Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Americium
Pages: 1
#1 2025-09-29 17:33:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,105
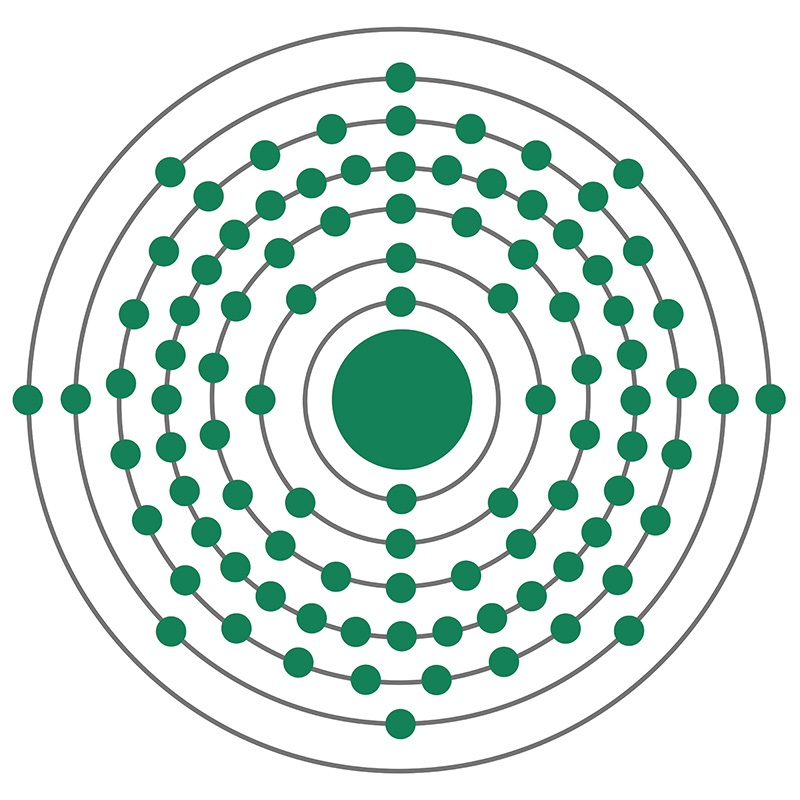
Americium
Americium
Gist
Americium - Periodic Table of VideosAmericium is a synthetic, radioactive, silvery-white metal and transuranic actinide element with symbol Am and atomic number 95. It was discovered in 1944 and named after the Americas, and is produced in nuclear reactors from plutonium. Its most stable and commercially important isotope, americium-241, is used in ionization smoke detectors, thickness gauges, and as a radiation source, but it is radiotoxic and poses health risks if inhaled or ingested.
Americium is primarily used in smoke detectors as Americium-241, where it serves as an ionization source to detect smoke. It also has industrial uses in thickness and density gauges for materials like glass and asphalt, as a portable source of alpha and gamma radiation for research, and in neutron sources for radiography and materials testing. Looking ahead, it's being developed as a power source for spacecraft in radioisotope thermoelectric generators (RTGs) due to its radioactive decay properties.
Summary
Americium is a synthetic chemical element; it has symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy.
Americium was first produced in 1944 by the group of Glenn T. Seaborg from Berkeley, California, at the Metallurgical Laboratory of the University of Chicago, as part of the Manhattan Project. Although it is the third element in the transuranic series, it was discovered fourth, after the heavier curium. The discovery was kept secret and only released to the public in November 1945. Most americium is produced by uranium or plutonium being bombarded with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains about 100 grams of americium. It is widely used in commercial ionization chamber smoke detectors, as well as in neutron sources and industrial gauges. Several unusual applications, such as nuclear batteries or fuel for space ships with nuclear propulsion, have been proposed for the isotope 242Am, but they are as yet hindered by the scarcity and high price of this nuclear isomer.
Americium is a relatively soft radioactive metal with a silvery appearance. Its most common isotopes are 241Am and 243Am. In chemical compounds, americium usually assumes the oxidation state +3, especially in solutions. Several other oxidation states are known, ranging from +2 to +7, and can be identified by their characteristic optical absorption spectra. The crystal lattices of solid americium and its compounds contain small intrinsic radiogenic defects, due to metamictization induced by self-irradiation with alpha particles, which accumulates with time; this can cause a drift of some material properties over time, more noticeable in older samples.
Details
americium (Am), synthetic chemical element (atomic number 95) of the actinoid series of the periodic table. Unknown in nature, americium (as the isotope americium-241) was artificially produced from plutonium-239 (atomic number 94) in 1944 by American chemists Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso in a nuclear reactor. It was the fourth transuranium element to be discovered (curium, atomic number 96, was discovered a few months previously). The element was named after the United States of America.
The metal is silvery white and tarnishes slowly in dry air at room temperature. The isotope americium-241 is the most important because of its availability. This isotope is produced by multiple neutron capture in nuclear reactors and has been isolated in kilogram amounts from plutonium and other actinoids in used nuclear fuel. Americium-241 has been used industrially in fluid-density gauges, thickness gauges, aircraft fuel gauges, and distance-sensing devices, all of which use its gamma radiation. The isotope’s alpha-particle emission is exploited in smoke detectors. All isotopes of americium are radioactive; the stablest isotope, americium-243, has proved more convenient for chemical investigations because of its longer half-life (7,370 years, compared with 433 years for americium-241).
Americium reacts with oxygen to form the dioxide AmO2, with halogen elements to form compounds such as the tetrafluoride AmF4 and all the trihalides, and with hydrogen to form the hydride AmH2+x. Americium has four well-characterized oxidation states, from +3 to +6, in acidic aqueous solution with the following ionic species: Am3+, pink; Am4+, rose (very unstable); AmO2 +, yellow; and AmO2+, light tan. In the common +3 state, americium is very similar to the other actinoid and lanthanoid elements. There is some evidence that the ion Am2+ has been prepared in trace amounts; its existence suggests that americium is similar to its lanthanoid homologue, europium, which can be reduced to its +2 oxidation state. There is also evidence for heptavalent americium in strongly basic aqueous solution.
Element Properties
atomic number : 95
stablest isotope : 243
melting point : above 850 °C (1,550 °F)
specific gravity : 13.67 (20 °C, or 68 °F)
oxidation states : +2, +3, +4, +5, +6.
Additional Information:
Appearance
Americium is a silvery, shiny radioactive metal.
Uses
Americium is commonly used in smoke alarms, but has few other uses.
It has the potential to be used in spacecraft batteries in the future. Currently plutonium is used but availability is poor so alternatives are being considered.
It is of interest as part of the decay sequence that occurs in nuclear power production.
Biological role
Americium has no known biological role. It is toxic due to its radioactivity.
Natural abundance
Americium occurs naturally in uranium minerals, but only in trace amounts. The main source of the element is the neutron bombardment of plutonium in nuclear reactors. A few grams are produced in this way each year.
It is also formed when nuclear weapons are detonated.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Americium