are more protected by the foliage and are less likely to be damaged.
In midsummer the strigs of green fruit ripen to edible berries, very dark purple in colour, almost black, with glossy skins and calyxes at the apex (the calyxes being persistent), each containing many seeds. An established bush can produce about 4.5 kilograms (10 pounds) of fruit each year.
Plants from Northern Asia are sometimes distinguished as a separate variety, Ribes nigrum var. sibiricum, of which R. cyathiforme is considered a synonym.
Phytochemicals
Polyphenol phytochemicals present in the fruit, seeds and leaves, are being investigated for their potential biological activities. Major anthocyanins in blackcurrant pomace – delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside, and cyanidin-3-O-rutinoside, which are retained in the juice concentrate – are among other polyphenols.
Distribution and habitat
The blackcurrant is native to northern Europe and Asia.
Cultivation:
Cultivated specimen
Cultivation in Europe is thought to have started around the last decades of the 17th century.
Site selection and planting
Blackcurrants can grow well on sandy or heavy loams, or forest soils, as long as their nutrient requirements are met. They prefer damp, fertile but not waterlogged ground and are intolerant of drought. Although the bushes are winter hardy, frosts during the flowering period may adversely affect the yield and cold winds may restrict the number of flying insects visiting and pollinating the flowers. A soil pH of about 6 is ideal for blackcurrants and the ground can be limed if the soil is too acidic. Planting is usually done in the autumn or winter to allow the plants to become established before growth starts in the spring, but container-grown stock can be planted at any time of year.
Two-year-old bushes are usually planted but strong one-year-old stock can also be used. Planting certified stock avoids the risk of introducing viruses. On a garden scale the plants can be set at intervals of 1.5 to 1.8 m (5 to 6 ft) or they can be set in rows with planting intervals of 1.2 m (4 ft) and row separations of 2.5 m (8 ft) or more. In the UK, young bushes are generally planted deeper than their initial growing level to encourage new stems to grow from the base.
Manures and fertilizers
The blackcurrant requires a number of essential nutrients to thrive; nitrogen provides strong plant growth and stimulates the production of flower sprigs; phosphorus aids growth, the setting of fruit and crop yield; potassium promotes growth of individual shoots and increases the weight of individual fruits; magnesium is a constituent of chlorophyll and helps increase yields through interaction with potassium; calcium is required for cell division and enlargement and is particularly important for young plants and buds.
An annual spring mulch of well rotted manure is ideal and poultry manure can also be used but needs prior composting with straw or other waste vegetable material. Spent mushroom compost can be used but care should be taken as it often contains lime and blackcurrants prefer slightly acidic soils. The blackcurrant is a gross feeder and benefits from additional nitrogen, and phosphatic and potash fertilisers should also be applied annually. A balanced artificial fertilizer can be used and a 10-10-10 granular product can be spread around the bushes at the rate of 100 to 240 grams (3+1⁄2 to 8+1⁄2 ounces) per plant. Weed growth can be suppressed with an organic mulch such as sawdust, bark, mushroom compost or straw, heavy plastic topped with an organic mulch cover or landscape fabric.
Pruning
Blackcurrant fruit is borne primarily on one-year-old shoots. Newly planted bushes should be pruned severely, cutting all shoots back to two buds above ground level. This gives the plant a chance to get properly established before needing to put its energy into producing fruit. The general rule when pruning is to remove all weak shoots and those growing out sideways which may get weighed down when fruiting. The remaining branches should be thinned to remove old unproductive wood and to encourage new shoots. An established bush should not be allowed to become overcrowded and should have about one third of its main branches or stems removed each year. When harvesting by machine, plants with an upright growth habit are encouraged.
Harvesting
On a garden scale, the berries should be picked when dry and ripe. Commercially, most harvesting is done mechanically by straddle harvesters. These move continually down the rows, straddling a row of bushes, shaking the branches and stripping off the fruit. The blackcurrants are placed into half tonne bins and to minimise stoppage time, some machines have cross conveyors which direct the fruit into continuously moving trailers in the adjoining row. A modern machine can pick up to fifty tonnes of blackcurrants in a day using only one operator and two tractor drivers. The bins should be stored in a cool place. Some fruit is still picked by hand for use in the fresh fruit market.
Diseases and pests
Ribes plants are susceptible to several diseases and a number of insect pests. However, new varieties have been or are being developed to overcome some of these problems.
Reversion is a serious disease transmitted by the blackcurrant gall mite (Cecidophyopsis ribis). It causes a decline in yield and is quite widespread in Europe but is rarely encountered on other continents. Symptoms include a modification of leaf shape in summer and swollen buds ("big bud") in winter, each housing thousands of microscopic mites. As pest control has limited effectiveness, severely infected bushes should be destroyed. All new plants purchased should be certified as virus-free.
White pine blister rust (Cronartium ribicola) needs two alternate hosts to complete its life cycle. One host is plants in the genus Ribes. On the blackcurrant, it causes the leaves to become pale and later develop tiny orange pustules and sometimes a yellow filamentous coating on some leaves. The fruit crop is little affected but the leaves fall early and growth is slowed the following year. The other host is any of the white pines, in which it causes serious disease and mortality for the North American species that have not co-evolved with the rust. As a result, the blackcurrant was banned in the United States as a disease vector for much of the 20th century, and even after the federal ban was lifted in 1966, several U.S. states continued their own bans, some of which remain in force as of August 2021. The effectiveness of these restrictions is questionable, since other Ribes species also host the disease, some are native to North America, and others such as red currants and Ribes uva-crispa were never banned.
American gooseberry mildew and powdery mildew can infect the leaves and shoot tips, and botrytis may cause the fruit to rot in a wet season. Currant and gooseberry leaf spot (Drepanopeziza ribis) is another disease of blackcurrants, but it is not usually a serious problem as most cultivars now have some resistance.
The blackcurrant leaf midge can cause browning, crimping and distortion of leaves at the tips of shoots but it is seldom a serious problem. The blackcurrant sawfly (Nematus ribesii) lays its eggs on the underside of the leaves and the voracious larvae work their way along the shoots, stripping off leaf after leaf. In a serious attack, the bush can be denuded of leaves. Larvae of the currant borer drill their way along the centres of shoots, which wilt and die back. Other insect pests include scale insects, aphids and earwigs.
Research and cultivars
Green currant is a variant of blackcurrant cultivated in Finland; its berries lack the dark color and strong aroma typical of blackcurrant. This particular cultivar is 'Vertti'.
There are many cultivars of blackcurrant. 'Baldwin' was the mainstay of the industry for many years but it has now largely been superseded by more productive and disease-resistant varieties. During the 20th century in Europe, much hybridisation work has been carried out in order to reduce the plant's susceptibility to disease and frost and also to increase yields. This effort centered mainly in Scotland, Poland, and New Zealand.
In Britain the Scottish Crop Research Institute was tasked with developing new varieties suitable for growing in the north of the country. They produced new cultivars that had greater cold tolerance, especially in the spring, ripened earlier and more evenly and had greater fungal disease resistance. Frost tolerance was improved by selecting for late flowering and genetic research identified genes involved in resistance to gall mite and the blackcurrant reversion virus. 'Ben Lomond' was the first of the 'Ben' varieties and was released in 1975. This was followed by several other cultivars for the juicing industry such as 'Ben Alder' and 'Ben Tirran'. The cultivar 'Ben Hope' was released in 1998 with increased tolerance to gall mite, and in the same year, 'Ben Gairn' became available. It shows resistance to the reversion virus. For gardeners and the pick-your-own market, 'Ben Sarek', 'Ben Connan' and 'Big Ben' were introduced and have large, sweet berries. The cultivars 'Ben Connan' and 'Big Ben' have gained the Royal Horticultural Society's Award of Garden Merit. and new varieties are being developed continually to improve frost tolerance, disease resistance, machine harvesting, fruit quality, nutritional content and fruit flavour.
Varieties producing green fruit, less strongly flavoured and sweeter than typical blackcurrants, are cultivated in Finland, where they are called "greencurrants" (viherherukka). In Poland, the Research Institute of Horticulture has done work on improving the blackcurrant with regard to disease and pest resistance, fruit quality, adaptations to local conditions and mechanical harvesting. Researchers have crossed various varieties and introduced inter-specific genetic material from the gooseberry (Ribes grossularia), the redcurrant (Ribes rubrum) and the flowering currant (Ribes sanguineum). The resulting offspring were further back-crossed to R. nigrum. Cultivars produced include 'Tisel' and 'Tiben' in 2000 and 'Ores', 'Ruben' and 'Tines' in 2005. Further cultivars 'Polares' and 'Tihope' are being tested. Since 1991, New Zealand has become an important centre for research and development, as its temperate climate is particularly suitable for cultivation of the crop. Breeding programmes are concentrating on yield, large fruit size, consistency of cropping and upright habit.
In North America, there is a need for this fruit to have resistance to white pine blister rust. New cultivars such as 'Crusader', 'Coronet' and 'Consort' have been developed there by crossing R. nigrum with R. ussuriense and these show resistance to the disease. However the quality and yield of these varieties are poor as compared to non-resistant strains and only Consort is reliably self-fertile. Back-crossing these varieties to a parent have produced new strains such as 'Titania' that have a higher yield, better disease resistance, are more tolerant of adverse weather conditions and are suitable for machine harvesting. Two new releases from a black currant breeding program in British Columbia, Canada, 'Blackcomb' and 'Tahsis', were selected for their immunity to white pine blister rust and their frost tolerance.
Uses:
Nutrition
Raw blackcurrants are 82% water, 15% carbohydrates, 1% protein and 0.4% fat (table). Per 100 g serving providing 63 kilocalories, the raw fruit has high vitamin C content (218% of the Daily Value, DV) and moderate levels of iron and manganese (12% DV each). Other nutrients are present in negligible amounts (less than 10% DV).
Blackcurrant seed oil is rich in vitamin E and unsaturated fatty acids, including alpha-linolenic acid and gamma-linolenic acid.
History
Decoction of the leaves, bark or roots was used as a traditional remedy.
During World War II, most fruits rich in vitamin C, such as oranges, became difficult to obtain in the United Kingdom. Since blackcurrant berries are a rich source of the vitamin, and blackcurrant plants are suitable for growing in the UK climate, the British Government encouraged their cultivation and soon the yield of the nation's crop increased significantly. From 1942 onwards, blackcurrant syrup was distributed free of charge to children under the age of two. This may have given rise to the lasting popularity of blackcurrant as a flavouring in Britain. In Britain the commercial crop is completely mechanised and about 1,400 hectares of the fruit are grown, mostly under contract to the juicing industry. Commercially, most large-scale cultivation of blackcurrants is done in eastern Europe for the juice and juice concentrate market. As of 2017, major cultivation efforts to improve fruit characteristics occurred in Scotland, New Zealand, and Poland.
Blackcurrants were once popular in the United States as well, but became less common in the 20th century after currant farming was banned in the early 1900s, when blackcurrants, as a vector of white pine blister rust, were considered a threat to the U.S. logging industry. The federal ban on growing currants was shifted to the jurisdictions of individual states in 1966, and was lifted in New York State in 2003 through the efforts of horticulturist Greg Quinn. As a result, currant growing is making a comeback in New York, Vermont, Connecticut, California, and Oregon. However, several statewide bans still exist as of August 2021. Since the American federal ban curtailed currant production nationally for nearly a century, the fruit remains largely unknown in the United States and has yet to regain its previous popularity to levels enjoyed in Europe or New Zealand. Owing to its unique flavour and richness in polyphenols, dietary fibre and essential nutrients, awareness and popularity of blackcurrant is once again growing, with a number of consumer products entering the U.S. market.
Culinary
The fruit of blackcurrants when eaten raw has a strong, tart flavour. It can be made into jams and jellies which set readily because of the fruit's high content of pectin and acid. For culinary use, the fruit is usually cooked with sugar to produce a purée, which can then be passed through muslin to separate the juice. The purée can be used to make blackcurrant preserves and be included in cheesecakes, yogurt, ice cream, desserts, sorbets, and many other sweet dishes. The exceptionally strong flavour can be moderated by combining it with other fruits, such as raspberries and strawberries in summer pudding, or apples in crumbles and pies. The juice can be used in syrups and cordials. Blackcurrants are a common ingredient of rødgrød, a popular kissel-like dessert in North German and Danish cuisines.
Blackcurrants are also used in savoury cooking. Their astringency creates added flavour in sauces, meats and other dishes. Blackcurrants are included in some unusual combinations of foods. They can be added to tomato and mint to make a salad. Blackcurrants may accompany roast beef, grilled lamb, duck, seafood and shellfish. Canvasback duck with blackcurrants was a delicacy in nineteenth century New York. They can provide a dipping sauce at barbecues. They can be blended with mayonnaise, and used to invigorate bananas and other tropical fruits. Blackcurrants can be combined with dark chocolate or added to mincemeat in traditional mince pies at Christmas.
Japan imports US$3.6 million of New Zealand blackcurrants for uses as dietary supplements, snacks, functional food products and as quick-frozen (IQF) produce for culinary production as jams, jellies or preserves.
Additional Information
Blackcurrants (Ribes nigrum) have been called “the forbidden fruit” in the United States. They help spread a fungus that infects white pine trees. For this reason, blackcurrants have been removed from many areas and Americans have missed out on these nutritious berries.
Blackcurrants have a high concentration of:
* anthocyanins
* polyphenolic substances
* antioxidants
* vitamin C
* gamma-linolenic acid (GLA)
Many health foods and drinks in the United Kingdom use these berries. Their tartness also lends itself to mixing with other fruits, especially in jams and juices.
Herbalists’ honoree
People use the whole blackcurrant plant, from the leaves to the seeds, for many conditions. The most common form is blackcurrant seed oil, but you can also make infusions and teas out of the plant’s leaves, fresh or dried.
People take blackcurrant to help their:
* blood flow
* immune system
* eye health
* gut health
* kidney health
Blackcurrant extracts are shown to reduce risk factors for metabolic conditions such as type 1 and 2 diabetes.
Vitamin superstar
Blackcurrants contain many vitamins, such as:
* A
* B-5
* B-6
* B-1
* E
The most significant is vitamin C. In fact, blackcurrants carry four times the amount of vitamin C as oranges, and double the amount of antioxidants as blueberries.
The benefits of vitamin C are many. The body uses vitamin C to metabolize protein and form collagen, which is essential for skin care and anti-aging.
Boosts immune system
In addition to vitamin C, blackcurrants have plenty of antioxidants and anthocyanins. These can help strength your immune system, soothe sore throats, and ease flu symptoms.
Blackcurrant leaves also have a range of properties, including:
* antimicrobial
* anti-inflammatory
* antiviral
* antitoxic
* antiseptic
* anticancer
One study showed that blackcurrant supplements enhanced the immune response in people who exercised regularly. They could also train harder for longer periods of time.
Another study of healthy older adults showed that blackcurrant seed oil boosted the immune system.
Joint jump starter
Blackcurrants have a direct effect on your body’s inflammatory response.
Blackcurrant seed oil contains gamma-linolenic acid (GLA), a type of omega-6 fatty acid that’s been said to help ease inflammation in the body. The high GLA and anthocyanin content can help reduce joint or muscle:
* pain
* stiffness
* soreness
* damage
In some studies, GLA supplements were so effective that participants with rheumatoid arthritis could reduce their usual pain medications.
Plaque punisher and heart helper
Grape-based drinks like wine and juice are known to help decrease plaque buildup, but blackcurrant juice, as well as pomegranate juice, is far more potent.
Blackcurrant is high in potassium and GLA, which can help lower your blood pressure too. The GLA also helps cells in your heart resist damage and slows down platelet clumping in your blood vessels.
In addition, one study found that blackcurrant powder increased heart blood flow and decreased overall peripheral resistance. This suggests that blackcurrant may help you recover after exercise.
While hard to find in most U.S. stores, the blackcurrant-based drink Ribena is very popular in the U.K.
Skin soother
Although there isn’t much scientific research about blackcurrant seed oil and its effectiveness for skin conditions, the National Psoriasis Foundation recommends the oil to help ease psoriasis symptoms.
Taken orally, blackcurrant seed oil can help slow the growth and development of psoriasis patches. It also can be applied directly to dry, itchy, or stinging skin.
Easy on the eyes
Research shows that GLA and linoleic acid, which are found in vitamin C, may be promising for treating dry eye. Thankfully, blackcurrants are packed with both of those.
Clinical trials with blackcurrants found that these berries improve eye function, including:
* the eyes’ ability to adapt to the dark
* blood flow to the eyes
* slowed progression of visual field deterioration in people with glaucoma
* symptoms of visual fatigue
People who do computer work every day may benefit from blackcurrant supplements. One study found that 1 tablespoon of blackcurrant berries reduced visual fatigue two hours afterward.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2218 2024-07-18 18:31:37
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2220) Petroleum jelly
Gist
For minor wounds such as cuts, scrapes, and scratches, use petroleum jelly to keep the wound moist. This helps prevent the wound from drying out and forming a scab, as scabs take longer to heal. This will also help prevent a scar from getting too large, deep or itchy.
Summary
Petroleum jelly has been used for years to help with skin moisturizing and healing. However, some types could contain carcinogenic ingredients. For safety, only buy triple-distilled products like Vaseline.
What is petroleum jelly made of?
Petroleum jelly (also called petrolatum) is a mixture of mineral oils and waxes, which form a semisolid jelly-like substance. This product hasn’t changed much since Robert Augustus Chesebrough discovered it in 1859. Chesebrough noticed that oil workers would use a gooey jelly to heal their wounds and burns. He eventually packaged this jelly as Vaseline.
Petroleum jelly’s benefits come from its main ingredient petroleum, which helps seal your skin with a water-protective barrier. This helps your skin heal and retain moisture. Read on to learn what else you can use petroleum jelly for.
Benefits and uses for petroleum jelly
1. Heal minor skin scrapes and burns
A study shows that petroleum jelly is effective in keeping skin moist during post-surgery healing. This may be particularly good for regular, less dramatic skin injuries. Make sure that the surface you apply petroleum jelly on is properly cleaned and disinfected. Otherwise, bacteria and other pathogens can get trapped inside and delay the healing process.
2. Moisturize your face, hands, and more
Face and body lotion: Apply petroleum jelly after a shower. As an occlusive moisturizer, it prevents your skin from drying out. You can also use it for dry noses during cold or allergy season.
Cracked heels: Soak your feet in warm water with some salt added to it. Towel-dry thoroughly and apply petroleum jelly and clean cotton socks.
Improve your gardening hands: After washing and drying, use some petroleum jelly and a clean pair of gloves to help lock in moisture and accelerate healing.
Chapped lips: Apply to chapped lips as you would any chapstick.
3. Help for pet paws
Your dog’s pad skin can crack and produce a great deal of discomfort. Clean their paws with cotton gauze, dry, and apply the jelly. Ideally this should be done after a walk or when your pet is resting. Only use a small amount as your pet may lick their paws, and consuming too much could cause an upset stomach.
4. Prevent diaper rash
Petroleum jelly has been shown to reduce the incidence of diaper rash in babies. Clean and towel-dry your little one’s skin properly before applying. Petroleum jelly will form a protective barrier that will help protect the skin from constant exposure to moisture. Make an appointment with the doctor if there is a persistent rash.
5. Remove eye makeup
Oil is an effective way to remove makeup, and petroleum jelly is safe to use in the eye area, according to a study on eye ultrasounds. Use a cotton pad or Q-tip (for hard to reach areas), and press gently without tugging too hard on your skin. Make sure to close your eyes as you wipe. Some people also swear by using it on crow’s feet lines.
6. Save split ends
Sun and wind exposure as well as pool water can dry up your hair. Petroleum jelly can reduce the look of split ends and add shine to your hair. Rub a small amount of jelly between your palms and apply to hair ends.
7. Prevent skin stains from hair dye or nail polish
Apply petroleum jelly along your hairline to prevent hair dye from staining your skin. This also works if you like to paint your nails at home. A barrier of petroleum jelly is easy to wipe away when you’re done.
8. Preserve perfume scents
Using petroleum jelly as a base for your perfume can help it last longer.
9. Use as lube for stuck objects
If a ring is stuck on your finger, put some jelly on your finger, making sure you get some around and under the ring. This will help the ring slip off your finger.
For door hinges, apply a bit of jelly right on the hinge and swing the door a few times to spread evenly. Wipe off the excess.
Details
Petroleum jelly, petrolatum, white petrolatum, soft paraffin, or multi-hydrocarbon, CAS number 8009-03-8, is a semi-solid mixture of hydrocarbons (with carbon numbers mainly higher than 25), originally promoted as a topical ointment for its healing properties. Vaseline has been an American brand of petroleum jelly since 1870.
After petroleum jelly became a medicine-chest staple, consumers began to use it for cosmetic purposes and for many ailments including toenail fungus, genital rashes (non-STI), nosebleeds, diaper rash, and common colds. Its folkloric medicinal value as a "cure-all" has since been limited by a better scientific understanding of appropriate and inappropriate uses. It is recognized by the U.S. Food and Drug Administration (FDA) as an approved over-the-counter (OTC) skin protectant and remains widely used in cosmetic skin care, where it is often loosely referred to as mineral oil.
History
Marco Polo in 1273 described the oil exportation of Baku oil by hundreds of camels and ships for burning and as an ointment for treating mange.
Native Americans discovered the use of petroleum jelly for protecting and healing skin. Sophisticated oil pits had been built as early as 1415–1450 in Western Pennsylvania. In 1859, workers operating the United States's first oil rigs noticed a paraffin-like material forming on rigs in the course of investigating malfunctions. Believing the substance hastened healing, the workers used the jelly on cuts and burns.
Robert Chesebrough, a young chemist whose previous work of distilling fuel from the oil of sperm whales had been rendered obsolete by petroleum, went to Titusville, Pennsylvania, to see what new materials had commercial potential. Chesebrough took the unrefined green-to-gold-colored "rod wax", as the drillers called it, back to his laboratory to refine it and explore potential uses. He discovered that by distilling the lighter, thinner oil products from the rod wax, he could create a light-colored gel. Chesebrough patented the process of making petroleum jelly by U.S. patent 127,568 in 1872. The process involved vacuum distillation of the crude material followed by filtration of the still residue through bone char. Chesebrough traveled around New York demonstrating the product to encourage sales by burning his skin with acid or an open flame, then spreading the ointment on his injuries and showing his past injuries healed, he said, by his miracle product. He opened his first factory in 1870 in Brooklyn using the name Vaseline.
Physical properties
Petroleum jelly is a mixture of hydrocarbons, with a melting point that depends on the exact proportions. The melting point is typically between 40 and 70 °C (105 and 160 °F). It is flammable only when heated to liquid; then the fumes will light, not the liquid itself, so a wick material is needed to ignite petroleum jelly. It is colorless (or of a pale yellow color when not highly distilled), translucent, and devoid of taste and smell when pure. It does not oxidize on exposure to the air and is not readily acted on by chemical reagents. It is insoluble in water. It is soluble in dichloromethane, chloroform, benzene, diethyl ether, carbon disulfide and turpentine. Petroleum jelly is slightly soluble in alcohol. It acts as a plasticizer on polypropylene (PP), but is compatible with most other plastics[citation needed]. It is a semi-solid, in that it holds its shape indefinitely like a solid, but it can be forced to take the shape of its container without breaking apart, like a liquid, though it does not flow on its own. At room temperature, it has 20.9% solid fat content. Its partially crystalline stacks of lamellar sheets, which immobilize the liquid portion, make up its microstructure. In general, only 7–13% of it is made up of high molecular weight paraffins, 30–45% of smaller paraffins, and 48–60% of small paraffins.
Depending on the specific application of petroleum jelly, it may be USP, B.P., or Ph. Eur. grade. This pertains to the processing and handling of the petroleum jelly so it is suitable for medicinal and personal-care applications.
Uses
Petroleum jelly has lubricating and coating properties, including use on dry lips and dry skin. Below are some examples of the uses of petroleum jelly.
Medical treatment
Vaseline brand First Aid Petroleum Jelly, or carbolated petroleum jelly containing phenol to give the jelly additional antibacterial effect, has been discontinued.
During World War II, a variety of petroleum jelly called red veterinary petrolatum, or Red Vet Pet for short, was often included in life raft survival kits. Acting as a sunscreen, it provides protection against ultraviolet rays.
The American Academy of Dermatology recommends keeping skin injuries moist with petroleum jelly to reduce scarring. A verified medicinal use is to protect and prevent moisture loss of the skin of a patient in the initial post-operative period following laser skin resurfacing.
Petroleum jelly is used extensively by otorhinolaryngologists—ear, nose, and throat doctors—for nasal moisture and epistaxis treatment, and to combat nasal crusting. Large studies have found petroleum jelly applied to the nose for short durations to have no significant side effects.
Historically, it was also consumed for internal use and even promoted as "Vaseline confection".
Skin and hair care
Most petroleum jelly today is used as an ingredient in skin lotions and cosmetics, providing various types of skin care and protection by minimizing friction or reducing moisture loss, or by functioning as a grooming aid (e.g., pomade). It is also used for treating dry scalp and dandruff. Although long known as just an occlusive, recent studies show that it is actually able to penetrate into the stratum corneum and helps in better absorption of other cosmetic products.
Preventing moisture loss
By reducing the loss of moisture via transepidermal water loss, petroleum jelly can prevent chapped hands and lips, and soften nail cuticles.
This property is exploited to provide heat insulation: petroleum jelly can be used to keep swimmers warm in water when training, or during channel crossings or long ocean swims. It can prevent chilling of the face due to evaporation of skin moisture during cold weather outdoor sports.
Hair grooming
In the first part of the twentieth century, petroleum jelly, either pure or as an ingredient, was also popular as a hair pomade. When used in a 50/50 mixture with pure beeswax, it makes an effective moustache wax.
Skin lubrication
Petroleum jelly can be used to reduce the friction between skin and clothing during various sport activities, for example to prevent chafing of the seat region of cyclists, or the nipples of long distance runners wearing loose T-shirts, and is commonly used in the groin area of wrestlers and footballers.
Petroleum jelly is commonly used as a personal lubricant, because it does not dry out like water-based lubricants, and has a distinctive "feel", different from that of K-Y and related methylcellulose products. However, it is not recommended for use with condoms during sexual activity, as it increases the chance of rupture. In addition, petroleum jelly is difficult for the body to break down naturally, and may cause vaginal health problems when used for intercourse.
Product care and protection:
Coating
Petroleum jelly can be used to coat corrosion-prone items such as metallic trinkets, non-stainless steel blades, and gun barrels prior to storage as it serves as an excellent and inexpensive water repellent. It is used as an environmentally friendly underwater antifouling coating for motor boats and sailing yachts. It was recommended in the Porsche owner's manual as a preservative for light alloy (alleny) anodized Fuchs wheels to protect them against corrosion from road salts and brake dust.
Finishing
It can be used to finish and protect wood, much like a mineral oil finish. It is used to condition and protect smooth leather products like bicycle saddles, boots, motorcycle clothing, and used to put a shine on patent leather shoes (when applied in a thin coat and then gently buffed off).
Lubrication
Petroleum jelly can be used to lubricate zippers and slide rules. It was also recommended by Porsche in maintenance training documentation for lubrication (after cleaning) of "Weatherstrips on Doors, Hood, Tailgate, Sun Roof". It is used in bullet lubricant compounds.
Industrial production processes
Petroleum jelly is a useful material when incorporated into candle wax formulas. It softens the overall blend, allows the candle to incorporate additional fragrance oil, and facilitates adhesion to the sidewall of the glass. Petroleum jelly is used to moisten nondrying modelling clay such as plasticine, as part of a mix of hydrocarbons including those with greater (paraffin wax) and lesser (mineral oil) molecular weights. It is used as a tack reducer additive to printing inks to reduce paper lint "picking" from uncalendered paper stocks. It can be used as a release agent for plaster molds and castings. It is used in the leather industry as a waterproofing cream.
Other:
Explosives
Petroleum jelly can be mixed with a high proportion of strong inorganic chlorates due to it acting as a plasticizer and a fuel source. An example of this is Cheddite C which consists of a ratio of 9:1, KClO3 to petroleum jelly. This mixture is unable to detonate without the use of a blasting cap. It is also used as a stabiliser in the manufacture of the propellant Cordite.
Mechanical, barrier functions
Petroleum jelly can be used to fill copper or fibre-optic cables using plastic insulation to prevent the ingress of water, see icky-pick.
Petroleum jelly can be used to coat the inner walls of terrariums to prevent animals from crawling out to escape.
A stripe of petroleum jelly can be used to prevent the spread of a liquid (retain or confine a liquid to a specific area). For example, it can be applied close to the hairline when using a home hair dye kit to prevent the hair dye from irritating or staining the skin. It is also used to prevent diaper rash.
Petroleum jelly is sometimes used to protect the terminals on batteries. However, automobiles batteries require a silicone-based battery grease because it is less likely to melt and thus offers better protection.
Surface cleansing
Petroleum jelly is used to gently clean a variety of surfaces, ranging from makeup removal from faces to tar stain removal from leather.
Pet care
Petroleum jelly is used to moisturize the paws of dogs. It is a common ingredient in hairball remedies for domestic cats.
Sports
Some goalkeepers in association football put petroleum jelly on their gloves to make them stickier.
Health
Petroleum jelly contains mineral oil aromatic hydrocarbons (MOAH). Many MOAH, mainly polycyclic aromatic hydrocarbons (PAH), are considered carcinogenic. The content of both MOAH and PAH in petroleum jelly products varies. The EU limits PAH content in cosmetics to 0.005%. The risks of PAH exposure through cosmetics have not been comprehensively studied, but food products with low levels (<3%) are not considered carcinogenic (by the EU).
A 2012 scientific opinion by the European Food Safety Authority stated that mineral oil aromatic hydrocarbons (MOAH) and polyaromatics were potentially carcinogenic and may present a health risk.
In 2015, German consumer watchdog Stiftung Warentest analyzed cosmetics containing mineral oils, finding significant concentrations of MOAH and polyaromatics in products containing mineral oils. Vaseline products contained the most MOAH of all tested cosmetics (up to 9%). Based on the 2015 results, Stiftung Warentest warned consumers not to use Vaseline or any product that is based on mineral oils for lip care.
A study published in 2017 found levels of MOAH levels to be up to 1% in petroleum jelly and likewise to be less than 1% in petroleum jelly-based beauty products.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2219 2024-07-19 14:21:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2221) Guava
Gist
Guava is native to tropical America. It was introduced in India in the early 17 century. In addition it contains various vitamins, proteins and dietary fibres. It can be used for making jam, jelly, juice and nectar. This fruit also has antioxidant properties.
Details
Guava is a common tropical fruit cultivated in many tropical and subtropical regions. The common guava Psidium guajava (lemon guava, apple guava) is a small tree in the myrtle family (Myrtaceae), native to Mexico, Central America, the Caribbean and northern South America. The name guava is also given to some other species in the genus Psidium such as strawberry guava (Psidium cattleyanum) and to the pineapple guava, Feijoa sellowiana. In 2019, 55 million tonnes of guavas were produced worldwide, led by India with 45% of the total. Botanically, guavas are berries.
Etymology
The term guava appears to have been in use since the mid-16th century. The name derived from the Taíno, a language of the Arawaks as guayabo for guava tree via the Spanish for guayaba. It has been adapted in many European and Asian languages, having a similar form.
Origin and distribution
Guavas originated from an area thought to extend from Mexico, Central America or northern South America throughout the Caribbean region. Archaeological sites in Peru yielded evidence of guava cultivation as early as 2500 BC.
Guava was adopted as a crop in subtropical and tropical Asia, parts of the United States (from Tennessee and North Carolina, southward, as well as the west and Hawaii), tropical Africa, and Oceania. Guavas were introduced to Florida, US in the 19th century and are grown there as far north as Sarasota, Chipley, Waldo and Fort Pierce. However, they are a primary host of the Caribbean fruit fly and must be protected against infestation in areas of Florida where this pest is present.
Guavas are cultivated in several tropical and subtropical countries. Several species are grown commercially; apple guava and its cultivars are those most commonly traded internationally. Guavas also grow in southwestern Europe, specifically the Costa del Sol on Málaga, (Spain) and Greece where guavas have been commercially grown since the middle of the 20th century and they proliferate as cultivars. Mature trees of most species are fairly cold-hardy and can survive temperatures slightly colder than −4 °C (25 °F) for short periods of time, but younger plants will likely freeze to the ground.
Guavas are of interest to home growers in subtropical areas as one of the few tropical fruits that can grow to fruiting size in pots indoors. When grown from seed, guava trees can bear fruit in two years, and can continue to do so for forty years.
Types
The most frequently eaten species, and the one often simply referred to as "the guava", is the apple guava (Psidium guajava). Guavas are typical Myrtoideae, with tough dark heavy leaves that are opposite, simple, elliptic to ovate, and 5–15 centimetres (2–6 in) long. The flowers are white, with five petals and numerous stamens. The fruits are many-seeded berries.
Ecology
Psidium species are eaten by the caterpillars of some Lepidoptera, mainly moths like the Ello Sphinx (Erinnyis ello), Eupseudosoma aberrans, E. involutum, and Hypercompe icasia. Mites, like Pronematus pruni and Tydeus munsteri, are known to be crop pests of the apple guava (P. guajava) and perhaps other species. The bacterium Erwinia psidii causes rot diseases of the apple guava.
The fruit is cultivated and favored by humans, and many other animals such as birds consume it, readily dispersing the seeds in their droppings. In Hawaii, strawberry guava (P. littorale) has become an aggressive invasive species threatening extinction to more than 100 other plant species. By contrast, several guava species have become rare due to habitat destruction and at least one (Jamaican guava, P. dumetorum), is already extinct.
Guava wood is used for meat smoking in Hawaii, and is used at barbecue competitions across the United States. In Cuba and Mexico, the leaves are used in barbecues.
Fruit
Guava fruits, usually 4 to 12 centimetres (1+1⁄2 to 4+1⁄2 in) long, are round or oval depending on the species. They have a pronounced and typical fragrance, similar to lemon rind but less sharp. The outer skin may be rough, often with a bitter taste, or soft and sweet. Varying between species, the skin can be any thickness, is usually green before maturity, but may be yellow, maroon, or green when ripe. The pulp inside may be sweet or sour and off-white ("white" guavas) to deep pink ("red" guavas). The seeds in the central pulp vary in number and hardness, depending on species.
In 2019, world production of guavas was 55 million tonnes, led by India with 45% of the total. Other major producers were China and Thailand.
Uses:
Culinary
In Mexico and other Latin American countries, the beverage agua fresca is often made with guava. The entire fruit is a key ingredient in punch, and the juice is often used in culinary sauces (hot or cold), ales, candies, dried snacks, fruit bars, and desserts, or dipped in chamoy. Pulque de guayaba ("guayaba" is Spanish for guava) is a common alcoholic beverage in these regions.
In many countries, guava is eaten raw, typically cut into quarters or eaten like an apple; it is also eaten with a pinch of salt and pepper, cayenne powder or a mix of spices (masala). In the Philippines, ripe guava is used in cooking sinigang. Guava is a snack in Cuba as pastelitos de guayaba; and in Taiwan, sold on many street corners and night markets during hot weather, accompanied by packets of dried plum powder mixed with sugar and salt for dipping. In east Asia, guava is commonly eaten with sweet and sour dried plum powder mixtures. Guava juice is consumed in many countries. The fruit is also often included in fruit salads.
Because of its high level of pectin, guavas are extensively used to make candies, preserves, jellies, jams, and marmalades (such as Brazilian goiabada and Colombian and Venezuelan bocadillo), and as a marmalade jam served on toast.
Red guavas can be used as the base of salted products such as sauces, substituting for tomatoes, especially to minimize the acidity. A drink may be made from an infusion of guava fruits and leaves, which in Brazil is called chá-de-goiabeira, i.e., "tea" of guava tree leaves.
Nutrition
A raw common guava is 81% water, 14% carbohydrates, 3% protein, and 0.5% fat (table). In a reference amount of 100 grams (3.5 oz), raw guava supplies 68 calories and is a rich source of dietary fiber and vitamin C (275% of the Daily Value, DV), with moderate levels of folic acid (12% DV, table). Raw guava contains lycopene
Phytochemicals
Guava leaves contain both carotenoids and polyphenols, such as (+)-gallocatechin and leucocyanidin.[21] As some of these phytochemicals produce the fruit skin and flesh color, guavas that are red-orange tend to have more polyphenol and carotenoid content than yellow-green ones.
Seed oil
Guava seed oil may be used for culinary or cosmetics products. It is rich in linoleic acid.
Folk medicine
Since the 1950s, guavas – particularly the leaves – have been studied for their constituents, potential biological properties and history in folk medicine.
Parasites
Guavas are one of the most common hosts for fruit flies like A. suspensa, which lay their eggs in overripe or spoiled guavas. The larvae of these flies then consume the fruit until they can proceed into the pupa stage. This parasitism has led to millions in economic losses for nations in Central America.
Fungal pathogens, Neopestalotiopsis and Pestalotiopsis species are causal agents of guava scab in Colombia.
Additional Information
Guava, (Psidium guajava), small tropical tree or shrub of the family Myrtaceae, cultivated for its edible fruits. Guava trees are native to tropical America and are grown in tropical and subtropical areas worldwide. Guava fruits are processed into jams, jellies, and preserves and are common pastry fillings. Fresh guavas are rich in vitamins A, B, and C; they are commonly eaten raw and may be sliced and served with sugar and cream as a dessert.
Physical description and cultivation
The common guava has quadrangular branchlets, oval to oblong leaves about 7.6 cm (3 inches) in length, and four-petaled white flowers about 2.5 cm (1 inch) broad. The fruits are round to pear-shaped and measure up to 7.6 cm in diameter; their pulp contains many small hard seeds (more abundant in wild forms than in cultivated varieties). The fruit has a yellow skin and white, yellow, or pink flesh. The musky, at times pungent, odour of the sweet pulp is not always appreciated.
Propagation is usually by seeds, but improved varieties must be perpetuated by plant parts. The plant’s hard dry wood and thin bark prevent cutting and conventional methods of grafting. Veneer grafting, using as rootstocks young plants in vigorous growth, gives excellent results.
The plant is not frost-resistant but is successfully grown throughout southern Florida; in several tropical regions it grows so abundantly in a half-wild state as to have become a pest.
Related species
The cattley, or strawberry, guava (Psidium cattleianum) is considerably more frost-resistant than the common guava. It occurs in two forms: one has fruits with a bright yellow skin, and the other has fruits with a purplish red skin. The plant is a large shrub with thick glossy green oval leaves and white flowers. The fruits are round, up to 5 cm (2 inches) in diameter, and contain many hard seeds. The soft pulp has a strawberry-like flavour. This species is frequently planted in gardens throughout southern California and other subtropical regions but is not commercially important.
Other guavas include the cás, or wild guava, of Costa Rica (P. friedrichsthalianum) and the guisaro, or Brazilian guava (P. guineense), both of which have acidic fruits.
:max_bytes(150000):strip_icc():format(webp)/Guava-15d1050d22034909bfca038ef1f8aaa2.jpg)
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2220 2024-07-20 14:23:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2222) Honeydew (melon)
Details
The honeydew melon is one of the two main cultivar types in Cucumis melo Inodorus Group. It is characterized by the smooth, often green or yellowish rind and lack of musky odor. The other main type in the Inodorus Group is the wrinkle-rind casaba melon.
Characteristics
A honeydew has a round to slightly oval shape, typically 15–22 cm (5.9–8.7 in) long. It generally ranges in weight from 1.8 to 3.6 kg (4.0 to 7.9 lb). The flesh is usually pale green in color, while the smooth peel ranges from greenish to yellow. Like most fruit, honeydew has seeds. The inner flesh is eaten, often for dessert, and honeydew is commonly found in supermarkets across the world alongside cantaloupe melons and watermelons. In California, honeydew is in season from August until October.
This fruit grows best in semiarid climates and is harvested based on maturity, not size. Maturity can be hard to judge, but it is based upon the rind color ranging from greenish white (immature) to creamy yellow (mature). Quality is also determined by the honeydew having a nearly spherical shape with a surface free of scars or defects. A honeydew should also feel heavy for its size and have a waxy rather than a fuzzy surface. This reflects the integrity and quality of its flesh as the weight can be attributed to the high water content of the ripened fruit.
Nutrition
The honeydew is 90% water, 9% carbohydrates, 0.1% fat, and 0.5% protein. Like most melons, it is an excellent source of vitamin C, with one cup containing 56% of the recommended daily value. The honeydew is also a good source of vitamin B thiamine, as well as other B vitamins and the mineral potassium. In addition, it is low in calories compared to many other high potassium fruits such as bananas, with only 36 calories per 100g. However, the honeydew contains only negligible amounts of most other vitamins and minerals.
Origin and alternate names
"Honeydew" is the American name for the White Antibes cultivar which has been grown for many years in southern France and Algeria.
In China, honeydews are known as Bailan melons. They are famous locally near Lanzhou, the capital city of Gansu province in China's northwest.
According to Chinese sources, the melons were introduced to China by American Secretary of Agriculture, Henry A. Wallace, who donated melon seeds to the locals while visiting in the 1940s (probably 1944). Wallace served as Secretary of Agriculture and Vice President under president Franklin D. Roosevelt. In 1926, Wallace had founded a major seed company (Pioneer Hi-Bred) and popularized the use of hybridized corn. He also had a general background and interest in agriculture.
As a result of Wallace's introduction of the crop, in China the melon is sometimes called the Wallace. The Mizo people use the name Hmazil, the Garo people and the Chakma people of Chittagong Hill Tracts use the name Chindire and the Tanchangya people call it Te'e in their local language.
Additional Information
Honeydew melons are the sweetest of all melons and are typically in season from July to September. The sweet summer fruit is light green and soft on the inside, and can have a white or yellow skin on the outside, although white skinned versions are generally sweeter. Honeydew is also related to squash and cucumbers.
Honeydew melon contains plenty of water as well as vitamin C, B vitamins, fiber, antioxidants, and smaller amounts of other key nutrients. Eating the fruit may offer several health benefits.
Supports Hydration
One cup of diced honeydew melon provides over five ounces (oz) of water. Consuming adequate water helps you stay hydrated and prevent dehydration.
When you become dehydrated, your body overheats and you can experience unclear thinking, mood changes, constipation, and kidney stones. Drinking water also helps to lubricate and cushion your joints.
One study found adults who stay well-hydrated appear to be healthier, develop fewer chronic conditions, such as heart and lung disease, and live longer compared to people who may not consume enough fluids.
Supports Immune Function
Apart from water, the standout nutrient in honeydew melon is vitamin C. One cup of diced melon provides about a third of the daily requirement for this immune-supporting nutrient.
The immune system needs vitamin C to respond to pathogens, such as bacteria, viruses, fungi, and parasites. Vitamin C’s antioxidant abilities also protect cells from damage known to increase chronic disease risk. The body can't store water-soluble vitamins for long periods, so a regular and adequate intake of vitamin C is required to support healthy immune function.
May Support Blood Pressure Regulation
Honeydews are very low in sodium and high in potassium, both of which are good for managing blood pressure. Potassium helps control blood pressure by causing your kidneys to excrete surplus sodium—a nutrient that can cause high blood pressure in excess. Potassium also eases tension in blood vessel walls, which further reduces blood pressure.
One study found melons, including honeydew, activate the production of a substance called nitric oxide (NO). NO helps smooth muscles in the body relax, including blood vessels, which results in a reduction in blood pressure.
Other research shows eating more fruits and vegetables that produce nitric oxide is part of a diet that can help prevent and treat lifestyle-related diseases, including high blood pressure.
May Protect Against Type 2 Diabetes
Research shows eating fruits like honeydew melon may be beneficial for people with or at risk for type 2 diabetes.
A research review found consuming 200 grams (about seven ounces) of fruit per day is linked to diabetes prevention. In addition, consuming up to 133 grams (about five ounces) of fresh fruit per day has been shown to decreased complications and death in people with type 2 diabetes.
Data also shows while fruits with a lower glycemic load (the amount of carbohydrates in a given portion) may be helpful for blood sugar control in those with type 2 diabetes, the glycemic index or glycemic load of individual fruits did not affect diabetes risk.
An Australian study found fruit consumption preserved insulin sensitivity, or how well insulin works to clear sugar from the blood, and was protective against type 2 diabetes.
After adjusting for other dietary and lifestyle factors, scientists concluded that compared to people with the lowest fruit intakes, those with a moderate total fruit consumption had a 36% lower risk of having type 2 diabetes after five years.
Eating fruit also changes gut microbiota, the collection of microbes that live in the gut, in ways that reduce type 2 diabetes risk.
The fluid and fiber in honeydew melon are an important combo for bowel regularity and the prevention of constipation.
Constipation is defined as having fewer than three bowel movements per week; stools that are hard, dry, or lumpy; stools that are difficult or painful to pass; or a feeling that not all stool has passed.
Common remedies for constipation include eating more fiber and consuming plenty of water, and honeydew provides some of both.
May Support Bone Health
Honeydew melon contains several nutrients involved with bone formation and maintenance, including vitamin C and smaller amounts of vitamin K, magnesium, potassium, and antioxidants.
Vitamin C alone, honeydew’s main nutrient, has been tied to a lower risk of hip fracture and osteoporosis (bone disease), as well as higher bone mineral density of both the neck and spine.
In addition, a study found close adherence to the Mediterranean diet—an eating pattern rich in fruits and vegetables—is protective against osteoporosis. Research shows postmenopausal women who most closely stuck with the Mediterranean diet had higher levels of bone mineral density and fewer hip fractures.
:max_bytes(150000):strip_icc():format(webp)/GettyImages-1206046080-8b478f5eb1914c23870050cb2d175839.jpg)
In some parts of Latin America, especially in Chile, the honeydew is nicknamed "Melón tuna" ("prickly pear melon").[
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2221 2024-07-21 14:30:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2223) Cantaloupe
Details
The cantaloupe is a type of food; a true melon (Cucumis melo) from the family Cucurbitaceae. Originally, cantaloupe referred only to the non-netted, orange-fleshed melons of Europe, but today may refer to any orange-fleshed melon of the C. melo species, including the netted muskmelon which is called cantaloupe in North America, rockmelon in Australia and New Zealand, and spanspek in Southern Africa. Cantaloupes range in mass from 0.5 to 5 kilograms (1 to 11 lb).
Etymology and origin
The name cantaloupe was derived in the 18th century via French cantaloup from The Cantus Region of Italian Cantalupo, which was formerly a papal county seat near Rome, after the fruit was introduced there from Armenia. It was first mentioned in English literature in 1739. The cantaloupe most likely originated in a region from South Asia to Africa. It was later introduced to Europe, and around 1890, became a commercial crop in the United States.
Melon derived from use in Old French as meloun during the 13th century, and from Medieval Latin melonem, a kind of pumpkin. It was among the first plants to be domesticated and cultivated.
The South African English name spanspek dates back at least as far as 18th-century Dutch Suriname: J. van Donselaar wrote in 1770, "Spaansch-spek is the name for the form that grows in Suriname which, because of its thick skin and little flesh, is less consumed." A common etymology involves the Spanish-born Juana María de los Dolores de León Smith, who ate canteloupe for breakfast while her husband and 19th-century governor of Cape Colony, Sir Harry Smith, ate bacon and eggs; the fruit was termed Spanish bacon (Afrikaans Spaanse spek) by locals as a result. However, the term had been in use long before that point.
Types
The European cantaloupe or true cantaloupe, C. melo var. cantalupensis, is lightly ribbed with a sweet and flavorful flesh and a gray-green skin that looks quite different from that of the North American cantaloupe.
The North American cantaloupe or muskmelon, C. melo var. reticulatus, common in the United States, Mexico, and some parts of Canada, is a different variety of C. melo, a melon with a reticulated ("net-like") peel. It is a round melon with firm, orange, moderately sweet flesh.
Production
In 2016, global production of melons, including cantaloupes, totaled 31.2 million tons, with China accounting for 51% of the world total (15.9 million tons). Other significant countries growing cantaloupe were Turkey, Iran, Egypt, and India producing 1 to 1.9 million tons, respectively.
California grows 75% of the cantaloupes in the US.
Uses:
Culinary
Cantaloupe is normally eaten as a fresh fruit, as a salad, or as a dessert with ice cream or custard. Melon pieces wrapped in prosciutto are a familiar antipasto. The seeds are edible and may be dried for use as a snack.
Because the surface of a cantaloupe can contain harmful bacteria - in particular, Salmonella - it is recommended that a melon be washed and scrubbed thoroughly before cutting and consumption to prevent risk of Salmonella or other bacterial pathogens.
A moldy cantaloupe in a Peoria, Illinois, market in 1943 was found to contain the highest yielding strain of mold for penicillin production, after a worldwide search.
Nutrition
Raw cantaloupe is 90% water, 8% carbohydrates, 0.8% protein and 0.2% fat. In a reference amount of 100 grams (3.5 oz), raw cantaloupe supplies 140 kJ (34 kcal) of food energy, and is a rich source (20% or more of the Daily Value, DV) of vitamin A (29% DV) and a moderate source of vitamin C (13% DV). Other micronutrients are in negligible amounts (less than 10% DV).
Additional Information
What Is a Cantaloupe?
It's a juicy, orange summer fruit that's related to the watermelon and honeydew melon. It also belongs to the same plant family as cucumbers, pumpkins, squashes, and gourds.
The semisweet cantaloupes most familiar to people in the U.S. are a type of muskmelon called Cucumis melo reticulatus. Reticulatus means "net-like" in Latin and refers to the cantaloupe's rough, webbed outer skin.
Cantaloupe Nutrition
Cantaloupes can be a great addition to your diet. One cup of fresh cubes of cantaloupe counts as one serving. It has 53 calories, 6% of your daily serving of fiber, about 1 gram of protein, and zero fat and cholesterol.
Cantaloupes are also low in carbohydrates, with 13 grams per 1-cup serving. When you eat fruits that are low in carbohydrates, you can eat larger amounts and better manage your blood glucose levels.
They pack:
* 100% of the daily value of vitamin C, a powerful antioxidant that protects your cells from damage
* All your daily needs for vitamin A, which helps keep your eyes, skin, bones, and immune system healthy
About 12% of your recommended daily potassium, important for your heart, muscles, and blood pressure
Cantaloupes are also full of other vitamins and minerals, including:
* Folate
* Calcium
* Zinc
* Copper
* Iron
* Vitamin K
* Niacin
* Choline
* Magnesium
* Phosphorus
* Manganese
* Selenium.
Cantaloupe Health Benefits
Cantaloupes contain compounds called phytonutrients with anti-inflammatory properties. Long-term inflammation can damage your cells and lead to diabetes, cancer, and other diseases.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2222 2024-07-22 14:09:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2224) Avocado
Details
The avocado, alligator pear or avocado pear (Persea americana) is a medium-sized, evergreen tree in the laurel family (Lauraceae). It is native to the Americas and was first domesticated in Mesoamerica more than 5,000 years ago. It was prized for its large and unusually oily fruit. The tree likely originated in the highlands bridging south-central Mexico and Guatemala. Avocado trees have a native growth range from Mexico to Costa Rica. Its fruit, sometimes also referred to as an alligator pear or avocado pear, is botanically a large berry containing a single large seed. Sequencing of its genome showed that the evolution of avocados was shaped by polyploidy events and that commercial varieties have an hybrid origin. Avocado trees are partly self-pollinating, and are often propagated through grafting to maintain consistent fruit output. Avocados are presently cultivated in the tropical and Mediterranean climates of many countries. Mexico is the world's leading producer of avocados as of 2020, supplying nearly 30% of the global harvest in that year.
The fruit of domestic varieties have smooth, buttery, golden-green flesh when ripe. Depending on the cultivar, avocados have green, brown, purplish, or black skin, and may be pear-shaped, egg-shaped, or spherical. For commercial purposes the fruits are picked while unripe and ripened after harvesting. The nutrient density and extremely high fat content of avocado flesh are useful to a variety of cuisines and are often eaten to enrich vegetarian diets.
In major production regions like Chile, Mexico and California the water demands of avocado farms place strain on local resources. Avocado production is also implicated in other externalities, including deforestation and human rights concerns associated with the partial control of their production in Mexico by organized crime. Global warming is expected to result in significant changes to the suitable growing zones for avocados, and place additional pressures on the locales in which they are produced due to heat waves and drought.
Description
Persea americana is a tree that grows to 9–20 m (30–66 ft) with a trunk diameter between 0.3–0.6 m (1–2 ft). The leaves are 8–25 cm (3–10 in) long and alternately arranged.
Flower
Panicles of flowers with deciduous bracts arise from new growth or the axils of leaves. The tree flowers thousands of blossoms every year. Avocado blossoms sprout from racemes near the leaf axils; they are small and inconspicuous 5–10 mm (3⁄16–3⁄8 in) wide. They have no petals but instead 2 whorls of 3 pale-green or greenish-yellow downy perianth lobes, each blossom has 9 stamens with 2 basal orange nectar glands.
Fruit
The avocado fruit is a climacteric, single-seeded berry, due to the imperceptible endocarp covering the seed, rather than a drupe. The pear-shaped fruit is usually 7–20 cm (3–8 in) long, weighs between 100 and 1,000 g (3+1⁄2 and 35+1⁄2 oz), and has a large central seed, 5–6.4 cm (2–2+1⁄2 in) long.
The species produces various cultivars with larger, fleshier fruits with a thinner exocarp because of selective breeding by humans.
Taxonomy
Persea americana is regarded as an evolutionary anachronism, having likely coevolved dispersal of its large seed by now-extinct megafauna in South America, notably giant ground sloths and the gomphothere genus of the elephant lineage. Following extinction of these original seed dispersers, humans migrating into the region are thought to have become primary long-distance dispersers, eventuating in domestication of the species.
In 1982, evolutionary biologist Daniel H. Janzen concluded that the avocado is an example of an "evolutionary anachronism", a fruit adapted for ecological relationship with now-extinct large mammals (such as giant ground sloths or gomphotheres). Most large fleshy fruits serve the function of seed dispersal, accomplished by their consumption by large animals. There are some reasons to think that the fruit, with its mildly toxic pit, may have coevolved with Pleistocene megafauna to be swallowed whole and excreted in their dung, ready to sprout. No extant native animal is large enough to effectively disperse avocado seeds in this fashion.
The earliest known written account of the avocado in Europe is that of Martín Fernández de Enciso (c. 1470 – 1528) in 1519 in his book, Suma De Geographia Que Trata De Todas Las Partidas Y Provincias Del Mundo. The first detailed account that unequivocally describes the avocado was given by Gonzalo Fernández de Oviedo y Valdés in his work Sumario de la natural historia de las Indias in 1526. The first written record in English of the use of the word 'avocado' was by Hans Sloane, who coined the term, in a 1696 index of Jamaican plants.
Etymology
The modern English name comes from a rendering of the Spanish aguacate as avogato. The earliest known written use in English is attested from 1697 as avogato pear, later avocado pear (due to its shape), a term sometimes corrupted to alligator pear.
Regional names
In Central American, Caribbean Spanish-speaking countries, and Spain it is known by the Mexican Spanish name aguacate, while South American Spanish-speaking countries Argentina, Chile, Perú and Uruguay use a Quechua-derived word, palta. In Portuguese, it is abacate. The Nahuatl āhuacatl can be compounded with other words, as in ahuacamolli, meaning avocado soup or sauce, from which the Spanish word guacamole derives.
In the United Kingdom the term avocado pear, applied when avocados first became commonly available in the 1960s, is sometimes used.
Originating as a diminutive in Australian English, a clipped form, avo, has since become a common colloquialism in South Africa and the United Kingdom.
It is known as "butter fruit" in parts of India and Hong Kong.
Additional Information
An avocado is a bright green fruit with a large pit and dark leathery skin. It's also known as alligator pear or butter fruit. Avocados are a favorite of the produce section. They're the go-to ingredient for guacamole dips. And they're turning up in everything from salads and wraps to smoothies and even brownies.
Avocado Nutrition Facts:
Avocado calories
Avocados have a lot of calories. The recommended serving size is smaller than you'd expect: one-third of a medium avocado (50 grams, or 1.7 ounces). One ounce has 50 calories.
Avocados are high in fat. But it's monounsaturated fat, which is "good" fat that helps lower bad cholesterol as long as you eat them in moderation.
Avocados offer nearly 20 vitamins and minerals. So in a 100-gram serving, you get:
* 485 milligrams of potassium
* 81 micrograms of folate
* 0.257 milligrams of vitamin B6
* 10 milligrams of vitamin C
* 2.07 milligrams of vitamin E
Avocados are low in sugar. And they contain fiber, which helps you feel full longer. In one study, people who added a fresh avocado half to their lunch were less interested in eating during the next 3 hours than those who didn't have the fruit.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2223 2024-07-23 14:10:38
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2225) Cranberry juice
Details
Cranberry juice is the liquid juice of the cranberry – a fruit recognized for its bright red color, tart taste, and versatility for product manufacturing. Major cranberry products include cranberry juice, dried cranberry, cranberry sauce, frozen cranberry, cranberry powder, and dietary supplements containing cranberry extracts.
The term "cranberry juice" or "cranberry juice blend" refers to products that contain about 28% cranberry juice, with the remainder either from other fruit juice concentrates (typically grape, apple or pear), water, and added sugar to improve palatability. Low-calorie cranberry juice products use non-caloric sweeteners.
Despite a long-held reputation for providing antibacterial activity against urinary tract infections (UTIs), cranberry juice has no proven effects on UTIs due to uncertainty about the quality of research, as determined by a Cochrane review of completed clinical research. A scientific panel for the European Food Safety Authority concluded a cause-and-effect relationship could not be established between cranberry consumption and risk of UTIs.
Nutrition and composition
Cranberry juice is 86% water, 11% carbohydrates, and less than 1% fat or protein. A cup of standard (fortified) cranberry juice, amounting to 248 grams or 8 ounces, provides 107 calories and contains vitamin C as an ingredient to preserve freshness, with other micronutrients that may be added during manufacturing. Other than vitamin C and folate having more than 10% of the Daily Value, a typical serving of cranberry juice provides no micronutrients in significant content.
One half cup of cranberry juice provides 60 calories, 20% of the Daily Value for vitamin C, and counts as one-half of a fruit serving toward the United States MyPlate daily nutrition guide.
Effect on health
Cranberry juice is an acidic drink with a pH of about 2.6. Some cranberry juice products contain large amounts of sugar used in manufacturing to make the drink more palatable, but their consumption may increase the risk of hyperglycemia and reduced control of blood glucose in people with diabetes or glucose intolerance.
Cranberry juice and urinary tract infection
In 2008, there was tentative evidence that long-term use of cranberry juice might help prevent symptomatic urinary tract infections (UTIs), but this finding was refuted in 2012 with a conclusion that "14 further studies suggest that cranberry juice is less effective than previously indicated," and "cranberry juice cannot currently be recommended for the prevention of UTIs." The European Food Safety Authority reviewed the evidence and concluded a cause and effect relationship has not been established between the consumption of cranberry products and reducing the risk of UTIs.
One systematic review in 2017 showed that cranberry products significantly reduced the incidence of UTIs, indicating that cranberry products may be effective particularly for individuals with recurrent infections. When the quality of meta-analyses on the efficacy of cranberry products for preventing or treating UTIs was examined, large variation was evident, resulting from inconsistencies in clinical research methods. A further systematic review published in 2023 concluded there is evidence that consuming cranberry products is effective for reducing the risk of UTIs in women with recurrent UTIs, in children, and in people susceptible to UTIs following clinical interventions; in this same review, there was little evidence of effect in elderly people, those with urination disorders, or pregnant women.
In September 2017, Ocean Spray, a major cranberry juice manufacturer, submitted a health claim petition to the FDA. According to the FDA's February 2018 response letter, the company had "requested that FDA authorize a health claim for the relationship between the consumption of cranberry juice products and a reduced risk of recurrent urinary tract infection in healthy women." The FDA stated that they would consider the petition for a "qualified health claim." This type of health claim label does not require "significant scientific agreement" as the FDA's label with a higher standard, the "authorized health claim," does. Rather, "qualified health claims" only require that the claim be "supported by some scientific evidence." These types of health claims also do not need to "meet the significant scientific agreement standard" and must be accompanied by a disclaimer stating that there is insufficient evidence of the product affecting a disease, such as UTI. Further FDA guidance in 2019 notified cranberry juice manufacturers that added sugars necessary for making the juice appealing to consumers had to be declared on the nutrition facts panel of product labels, and the amount of sugar used would be reviewed by the FDA under its "enforcement discretion".
In October 2018, the National Institute for Health and Care Excellence's recommendation for self-care for lower UTIs in people aged 16 and over states that "no evidence was found on cranberry products or urine alkalinising agents to treat lower UTI".
Dental health
Cranberry juice has a level of acidity (pH 2.6) that may erode tooth enamel.
Manufacturing and processing:
Manufacturing
Cranberries are a kind of tart red berry produced by various plant species, but it is the large-fruited, or "American cranberry" (Vaccinium macrocarpon), that is farmed for commercial production. Currently, the main cranberry farming Canadian provinces are British Columbia, Québec, New Brunswick, Nova Scotia and Prince Edward Island. However, the lower temperatures present in the Eastern provinces require the use of irrigation and flooding to prevent frost damage and moisture loss. Wet harvesting is the common harvesting method used for cranberries that are to become cranberry juice.[20] A paddled machine called a water reel harvester is used to separate the ripe cranberries from the vines, then collected through a large suction pipe and transported by truck to a processing plant. At the processing plant, the cranberries go through a sequence of fruit crushing, mash maceration, mash heating, juice pressing, and pasteurization to produce a cranberry concentrate that is separated from pulp. To prepare a cranberry juice/math product, cranberry juice concentrate is reconstituted with varying amounts of water, specified by the solicitation, contract, or purchase order.
Sterilization
Traditionally, cranberry juice is commercially sterilized though thermal processing to eliminate any pathogenic or spoilage-causing microorganisms and spores. The prepared cranberry juice product is heat treated by high temperature-short time (HTST) or ultra-high temperature (UHT) techniques and packaged into aseptic, hermetically sealed containers. During thermal processing, the cranberry juice receives a heat treatment time equivalent to a 5-log pathogen reduction. Often, the bacterium Clostridium botulinum is given special attention during thermal processing techniques of food. However, C. botulinum does not grow and produce toxins below a pH of 4.6 and cranberry juice is classified as a high-acid food with a pH of 2.3 to 2.9.
Improved sterilization
In 2017, new methods of cranberry juice processing included high pressure processing (HHP) and pulsed electric field (PEF) technology. HHP treatment involves applying pressure (80,000 psi or 550 MPa) to cranberry juice for 1 to 9 minutes to eliminate any harmful bacteria, moulds and viruses. The resulting raw cranberry juice, without thermal processing, is classified as a novel food item by Health Canada. PEF treatment involves generating a high-intensity electric field inducing a flux of electric current to flow through the food product to eliminate harmful microorganisms. PEF treated cranberry juice does not alter the flavor, color, or aroma profile of the cranberries used, unlike the traditional thermally processed method.
Tartness
Naturally, cranberries are low in sugar content and have a tart taste. As a result, unsweetened cranberry juice is generally considered unpalatable by consumers. To make the juice more palatable to consumers, the tart flavor can be made less acidic by blending with other fruit juices or the addition of sugar or sugar substitutes.
The tartness of cranberry juice derives from its mixed content of polyphenols, including flavonoids, proanthocyanidins, anthocyanins, phenolic acids, and ellagitannins.
Packaging
All cranberry juice products are required to be packed in aseptic, hermetically seal containers (plastic bottles, cans, cartons) in accordance with good manufacturing practices of their country. The typical container size used are 11.5 or 64 fluid ounce, and each must be filled with the product by at least 90 percent. Cranberry juice products should also not be packaged more than 90 days prior to their delivery, unless specified in the order. Ocean Spray and Fruit d'Or cranberry juice products have a frozen shelf life of 24 months and 36 months, respectively.
Regulations:
Labeling
In 2020, the US Food and Drug Administration (FDA) announced that a qualified health claim would be allowed on cranberry juice product labels for reduced risk of recurrent urinary tract infection in healthy women consuming 8 US fluid ounces (240 mL) per day of a fruit juice product containing at least 27% cranberry juice. An example of the label claim provided by the FDA was: "Consuming one serving (8 oz) each day of a cranberry juice beverage may help reduce the risk of recurrent urinary tract infection (UTI) in healthy women. FDA has concluded that the scientific evidence supporting this claim is limited and inconsistent."
Cranberry juice container labels have the following information printed: product name and code, nutrition facts table, lot/drum number, date of packaging, brix, acidity, net weight, manufacturer name, manufacturer address and country of origin. According to Canada's composition claims, a "no preservatives" claim can be added to cranberry juice products if it only contains naturally-occurring constituents that provide a preservative function such as benzoates.
United States
For many US markets, cranberry juice from concentrate is a blended mixture of cranberry juice or cranberry juice concentrate, water, sweeteners, and vitamin C (as ascorbic acid). The cranberry juice or concentrate in the mixture must be produced from clean, sound, mature, well-colored, and washed, fresh or frozen cranberries (Vaccinium macrocarpon). One or more of the following sweetening ingredients may be added: sucrose, liquid sugar, invert sugar syrup, or high-fructose corn syrup (40% or greater). The use of food additives (color, flavours, or acids) into cranberry juice depends on the percentage of cranberry juice or concentrate by volume. Cranberry juice mixtures with 25% or 27% contain none of the mentioned additives, except for ascorbic acid. Cranberry juice mixtures with 22% contain no added color or flavors, but citric acid may be added. Cranberry juice mixtures with 20% may contain color, flavors, and citric acid. The finished cranberry juice from concentrate product should yield a minimum of one part cranberry juice concentrate to three parts water with a minimum brix level of 12°. Additionally, each cranberry juice product should be fortified with vitamin C, with each serving size delivering not less than 100% of the current US Reference Daily Intake. The minimum titratable acidity of the cranberry juice product must be 1.67% wt/wt, measured as citric acid.
Canada
For Canadian markets, cranberry juice is regulated as a processed product under fruit juices. Cranberry juice must be made from sound, clean, and ripe cranberries. One or more of the following dry sweetening ingredients may be added: sugar, invert sugar, and dextrose. According to the Canadian Food Inspection Agency (CFIA), the common name of this product may appear as "cranberry juice drink/cooler" if at least 25% of the named juice is contained within the net quantity of the product.
In Canada, cranberries are graded into two categories: Canada No. 1 and Canada Domestic. The cranberries of Canada No. 1 grade are required to be fairly clean; be uniform in size; and free from any damage and/or defect that affects the appearance, edibility, or shipment quality. The cranberries of Canada Domestic grade are required to be reasonably clean; and be free from any damage and/or defect that seriously affects the appearance, edibility, or shipment quality. Furthermore, all grades must be properly packaged; be sound; have a minimum surface area of 65% coloured red; and be free of insects and insect larvae.
Interaction with blood thinners
Cranberry juice may interfere with coumarins used as blood thinners, such as warfarin, causing an unstable INR. The British National Formulary (BNF) and the Food and Drug Administration (FDA) both advise avoiding concomitant use.
Market
Commercially cultivated in the United States and Canada, cranberries are harvested in the autumn for manufacturing of juice and other products. A barrel of US cranberries weighing 100 pounds (45 kg) cost US$57.60 in 2017, but the price fell to $22.30 per barrel in 2019 due to international trade wars with the United States, causing the market to shift to more purchases from Canada.
Including cranberries used for juice production, Americans consume some 400 million pounds (180 million kg) of cranberries per year. About 95% of the annual US cranberry harvest is used to make juice or juice blends. Wisconsin was the leading producer of cranberries in the United States in 2017. Cranberries and juice concentrate exported from the United States were the objects of imposed tariffs during trade wars with the European Union, China, Mexico, and Canada over the period 2017–19.
Additional Information
Cranberries (Vaccinium oxycoccos or Vaccinium macrocarpon) are small, red berries native to the U.S. and Canada. They grow on creeping, low-lying vines and do best in peat-based soil and damp conditions. Cranberries are closely related to blueberries, bilberries, and huckleberries.
The U.S. is the world’s largest producer of cranberries. The state of Wisconsin alone produced 5.01 million barrels in 2023.
Cranberry juice might not be as popular as orange or apple juice, but it’s a delicious beverage with many health benefits. Some people drink it to help prevent urinary tract infections, but cranberry juice also offers many other health benefits.
Cranberry Juice Nutritional Information
Cranberry juice is a nutritious drink packed with antioxidants and essential vitamins and minerals that support the immune system and overall health. One cup of cranberry juice contains:
* 23.5 milligrams of vitamin C
* 3.04 milligrams of vitamin E
* 32.9 milligrams of phosphorus
* 20.2 milligrams of calcium
* 15.2 milligrams of magnesium
* 195 milligrams of potassium
* 8.35 milligrams of choline
One cup of unsweetened cranberry juice also contains:
* 116 calories
* 1 gram of protein
* 0 grams of fat
* 31 grams of carbohydrates
* 0 grams of fiber
* 31 grams of sugar.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2224 2024-07-23 18:18:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2226) Tarbela Dam
Gist
Tarbela Dam, giant rock-fill dam on the Indus River, Pakistan. Built between 1968 and 1976, it has a volume of 138,600,000 cubic yards (106,000,000 cubic m). With a reservoir capacity of 11,098,000 acre-feet (13,690,000,000 cubic m), the dam is 469 feet (143 m) high and 8,997 feet (2,743 m) wide at its crest. Tarbela Dam is one of two main structures (the other is Mangla Dam on the Jhelum River) in the Indus Basin project, which resulted from the Indus Waters Agreement between India and Pakistan. Together with their subsidiary dams, Tarbela and Mangla were built to control seasonal fluctuations on the Indus River.
Summary
The Indus River basin extends from the Himalaya Mountains that form the northeastern boundary of Pakistan to the alluvial plains of Sindh near the Arabian Sea coastline. Tarbela Dam is part of the Indus Basin Project, which resulted from a water treaty signed in 1960 between India and Pakistan. This treaty guaranteed Pakistan water supplies independent of upstream control by India. Designed primarily for water storage rather than power generation, the dam was completed in 1977.
Turquoise waters of the Indus River (to the south of the dam) reflect the high proportion of silt and clay suspended in waters released by the spillways (chutes on either of side of the main dam). With a volume of 142,000,000 cubic meters, the Tarbela Dam is the largest earth and rock fill dam in the world and stands 147 meters above the Indus riverbed. Its reservoir occupies an area of 37 square kilometers. While the dam has fulfilled its purpose in storing water for agricultural use in Pakistan, there have been environmental consequences to the Indus river delta. Reduction of seasonal flooding and reduced water flows to the delta have decreased mangrove stands and the abundance of some fish species.
Details
Tarbela Dam is an earth-filled dam along the Indus River in Pakistan's Khyber Pakhtunkhwa province. It is located mainly in the Haripur Tehsil Ghazi.
It is about 20 km (10 mi) from the city of Swabi KPK, 105 km (65 mi) northwest of Islamabad, and 125 km (80 mi) east of Peshawar. It is the largest earth-filled dam in the world. The dam is 143 metres (470 ft) high above the riverbed and its reservoir, Tarbela Lake, has a surface area of approximately 250 square kilometres (97 sq mi).
The Tarbela Dam is located on the Indus River near the village of Tarbela in Bara, approximately 30 kilometers from the town of Attock. Positioned where the Indus River emerges from the foothills of the Himalayas and enters the Potwar Plateau, the dam features a reservoir for water storage. The average annual flow available is 101 billion cubic meters (3221 m^3/sec). It stands 143 meters tall and covers an area of 243 square kilometers. It has a storage capacity of 11.9 billion cubic meters of water and has nine gates to control the outflow of water. The dam was completed in 1976 and was designed to utilize water from the Indus River for irrigation, flood control, and the generation of hydroelectric power by storing flows during the monsoon period while subsequently releasing stored water during the low flow period in winter. The installed capacity of the 4,888 MW Tarbela hydroelectric power stations will increase to 6,418 MW after completion of the planned fifth extension financed by Asian Infrastructure Investment Bank and the World Bank. Then, it will be the 12th largest hydroelectric dam in the world, for electricity production capacity.
Project description
The dam is at a narrow spot in the Indus River valley, named after the town of Tarbela in the Haripur District of the Hazara Division within the Khyber Pakhtunkhwa province of Pakistan.
The main dam wall, built of earth and rock fill, stretches 2,743 metres (8,999 ft) from the island to river right, standing 148 metres (486 ft) high. A pair of concrete auxiliary dams spans the river from the island to river left. The dam's two spillways are on the auxiliary dams rather than the main dam. The main spillway has a discharge capacity of 18,406 cubic metres per second (650,000 cu ft/s) and the auxiliary spillway, 24,070 cubic metres per second (850,000 cu ft/s). Annually, over 70% of water discharged at Tarbela passes over the spillways and is not used for hydropower generation.
Five large tunnels were constructed as part of the outlet works. Hydroelectricity is generated from turbines in tunnel 1 through 3, while tunnels 4 and 5 were designed for irrigation use. Both tunnels are to be converted to hydropower tunnels to increase Tarbela's electricity-generating capacity. These tunnels were originally used to divert the Indus River while the dam was being constructed.
MA hydroelectric power plant on the right side of the main dam houses 14 generators fed with water from outlet tunnels 1, 2, and 3. There are four 175 MW generators on tunnel 1, six 175 MW generators on tunnel 2, and four 432 MW generators on tunnel 3, for a total generating capacity of 3,478 MW.
Tarbela Reservoir is 80.5 kilometres (50.0 mi) long, with a surface area of 250 square kilometres (97 sq mi). The reservoir initially stored 11,600,000 acre-feet (14.3 km3) of water, with a live storage of 9,700,000 acre-feet (12.0 km^3), though this figure has been reduced over the subsequent 35 years of operation to 6,800,000 acre-feet (8.4 km^3) due to silting. The maximum elevation of the reservoir is 1,550 ft (470 m) above MSL and the minimum operating elevation is 1,392 ft (424 m) above MSL. The catchment area upriver of the Tarbela Dam is spread over 168,000 square kilometres (65,000 sq mi) of land largely supplemented by snow and glacier melt from the southern slopes of the Himalayas. There are two main Indus River tributaries upstream of the Tarbela Dam. These are the Shyok River, joining near Skardu, and the Siran River near Tarbela.
Background
Tarbela Dam was constructed as part of the Indus Basin Project after signing of the 1960 Indus Waters Treaty between India and Pakistan. The purpose was to compensate for the loss of water supplies of the eastern rivers (Ravi, Sutlej and Beas) that were designated for exclusive use by India per terms of the treaty. By the mid-1970s, power generation capacity was added in three subsequent hydro-electrical project extensions which were completed in 1992, installing a total of 3,478 MW generating capacity.
Construction
Construction of Tarbela Dam was carried out in three stages to meet the diversion requirements of the river. Construction was undertaken by the Italian firm Salini Impregilo.
Stage 1
In the first stage, the Indus River was allowed to flow in its natural channel, while construction works commenced on the right bank where a 1,500 feet (460 meters) long and 694.8 feet (211.8 meters) wide diversion channel was being excavated along with a 105 feet (32 meters) high buttress dam that was also being constructed. Stage 1 construction lasted approximately 2½ years.
Stage 2
The main embankment dam and the upstream blanket were constructed across the main valley of the river Indus as part of the second stage of construction. During this time, water from the Indus river remained diverted through the diversion channel. By the end of construction works in stage 2, tunnels had been built for diversion purposes. Stage 2 construction took 3 years to complete.
Stage 3
Under the third stage of construction, works were carried out on the closure of the diversion channel and construction of the dam in that portion while the river was made to flow through diversion tunnels. The remaining portion of upstream blanket and the main dam at higher levels was also completed as part of stage 3 works, which were concluded in 1976.
An area of about 260 square kilometers and about 82,000 acres (33,000 ha) of land was acquired for construction. The large reservoir of the dam submerged 135 villages, which resulted in the displacement of a population of about 96,000 people, many of whom were relocated to townships surrounding the Tarbela Reservoir or in adjacent higher valleys.
For the land and built-up property acquired under the Land Acquisition Act of 1984, a cash compensation of Rs 469.65 million was paid to those affected. In the absence of a national policy, the resettlement concerns of the people displaced by the Tarbela Dam were addressed on an ad hoc basis. In 2011, many such people had still not been resettled or given land in compensation for their losses by the government of Pakistan, in accordance with its contractual obligations with the World Bank.
Lifespan
Because the source of the Indus River is glacial meltwater from the Himalayas, the river carries huge amounts of sediment, with an annual suspended sediment load of 200 million tons. Live storage capacity of Tarbela reservoir had declined more than 33.5 per cent to 6.434 million acre feet (MAF) against its original capacity of 9.679 MAF because of sedimentation over the past 38 years. The useful life of the dam and reservoir was estimated to be approximately 50 years. However, sedimentation has been much lower than predicted, and it is now estimated that the useful lifespan of the dam will be 85 years, to about 2060.
Pakistan plans to construct several large dams upstream of Tarbela, including the Diamer-Bhasha Dam. Upon completion of the Diamer-Bhasha dam, sediment loads into Tarbela will be decreased by 69%.
Project benefits
In addition to fulfilling the primary purpose of the dam, i.e., supplying water for irrigation, Tarbela Power Station has generated 341.139 billion kWh of hydro-electric energy since commissioning. A record annual generation of 16.463 billion kWh was recorded during 1998–99. Annual generation during 2007–08 was 14.959 billion kWh while the station shared peak load of 3702 MW during the year, which was 23.057% of total WAPDA system peak.
Tarbela-IV Extension Project
Tarbela dam extension-IV was planned in June, 2012, and PC-1 was developed for the project. US ambassador Richard Olson offered aid for construction of this project during his visit to Pakistan, in March, 2013. In September 2013, Pakistan's Water and Power Development Authority signed a Rs. 26.053 billion contract with Chinese firm Sinohydro and Germany's Voith Hydro for executing civil works on the 1,410 MW Tarbela-IV Extension Project. Construction commenced in February 2014, and was completed in February 2018.
This project was constructed on Tunnel No. 4 of Tarbela Dam. It consists of three turbine-generator units, each with a capacity of 470 MW. The project is expected to provide an average of 3.84 billion units of electricity annually to the National Grid. It is intended to help supplement electricity supply during the high-demand summer months.
Annual benefits of the project were estimated at Rs. 30.7 billion. On an annual basis, over 70% of water passing through Tarbela is discharged over spillways, while only a portion of the remaining 30% is used for hydropower generation.
The Water and Power Development Authority in Pakistan says the third and last unit at its 1,410-MW Tarbela 4th Extension Hydropower Project has been synchronized with the National Grid. With this extension, the installed capacity of the Tarbela Hydel Power Station has increased to 4,888 MW.
Financing
The project's cost was initially estimated to be $928 million, but the cost was revised downwards to $651 million. The World Bank had agreed to provide an $840 million loan for the project in June 2013.
The loan had two components: The first is a $400 million International Development Association loan, which will be lent as a concessional loan at low interest rates. The second portion consists of a $440 million from the World Bank's International Bank for Reconstruction and Development. Pakistan's Water and Power Development Authority was to provide the remaining $74 million required for construction, before the project's cost was downwardly revised by $277 million. Interest costs for the loans are estimated to cost $83.5 million.
Because of revised lower costs to $651 million from $928 million, the World Bank permitted Pakistani officials to expedite completion of the project by 8 months at a cost of an additional $51 million. Pakistani officials were also permitted to divert $126 million towards the Tarbela-V Extension Project.
Tarbela-V Extension Project
The Tarbela Dam was built with five original tunnels, with the first three dedicated to hydropower generation, and the remaining two slated for irrigation use. The fourth phase extension project uses the first of the two irrigation tunnels, while the fifth phase extension will use the second irrigation tunnel. Pakistan's Water and Power Development Authority sought expressions of interest for the Tarbela-V Extension Project in August 2014, and was given final consent for construction in September 2015.
The hydropower project of tunnel 5 has two major components: power generation facilities and power evacuation facilities. The major works included under the project are modifications to tunnel 5 and building a new power house and its ancillaries to generate about 1,800GWh of power annually, a new 50 km of 500kV double-circuit transmission line from Tarbela to the Islamabad West Grid Station for power evacuation, and a new 500kV Islamabad West Grid Station.
Construction commenced in August 2021 and will require an estimated 3.5 years for completion. The project will require the installation of three turbines with a capacity of 510 MW each in Tarbela's fifth tunnel which was previously dedicated to agricultural use. Upon completion, the total power generating capacity of Tarbela Dam will increase to 6,418 MW.
Financing
In November 2015, the World Bank affirmed that it would finance at least $326 million of the project's estimated $796 million cost which includes $126 million of funding that was diverted from the $840 million fourth phase extension project after costs for that project were revised downwards. In September 2016, the World Bank approved an additional financing of $390 million for the fifth extension hydropower project of Tarbela dam that will support the scaling up of the power generation capacity by adding 1,530 megawatts to the existing tunnel 5.
The project will be financed by the International Bank for Reconstruction and Development (IBRD), with a variable spread and 20-year maturity, including a six-year grace period. This will be the first World Bank-supported project in South Asia to be jointly financed with the Asian Infrastructure Investment Bank (AIIB) which will be providing $300m and the Government of Pakistan $133.5m. The total cost of the project is $823.5m.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2225 2024-07-24 13:50:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,376
Re: Miscellany
2227) Pitaya
Details
A pitaya or pitahaya is the fruit of several different cactus species indigenous to the region of southern Mexico and along the Pacific coasts of Guatemala, Costa Rica, and El Salvador. Pitaya is cultivated in East Asia, South Asia, Southeast Asia, the United States, the Caribbean, Australia, Brazil, and throughout tropical and subtropical regions of the world.
Pitaya usually refers to fruit of the genus Stenocereus, while pitahaya or dragon fruit refers to fruit of the genus Selenicereus (formerly Hylocereus), both in the family Cactaceae. The common name in English – dragon fruit – derives from the leather-like skin and scaly spikes on the fruit exterior. Depending on the variety, pitaya fruits may have sweet- or sour-tasting flesh that can be red, white, or yellow in color.
Vernacular names
These fruits are commonly known in English as "dragon fruit", a name used since 1963, apparently resulting from the leather-like skin and prominent scaly spikes on the fruit exterior. The fruit is often designated as "Vietnamese dragon fruit" as Vietnam is the lead exporter. The fruit may also be known as a strawberry pear.
The names pitahaya and pitaya derive from Mexico, and pitaya roja in Central America and northern South America, possibly relating to pitahaya for names of tall cacti species with flowering fruit.
Geography
Pitaya or dragon fruit is native to the region of southern Mexico and along the Pacific coasts of Guatemala, Costa Rica, and El Salvador. The dragon fruit is cultivated in East Asia, South Asia, Southeast Asia, the United States, the Caribbean, Australia, and throughout tropical and subtropical regions of the world.
Varieties:
Stenocereus
Stenocereus fruit (sour pitayas) are a variety that is commonly eaten in the arid regions of the Americas. They are more sour and refreshing, with juicier flesh and a stronger taste.
The sour pitaya or pitaya agria (S. gummosus) in the Sonoran Desert has been an important food source for indigenous peoples of the Americas. The Seri people of northwestern Mexico still harvest the fruit, and call the plant ziix is ccapxl "thing whose fruit is sour".
The fruit of related species, such as S. queretaroensis and the dagger cactus or pitaya de mayo (S. griseus), are also locally important foods. The fruit of the organ pipe cactus (S. thurberi, called ool by the Seris) is the pitaya dulce "sweet pitaya".
Dragon fruit, Selenicereus
Sweet pitayas come in three types, all with leathery, slightly leafy skin:[3]:
* Selenicereus undatus (Pitaya blanca or white-fleshed pitaya, also known as Hylocereus undatus) has pink-skinned fruit with white flesh. This is the most commonly seen "dragon fruit".
* Selenicereus costaricensis (Pitaya roja or red-fleshed pitaya, also known as Hylocereus costaricensis, and possibly incorrectly as Hylocereus polyrhizus) has red-skinned fruit with red flesh.
* Selenicereus megalanthus (Pitaya amarilla or yellow pitaya, also known as Hylocereus megalanthus) has yellow-skinned fruit with white flesh.
The fruit normally weighs from 150 to 600 grams (5+1⁄2 to 21 oz); some may reach 1 kg (2 lb 3 oz). Early imports from Colombia to Australia were designated "Hylocereus ocampensis" (or "Cereus repandus", the red fruit) and "Cereus triangularis" (supposedly, the yellow fruit or the three-sided cross-section of the stem).
Cultivation
After a thorough cleaning of the seeds from the pulp of the fruit, the seeds may be stored when dried. The ideal fruit is unblemished and overripe.
Seeds grow well in a compost or potting soil mix – even as a potted indoor plant. Pitaya cacti usually germinate after between 11 and 14 days after shallow planting. As they are cacti, overwatering is a concern for home growers. As their growth continues, these climbing plants will find something to climb on, which can involve putting aerial roots down from the branches in addition to the basal roots. Once the plant reaches a mature 4.5 kilograms (10 pounds) in weight, the plant may flower.
Commercial plantings can be done at high density with between 1,100 and 1,350 per hectare (445 and 546/acre). Plants can take up to 60 months/260 weeks to come into full commercial production, at which stage yields of 20 to 30 metric tons (22 to 33 short tons) can be expected.
Pitaya flowers bloom overnight and usually wilt by the evening. They rely on nocturnal pollinators such as bats or moths for fertilization. Self-fertilization will not produce fruit in some species and while crossbreeding has resulted in several "self-fertile" varieties, cross-pollinating with a second, genetically distinct plant of the same species generally increases fruit set and quality. This limits the capability of home growers to produce the fruit. However, the plants can flower between three and six times per year depending on growing conditions. Like other cacti, if a healthy piece of the stem is broken off, it may take root in the soil and become its own plant.
The plants can endure temperatures up to 40 °C (104 °F) and short periods of frost but will not survive long exposure to freezing temperatures. The cacti thrive most in USDA zones 10–11 but may survive outdoors in zone 9a or 9b.
Selenicereus has adapted to live in dry tropical climates with a moderate amount of rain. In numerous regions, it has escaped cultivation to become a weed and is classified as an invasive weed in some countries.
Pests and diseases
Stems and fruits are susceptible to several diseases caused by fungi, bacteria, a nematode, and a virus. Overwatering or excessive rainfall can cause the flowers to drop and fruit to rot. The bacterium Xanthomonas campestris causes the stems to rot. Dothiorella fungi can cause brown spots on the fruit. Other fungi known to infect pitaya include Botryosphaeria dothidea, Colletotrichum gloeosporioides and Bipolaris cactivora.
Pitaya, Raw
Nutritional value per 100 g (3.5 oz)
Energy : 240 kJ (57 kcal)
Carbohydrates : 15.2 g
Sugars : 9.75 g
Dietary fiber : 3.1 g
Fat : 0.14 g
Protein : 0.36 g
Vitamins Quantity%DV
Folate (B9) : 2%7 μg
Choline : 1%5.1 mg
Vitamin C : 5%4.3 mg
Vitamin K : 4%4.4 μg
Minerals : Quantity%DV
Calcium : 1%9 mg
Iron : 1%0.18 mg
Magnesium : 2%7 mg
Phosphorus : 1%12 mg
Potassium : 4%116 mg
Sodium : 0%1 mg
Other constituents : Quantity
Water : 84 g
Uses:
Culinary
The fruit's texture is sometimes likened to that of the kiwifruit because of its black, crunchy seeds. The seed oil contains the fatty acids linoleic acid and linolenic acid. Dragon fruit is used to flavor and color juices and alcoholic beverages, such as "Dragon's Blood Punch" and the "Dragotini". The flowers can be eaten or steeped as tea.
The red and purple colors of some Selenicereus fruits are due to betacyanins, a family of pigments that includes betanin, the same substance that gives beets, Swiss chard, and amaranth their red color.
Nutrients
The USDA FoodData Central database published their analysis of the nutritional contents of raw Pitaya in 2022. The majority of the fruit by weight is water (87g out of 100g). One serving of 100-gram (3+1⁄2-ounce) provides 240 kilojoules (57 kilocalories) of food energy.
The USDA also reports one limited product label entry from a manufacturer of a branded product, showing that a 100-gram (3+1⁄2-ounce) reference serving of dried pitaya provides 1,100 kilojoules (264 kilocalories) of food energy, 82% carbohydrates, 4% protein, and 11% of the Daily Value each for vitamin C and calcium.
Seed oils
The fatty acid compositions of the seed oils of Selenicereus costaricensis, syn. Hylocereus costaricensis (red-fleshed pitaya) and Selenicereus undatus, syn. Hylocereus undatus (white-fleshed pitaya) were similar: myristic acid (negligible), palmitic acid (17%), stearic acid (5%), palmitoleic acid (about 1%), oleic acid (22%), cis-vaccenic acid (3%), linoleic acid (50%), and α-linolenic acid (1%).
Additional Information
Dragon fruit is a tropical fruit that’s low in calories and high in fiber and antioxidants. Some people say it tastes like a cross between a pear and a kiwi. You can slice and eat the fruit as-is, try it with yogurt, or add it to a smoothie or salad.
Dragon fruit is a tropical fruit that has become increasingly popular in recent years.
Though people primarily enjoy it for its unique look and taste, evidence suggests it may provide health benefits as well.
Dragon fruit grows on the Hylocereus cactus, also known as the Honolulu queen, whose flowers only open at night.
The plant is native to southern Mexico and Central America. Today, it is grown all over the world.
It goes by many names, including pitaya, pitahaya, and strawberry pear.
The two most common types have bright red skin with green scales that resemble a dragon — hence the name.
The most widely available variety has white pulp with black seeds, though a less common type with red pulp and black seeds exists as well.
Another variety — referred to as yellow dragon fruit — has yellow skin and white pulp with black seeds.
Dragon fruit may look exotic, but its flavors are similar to other fruits. Its taste has been described as a slightly sweet cross between a kiwi and a pear.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline