Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » This is Cool
- » Cation
Pages: 1
#1 Yesterday 18:13:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,167
Cation
Cation
Gist
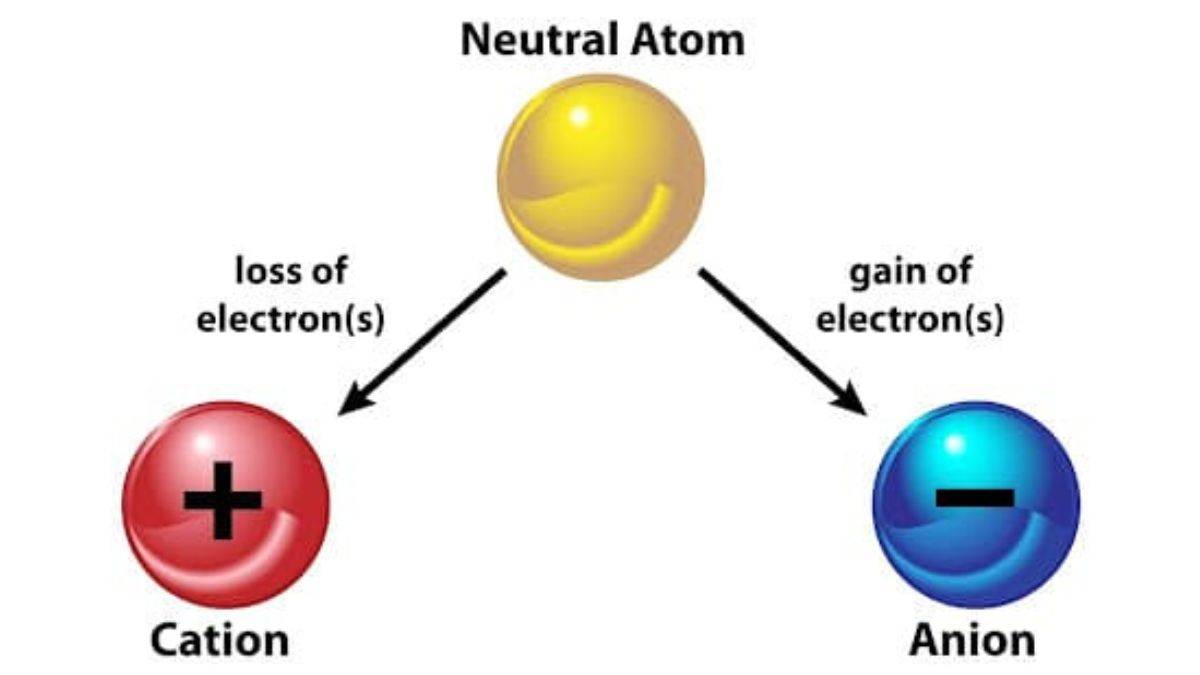
A cation is an atom or molecule with a net positive electrical charge, formed when a neutral atom loses one or more negatively charged electrons, resulting in more protons than electrons. Most metals readily form cations, such as sodium (Na⁺) or calcium (Ca²⁺), by losing electrons to achieve a stable electron configuration.
A cation is a positively charged ion, formed when a metal atom loses one or more electrons, resulting in more protons than electrons. Cations possess a net positive charge and are attracted to the cathode in an electric field. Common examples include sodium, calcium, and aluminum.
Cations are positively charged ions. They are formed when a metal loses its electrons. They lose one or more than one electron. It has fewer electrons than protons.
Summary
Cations are positively charged ions that result from an atom or group of atoms losing one or more valence electrons. The term "cation" is derived from "cathode ion," reflecting their attraction to the cathode in an electrolytic solution. Common examples of cations include sodium (Na⁺) and calcium (Ca²⁺), which typically form when alkali and alkaline earth metals lose their valence electrons to achieve a more stable electron configuration. The formation of cations is crucial to the creation of ionic compounds, such as sodium chloride (NaCl), where cations bond with negatively charged ions, or anions.
The electronic structure of atoms, including the arrangement of electrons in shells and orbitals, plays a significant role in determining the behavior of cations. Elements with similar valence electron configurations often exhibit similar chemical properties, a principle that underpins the organization of the periodic table. Naming conventions for cations are standardized by the International Union of Pure and Applied Chemistry (IUPAC), which includes the use of charge notation for monatomic and polyatomic cations. Understanding cations is fundamental to the study of chemistry, influencing reactions, bonding, and the properties of various compounds.
Details
A cation is a type of ion that has a positive electric charge. This means it has fewer electrons than protons. The opposite of a cation is an anion, which has a negative charge.
Cations can have only one atom (monatomic cations) or be made of multiple atoms together (polyatomic cations). Most metals form monatomic cations, while polyatomic cations are rarer.
Examples
Most metals make one or more monatomic cations. Alkali metals like sodium can lose one electron to make cations like Na+. Alkaline earth metals like calcium lose two electrons to make cations like Ca2+. These are the only ions these elements form, and so are just named after the element: the sodium cation Na+ is just called "sodium" in compounds like sodium chloride.
Transition metals and post-transition metals can make more than one type of cation: iron forms two cations, Fe2+ and Fe3+. The charge on transition metal cations is usually between +1 (such as silver in silver iodide) and +4 (such as titanium in titanium tetrachloride).
Because transition metal cations can have more than one charge, the charge (formally, the oxidation state), is included in the name of the cation in compounds using Roman numerals. Pyrite is made of Fe2+ and the sulfide anion S2−, so it is called iron(II) sulfide. Magnetite is made of Fe3+ and the oxide anion O2−, so it is called iron(III) oxide. Sometimes in older sources these cations have specific names: another name for iron(II) is the "ferrous" cation, while iron(III) is the "ferric" cation.
Ammonium is an example of a polyatomic cation. It is made of a nitrogen atom connected to four hydrogen atoms. The formula for ammonium is written NH4+. Ammonium is made when an acid gives a hydrogen ion to a molecule of ammonia.
Additional Information:
Frequently Asked Questions
What is an example of a cation?
Calcium in its most common state is a cation. It has a 2+ charge and thus has a net ratio of two more protons than electrons. Calcium is an important cation in the human body and its positive charge is needed to complete muscle contractions.
What is the difference between a cation and an anion?
A cation and an anion have a different net charge. Cations have more protons than electrons, and thus have an overall positive charge. Anions have more electrons than protons, and thus have an overall negative charge.
What is a cation?
A cation is any ion that is positively charged. This results in an atom or molecule which has a net positive charge due to the greater number of protons than electrons.
What are cations and anions?
Cations are atoms or molecules that have a positive charge due to a higher ratio of protons to electrons. Anions are atoms or molecules with a negative charge due to a higher ratio of electrons to protons.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » This is Cool
- » Cation