Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2023-04-17 20:52:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,716
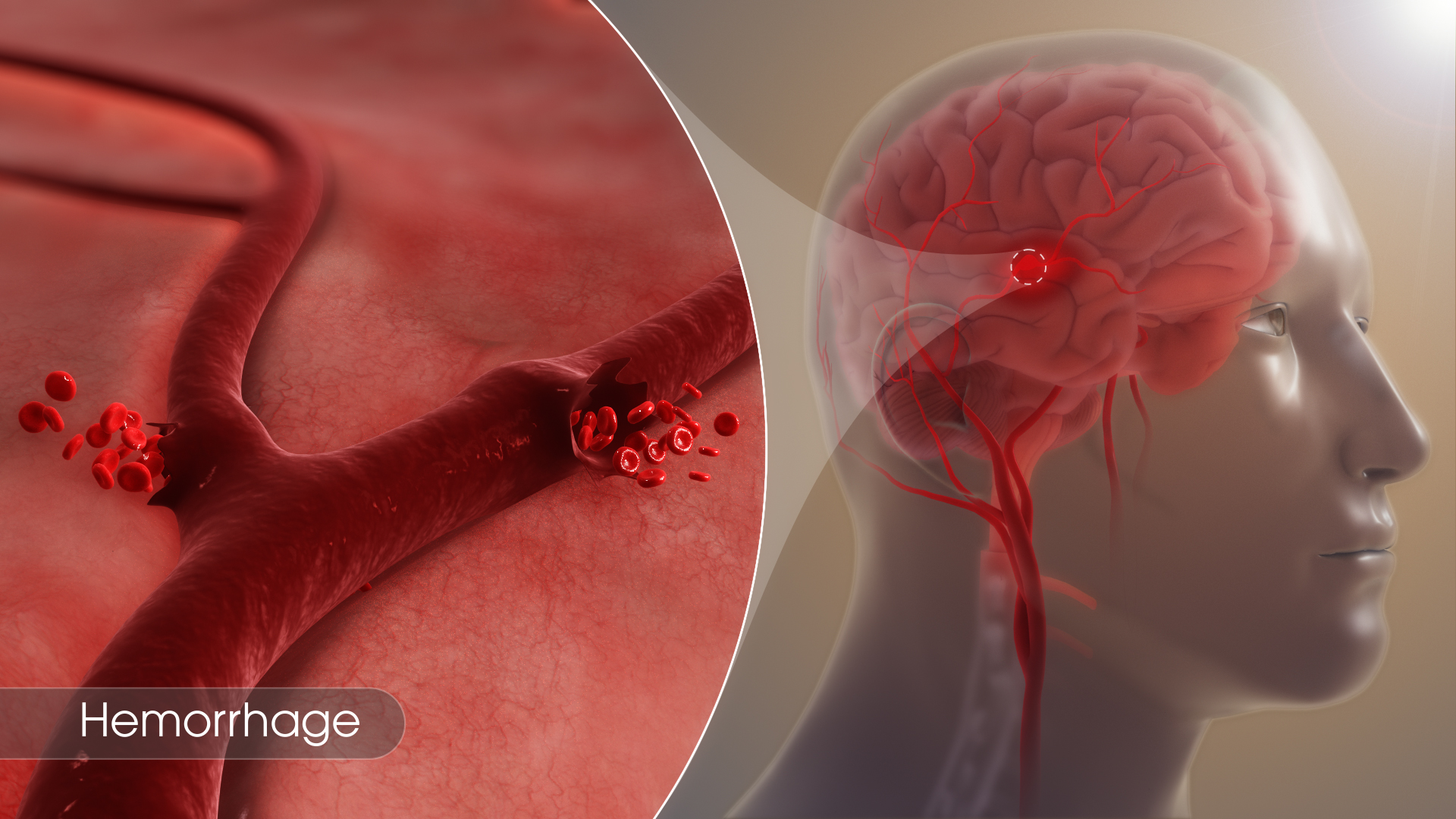
Hemorrhage
Hemorrhage
Gist
Hemorrhage: Escape of blood from blood vessels into surrounding tissue. When a vessel is injured, hemorrhage continues as long as the vessel remains open and the pressure in it exceeds the pressure outside of it. Normally, coagulation closes the vessel and stops the bleeding.
Summary
Bleeding and blood clotting, escape of blood from blood vessels into surrounding tissue and the process of coagulation through the action of platelets.
Significance of hemostasis
The evolution of high-pressure blood circulation in vertebrates has brought with it the risk of bleeding after injury to tissues. Mechanisms to prevent bleeding (i.e., hemostatic mechanisms) are essential to maintain the closed blood-circulatory system. Normal hemostasis is the responsibility of a complex system of three individual components: blood cells (platelets), cells that line the blood vessels (endothelial cells), and blood proteins (blood-clotting proteins). The blood platelet is a nonnucleated cell that circulates in the blood in an inactive, resting form. Endothelial cells line the wall of the blood vessel and inhibit blood from clotting on the vessel wall under normal conditions. Blood-clotting proteins circulate in the blood plasma in an inactive form, poised to participate in blood coagulation upon tissue injury. Blood-clotting proteins generate thrombin, an enzyme that converts fibrinogen to fibrin, and a reaction that leads to the formation of a fibrin clot.
The hemostatic mechanism involves three physiologically important reactions: (1) the formation of a blood clot, (2) the formation of a platelet plug, and (3) changes associated with the wall of the blood vessel after injury of its cells. In humans, defects in any of these processes may result in persistent bleeding from slight injuries, or, alternatively, in an overreaction that causes the inappropriate formation of blood clots (thrombosis) in blood vessels. When a blood vessel is injured, blood escapes for as long as the vessel remains open and the pressure within the vessel exceeds that outside. Blood flow can be stopped or diminished by closing the leak or by equalizing the pressure. The leak may be closed by contraction of the blood vessel wall or by the formation of a solid plug. Pressure may be equalized by an increase in external pressure as blood becomes trapped in the tissues (hematoma) or by a decrease in the intravascular pressure (the pressure within the blood vessel) caused by constriction of a supply vessel. The timing and relative importance of these events can vary with the scale of the injury. Bleeding from the smallest vessels can be stopped by platelet plugs; when bleeding is from larger vessels, blood clot formation is required; in still larger vessels the severe drop in pressure associated with shock is the last line of defense.
Details
The hemostatic process
Blood vessels that constitute the circulatory system include arterioles (the smallest arteries) and venules (the smallest veins) connected by capillaries (the smallest of all blood vessels). Blood cells, including red cells and platelets, normally have no tendency to adhere to each other or to the lining (endothelium) of the vessels. An injury too slight to rupture a vessel, however, may still bring about a hemostatic reaction that causes blood cells to adhere to each other. After minor tissue injury there may be partial vessel contraction and platelet adhesion in successive layers at the point of injury. A platelet mass is formed that grows until it blocks, or almost blocks, the vessel. Sometimes this platelet mass breaks down and then reforms, a cycle that repeats perhaps many times. These masses consist of minimally altered platelets. Even these slight injuries cause shedding of some endothelial cells from the vessel and the exposure of deeper layers to which the platelets adhere.
If the vessel is cut so that blood escapes, the hemostatic reaction is different. In muscular vessels there may be immediate contraction and narrowing of the vessel, but this usually only minimizes blood loss. A mass of activated platelets adheres to the site of vessel injury (a platelet plug) and normally stops the flow of blood out of the vessel. Unlike the platelets circulating in the blood and those adhering to minor tissue injuries, these platelets have undergone a biochemical and morphological change characteristic of platelet activation, a process that includes the secretion of the contents of platelet granules into the surrounding blood and the extension of pseudopodia. Between the platelets develop bundles of fibrin fibres (coagulation). These changes occur near damaged collagen, the fibrous protein found in connective tissue that underlies the endothelial cell. Later, normal healing of the wound occurs. The platelets subsequently degenerate into an amorphous mass and after several days, the fibrin itself is dissolved (fibrinolysis) by an enzyme, plasmin. The fibrin clot is replaced by a permanent framework of scar tissue that includes collagen, and healing is thus complete.
The normal hemostatic response to damage to the vascular endothelium can be organized into four stages: (1) initial vasoconstriction, (2) aggregation of platelets on and around the lesion and the formation of a platelet plug, (3) activation of the reactions of coagulation, and (4) the activation of fibrinolysis.
Vascular function
The most obvious hemostatic vascular reaction is constriction of the blood vessel after injury. This is important in large arteries because platelet adhesion and clotting are insufficient to arrest bleeding. Delayed surgical aid notwithstanding, the survival of some persons who have lost limbs in accidents is due to constriction of their main arteries. Other vascular reactions to injury have only a subsidiary hemostatic effect.
Platelets and their aggregation
Mammalian platelets are nonnucleate cells produced by large bone marrow cells called megakaryocytes and circulate in the blood in a resting, inactive form for an average of 10 days. The normal platelet count in humans is between 150,000 and 400,000 platelets per cubic millimetre of blood. The inactive platelet contains three types of internal granules: the alpha granules, the dense granules, and the lysosomes. Each of these granules is rich in certain chemicals that have an important role in platelet function. For example, dense granules contain large quantities of calcium ions and adenosine diphosphate (ADP). Upon release from the platelet, ADP stimulates other platelets to activate when it binds to the ADP receptor on the platelet membrane. The alpha granules contain many proteins, including fibrinogen, thrombospondin, fibronectin, and von Willebrand factor. Upon platelet activation, platelets alter their shape from discoid to spherical and extend long footlike projections called pseudopodia. The alpha granules and dense granules move to the surface of the platelet, fuse with the platelet membrane, and release their contents into the blood surrounding the platelet. The lysosomes contain enzymes that digest spent proteins and other metabolites of the cell.
Activated platelets strongly adhere to surfaces other than the lining of blood vessels, such as collagen, glass, metals, and fabrics. Adherent platelets themselves become adhesive for other activated platelets so that, in a flow system, a platelet plug develops. The propagation of this adhesiveness from one layer to the next is probably due to chemicals, such as ADP and thromboxane A2, secreted into the blood from the granules of the activated platelets. The ADP released from the dense granules binds to a receptor on the platelet surface, initiating the biochemical and morphological changes associated with platelet activation and secretion. The property of adhesiveness for normal platelets requires a protein on the surface of the platelet membrane, known as glycoprotein Ib, to bind von Willebrand factor, a large multimeric plasma protein released from the alpha granules. Von Willebrand factor, when bound to glycoprotein Ib on the platelet surface, facilitates the interaction of platelets with a variety of other surfaces (e.g., the damaged vessel lining).
Platelet aggregation is the property of platelets to clump with each other to form a platelet plug. Two proteins on the platelet membrane play an important role in platelet aggregation: glycoprotein IIb and glycoprotein IIIa. These proteins form a complex in the membrane and expose a receptor site after platelet activation that binds fibrinogen (a bivalent molecule with two symmetrical halves that is found in relatively high concentration in plasma). Fibrinogen can bind simultaneously to two platelets. Thus, fibrinogen links platelets together (aggregation) through the glycoprotein IIb–IIIa complex that serves as the fibrinogen receptor.
Injury to the vessel lining and contact of the blood with tissues outside the vessel stimulates thrombin production by the activation of the clotting system. Thrombin causes platelet aggregation. Platelets exposed to thrombin secrete their granules and release the contents of these granules into the surrounding plasma.
Blood coagulation
Coagulation is the replacement of a relatively unstable platelet plug with a stronger, more resilient blood clot through a series of interdependent, enzyme-mediated reactions that bring about the generation of thrombin and the formation of fibrin from fibrinogen. The intrinsic and the extrinsic pathways of coagulation are involved in regulating coagulation; each is activated by a different trigger, although they share many steps in the course of the generation of thrombin.
Intrinsic pathway of blood coagulation
All the components necessary for the clotting process to proceed are found in the blood. As such, the proteins required for such clotting to take place are part of the intrinsic pathway of blood coagulation. This pathway involves a series of proteins, protein cofactors, and enzymes, which interact in reactions that take place on membrane surfaces. These reactions are initiated by tissue injury and result in the formation of a fibrin clot.
The intrinsic pathway is initiated by the activation of factor XII by certain negatively charged surfaces, including glass. High-molecular-weight kininogen and prekallikrein are two proteins that facilitate this activation. The enzyme form of factor XII (factor XIIa) catalyzes the conversion of factor XI to its enzyme form (factor XIa). Factor XIa catalyzes the conversion of factor IX to the activated form, factor IXa, in a reaction that requires calcium ions. Factor IXa assembles on the surface of membranes in complex with factor VIII; the factor IXa–factor VIII complex requires calcium to stabilize certain structures on these proteins associated with their membrane-binding properties. Factor X binds to the factor IXa–factor VIII complex and is activated to factor Xa. Factor Xa forms a complex with factor V on membrane surfaces in a reaction that also requires calcium ions. Prothrombin binds to the factor Xa–factor V complex and is converted to thrombin, a potent enzyme that cleaves fibrinogen to fibrin, a monomer. The monomer fibrin molecules then link together (polymerize) to form long fibres. Later, additional bonding between the units of the polymer is promoted by an enzyme known as factor XIIIa, which stabilizes the newly formed clot by cross-linkages. Although the detailed mechanisms are not known, this cascade, or waterfall, effect offers the possibility for amplification of a small signal associated with tissue injury into a major biologic event—the formation of a fibrin clot. Furthermore, careful regulation of this system is possible with the participation of two protein cofactors, factor VIII and factor V.
Certain negatively charged surfaces, including glass, kaolin, some synthetic plastics, and fabrics, activate factor XII to its enzyme form, factor XIIa. In contrast, certain materials have little tendency to activate factor XII. Inactive surfaces include some oils, waxes, resins, silicones, a few plastics, and endothelial cells, the most inert surface of all. The physicochemical properties that determine activity are not known. The problem is important, for modern surgery requires a perfectly inactive material to make substitutes (prostheses) for heart valves and sections of blood vessels. The formation of clots (thrombi) on these surfaces can lead to serious or even fatal complications. Open-heart surgery requires pumping of blood through equipment that does not activate the blood-clotting process significantly. Similarly, blood filtration of waste products during kidney dialysis must not lead to the generation of fibrin clots. To minimize the activation of blood coagulation when blood flows over foreign surfaces, special drugs (anticoagulants) such as heparin are employed.
The activity of the intrinsic pathway may be assessed in a simple laboratory test called the partial thromboplastin time (PTT), or, more accurately, the activated partial thromboplastin time. Plasma is collected and anticoagulated with citrate buffer; the citrate binds and effectively removes functional calcium ions from the plasma. Under these conditions, a fibrin clot cannot be generated. A negatively charged material, such as the diatomaceous material kaolin, is added to the plasma. Kaolin activates factor XII to its enzyme form, factor XIIa, which then activates factor XI. The process is blocked from further activation because of the lack of calcium ions, which are required for the next reaction, the activation of factor IX. Upon the addition of calcium ions and a phospholipid preparation (which serves as an artificial membrane for the assembly of the blood-clotting protein complexes), the duration of time is recorded until a visible clot is formed. This reaction takes place in a matter of 25 to 50 seconds, depending upon the formulation of chemicals used. In practice, the clotting time of a test plasma is compared to the clotting time of normal plasma. Delayed clotting, measured as a prolonged partial thromboplastin time, may be due to a deficiency in the activity of one or more of the blood-clotting factors or to a chemical inhibitor of blood coagulation.
The extrinsic pathway of blood coagulation
Upon the introduction of cells, particularly crushed or injured tissue, blood coagulation is activated and a fibrin clot is rapidly formed. The protein on the surface of cells that is responsible for the initiation of blood clotting is known as tissue factor, or tissue thromboplastin. Tissue factor is found in many of the cells of the body but is particularly abundant in those of the brain, lungs, and placenta. The pathway of blood coagulation activated by tissue factor, a protein extrinsic to blood, is known as the extrinsic pathway.
Tissue factor serves as a cofactor with factor VII to facilitate the activation of factor X. Alternatively, factor VII can activate factor IX, which, in turn, can activate factor X. Once activated, factor X proceeds to activate prothrombin to thrombin in a reaction requiring factor V. The thrombin converts fibrinogen to fibrin. With the exception of factor VII, all components of the extrinsic pathway are also components of the intrinsic pathway.
The activity of the extrinsic pathway may be assessed in the laboratory using a simple test known as the prothrombin time. Tissue extract, or tissue thromboplastin, is extracted from animal tissues rich in tissue factor. Plasma, anticoagulated with citrate buffer, is allowed to clot with the simultaneous addition of phospholipid, calcium, and thromboplastin. The duration of time until clot formation, known as the prothrombin time, is usually between 10 and 12 seconds. In practice, the clotting time of a test plasma is compared to the clotting time of normal plasma. Delayed clotting, measured as a prolonged prothrombin time, may be due to a deficiency in the activity of one or more of the blood-clotting factors in the extrinsic pathway or to a chemical inhibitor of blood coagulation that interferes with the extrinsic pathway.
In summary, there are two independent mechanisms for initiating blood coagulation and for activating factor X: (1) negatively charged surfaces that initiate blood clotting through the intrinsic pathway (factors XII, XI, IX, and VIII), and (2) tissue factor on cells outside the blood that participates in the extrinsic pathway (factor VII). The common pathway (factor X, factor V, prothrombin, and fibrinogen) is shared by both systems. Although both pathways provide the opportunity to acquire meaningful information about clotting proteins using the partial thromboplastin time and the prothrombin time, it is most likely that the physiologically important pathway of blood coagulation is the extrinsic pathway initiated by tissue factor.
Biochemical basis of activation
The blood-clotting proteins circulate in the blood in their inactive, proenzyme form. The biochemical term for such proenzymes is zymogen. These zymogens are precursor enzymes that are converted to active enzymes by the cleavage of one or in some instances two peptide bonds. By splitting the protein into specific fragments, the zymogen is turned into an active enzyme that can itself split particular peptide bonds. This process, known generally as limited proteolysis, is equivalent to a molecular switch; by cutting a specific bond that connects two amino acids in the string of amino acids known as a polypeptide, an active enzyme is formed. Thus, the blood contains a system poised to become engaged instantaneously in the formation of blood clots if tissue is injured. Under normal conditions, however, blood clotting does not take place in the absence of tissue injury. The clotting proteins that function as zymogens in the blood include factor XII, factor XI, prekallikrein, factor IX, factor X, factor VII, and prothrombin.
Protein cofactors also play an important role in blood coagulation. Two protein cofactors, factor V and factor VIII, are large proteins that probably regulate blood coagulation. These proteins circulate in the blood as inactive cofactors. By the process of limited proteolysis, in which several cuts in the polypeptide chains of these cofactors are formed by the enzyme thrombin, factors V and VIII are converted to active cofactors. Factor V and factor VIII bind to membrane surfaces and form a focal point for the organization of certain protein complexes.
Inhibition of clotting
After the activation of the blood-clotting system, the active enzymes must be turned off and the clotting process contained locally to the area of tissue injury. The details of the regulation of blood coagulation remain obscure, but it is clear that a series of blood proteins play a specialized role in disengaging the activated blood-clotting system. Antithrombin III is a plasma protein that combines with thrombin as well as most of the other activated blood-clotting proteins (e.g., factors Xa and IXa) to form inert complexes. This action is greatly enhanced by the presence of heparin, a substance formed by mast cells of the connective tissue. The hereditary deficiency of antithrombin III is associated with an excessive tendency toward clot formation, and manifestations of this defect are recurrent thrombophlebitis and pulmonary embolism. Heparin cofactor II is another plasma protease inhibitor that specifically forms a complex with thrombin, thus inactivating this enzyme. Protein C, a vitamin K-dependent protein, is a zymogen that requires vitamin K for its activation by thrombin complexed to thrombomodulin, a protein on the endothelial cell membrane. Activated protein C is capable of inactivating the active cofactor forms of factors VIII and V. Its action is enhanced when bound to protein S, a vitamin K-dependent protein that is attached to cell membranes (platelet or possibly endothelial cells). A deficiency in the level of protein C or protein S is associated with an excessive tendency to form clots.
Another anticoagulant effect is the fibrinolytic (fibrin-splitting) action of plasmin, an enzyme that catalyzes the removal of old fibrin at injury sites and any which may be deposited in normal vessels. Plasmin is derived from plasminogen, an inert protein precursor that can be activated by tissue plasminogen activator. Streptokinase, urokinase, and tissue plasminogen activator are drugs that activate plasminogen and lead to the dissolution of clots.
Synthesis of blood-clotting proteins
Most of the blood coagulation proteins are synthesized in the liver. In addition, factor VIII is synthesized in a large number of other tissues. Six proteins involved in blood coagulation require vitamin K for their complete synthesis: factor IX, factor X, prothrombin, factor VII, protein C, and protein S. These proteins are synthesized in precursor form. In a region of the liver cell called the rough endoplasmic reticulum, specific glutamic acid residues in the protein are changed by an enzyme-mediated reaction to form a modified glutamic acid known as γ-carboxyglutamic acid. This enzyme reaction, known as γ-carboxylation, requires vitamin K as a cofactor. γ-Carboxyglutamic acid is a unique amino acid that binds to calcium. In the protein, γ-carboxyglutamic acids form the calcium-binding sites that characterize this form of calcium-binding protein, the vitamin K-dependent proteins. Calcium stabilizes certain structural forms of the vitamin K-dependent proteins, enabling these proteins to bind to cell membranes. In the absence of vitamin K or in the presence of vitamin K antagonists such as warfarin, γ-carboxylation is inhibited and proteins are synthesized that are deficient in γ-carboxyglutamic acid. These proteins have no biologic activity because they do not bind to calcium and do not interact with membrane surfaces.
Overview
Bleeding, also called hemorrhage, is the name used to describe blood loss. It can refer to blood loss inside the body, called internal bleeding, or to blood loss outside of the body, called external bleeding.
Blood loss can occur in almost any area of the body. Internal bleeding occurs when blood leaks out through a damaged blood vessel or organ. External bleeding happens when blood exits through a break in the skin.
Blood loss from bleeding tissue can also be apparent when blood exits through a natural opening in the body, such as the:
* mouth
* vaginas
* rectum
* nose
What are the common causes of bleeding?
Bleeding is a common symptom. A variety of incidents or conditions can cause bleeding. Possible causes include:
Traumatic bleeding
An injury can cause traumatic bleeding. Traumatic injuries vary in their severity.
Common types of traumatic injury include:
* abrasions (scrapes) that don’t penetrate too far below the skin
* hematoma or bruises
* lacerations (cuts)
* puncture wounds from items like needles, nails, or knives
* crushing injuries
* gunshot wounds
Medical conditions
There are also some medical conditions that can cause bleeding. Bleeding due to a medical condition is less common than traumatic bleeding.
Conditions that can cause bleeding include:
* hemophilia
* leukemia
* liver disease
* menorrhagia, heavy or prolonged menstrual bleeding, like what’s sometimes seen in endometriosis
* thrombocytopenia, low blood platelet count
* von Willebrand disease
* vitamin K deficiency
* brain trauma
* colon diverticulosis
* lung cancer
* acute bronchitis
Medicines
Some medicines and certain treatments can increase your chances of bleeding, or even cause bleeding. Your doctor will warn you about this when they first prescribe the therapy. And they’ll tell you what to do if bleeding occurs.
Medications that may be responsible for bleeding include:
* blood thinners
* antibiotics, when used on a long-term basis
* radiation therapy
* aspirin and other NSAIDs
When is bleeding a sign of an emergency?
If bleeding is severe, seek help immediately. You should seek emergency help if you suspect internal bleeding. This can become life-threatening.
People who have bleeding disorders or take blood thinners should also seek emergency help to stop bleeding.
Seek medical help if:
* the person has gone into shock or has a fever
* the bleeding cannot be controlled using pressure
* the wound requires a tourniquet
* the bleeding was caused by a serious injury
* the wound may need stitches to stop bleeding
* foreign objects are stuck inside the wound
* the wound appears to be infected, such as swelling or leaking a whitish-yellow or brown pus, or has redness
* the injury occurred due to a bite from an animal or human
When you call for help, emergency services will tell you what to do and when they’ll arrive.
In most cases, emergency services will tell you to continue to put pressure on the wound and keep reassuring the person who’s bleeding. You may also be told to lay the person down to reduce their risk of fainting.
How is bleeding treated?
A person can bleed to death in 5 minutes. Bystanders may be able to save a life before emergency personnel can arrive.
There is a national campaign called Stop the Bleed to teach anyone how to stop bleeding. People in mass casualty events have died from blood loss even when their wounds shouldn’t have been fatal.
First aid for traumatic bleeding
It’s possible to treat external traumatic bleeding. Seek emergency help if the person is having any of the emergency signs listed above and if you need help to stop the bleeding.
The person who’s bleeding should try to remain calm to keep their heart rate and blood pressure controlled. Either heart rate or blood pressure being too high will increase the speed of bleeding.
Lay the person down as soon as possible to reduce the risk of fainting, and try to elevate the area that’s bleeding.
Remove loose debris and foreign particles from the wound. Leave large items such as knives, arrows, or weapons where they are. Removing these objects can cause further harm and will likely increase the bleeding. In this case, use bandages and pads to keep the object in place and absorb the bleeding.
Use the following to put pressure onto the wound:
* a clean cloth
* bandages
* clothing
* your hands (after applying protective gloves)
Maintain medium pressure until the bleeding has slowed and stops.
Do not:
* remove the cloth when bleeding stops. Use an adhesive tape or clothing to wrap around the dressing and hold it in place. Then place a cold pack over the wound.
* look at the wound to see if bleeding has stopped. This can disturb the wound and cause it to begin bleeding again.
remove the cloth from the wound, even if blood seeps through the material. Add more material on top, and continue the pressure.
* move anyone with an injury to the head, neck, back, or leg
* apply pressure to an eye injury
Use tourniquets only as a last resort. An experienced person should apply the tourniquet. To apply a tourniquet, follow these steps:
* Identify where to place the tourniquet. Apply it to a limb between the heart and the bleeding.
* Make the tourniquet using bandages, if possible. Wrap them around the limb and tie a half knot. Ensure there is enough room to tie another knot with the loose ends.
* Place a stick or rod between the two knots.
* Twist the stick to tighten the bandage.
* Secure the tourniquet in place with tape or cloth.
* Check the tourniquet at least every 10 minutes. If the bleeding slows enough to be controlled with pressure, release the tourniquet and apply direct pressure instead.
What are the signs of a medical emergency?
You will need emergency medical care if:
* bleeding is caused by a serious injury
* bleeding can’t be controlled
* bleeding is internal
Paramedics will attempt to control the bleeding before rushing you to the hospital. In some cases, care might be given at home or while on a stretcher. The treatment required will depend on the cause of the bleeding.
In rare cases, surgery may be required to stop bleeding.
What are the consequences of untreated bleeding?
A medical professional should see anyone who experiences unexplained or uncontrolled bleeding.
Traumatic bleeding
If an injury or accident causes bleeding, it may be stopped with local first aid. If it’s just a minor wound, it may heal without further care.
More significant wounds may require sutures, medicated dressings, or corrective surgery.
Medical bleeding
If a medical condition causes bleeding, and the condition isn’t identified or diagnosed, the bleeding is likely to recur.
Any bleeding that continues without medical treatment could be fatal. For example, if someone has acute bleeding in a short period of time and loses 30 percent or moreTrusted Source of their blood volume, they could bleed to death very quickly and would require IV fluid and transfusion of packed red blood cells for resuscitation.
Even medical conditions that cause slow blood loss over time can add up and cause major organ injury, possibly leading to death.
Exsanguination, which is severe bleeding or bleeding to death, can occur without any visible external bleeding. Catastrophic internal hemorrhages can cause a great deal of blood loss, such as ruptured blood vessel aneurysms.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1