Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1076 2021-07-10 00:20:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1054) Covalent bond
Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same electrons. A covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms.
A brief treatment of covalent bonds follows.
Molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (H2, N2, Cl2, H2O, NH3) together with all organic compounds. In structural representations of molecules, covalent bonds are indicated by solid lines connecting pairs of atoms.
A single line indicates a bond between two atoms (i.e., involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e., involving two electron pairs), and triple lines (≡) represent a triple bond, as found, for example, in carbon monoxide (C≡O). Single bonds consist of one sigma (σ) bond, double bonds have one σ and one pi (π) bond, and triple bonds have one σ and two π bonds.
The idea that two electrons can be shared between two atoms and serve as the link between them was first introduced in 1916 by the American chemist G.N. Lewis, who described the formation of such bonds as resulting from the tendencies of certain atoms to combine with one another in order for both to have the electronic structure of a corresponding noble-gas atom.
Covalent bonds are directional, meaning that atoms so bonded prefer specific orientations relative to one another; this in turn gives molecules definite shapes, as in the angular (bent) structure of the H2O molecule. Covalent bonds between identical atoms (as in H2) are nonpolar—i.e., electrically uniform—while those between unlike atoms are polar—i.e., one atom is slightly negatively charged and the other is slightly positively charged. This partial ionic character of covalent bonds increases with the difference in the electronegativities of the two atoms.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1077 2021-07-11 00:31:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
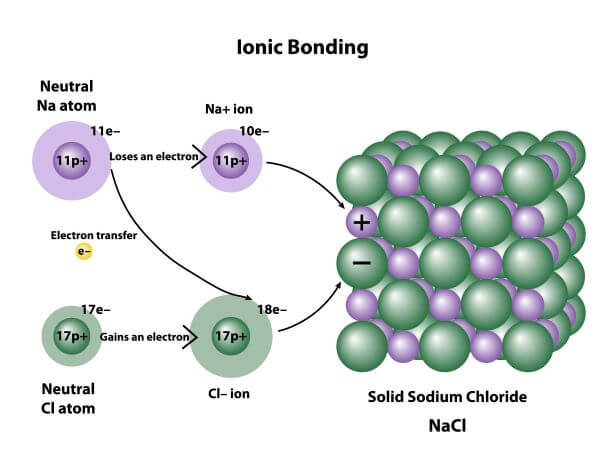
1055) Ionic bond
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. The atom that loses the electrons becomes a positively charged ion (cation), while the one that gains them becomes a negatively charged ion (anion). A brief treatment of ionic bonds follows.
Ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals and the alkali and alkaline-earth metals. In ionic crystalline solids of this kind, the electrostatic forces of attraction between opposite charges and repulsion between similar charges orient the ions in such a manner that every positive ion becomes surrounded by negative ions and vice versa. In short, the ions are so arranged that the positive and negative charges alternate and balance one another, the overall charge of the entire substance being zero. The magnitude of the electrostatic forces in ionic crystals is considerable. Accordingly, these substances tend to be hard and nonvolatile.
An ionic bond is actually the extreme case of a polar covalent bond, the latter resulting from unequal sharing of electrons rather than complete electron transfer. Ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1078 2021-07-12 00:20:05
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1056) Hard water and Soft water
Hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Ferrous iron may also be present; oxidized to the ferric form, it appears as a reddish brown stain on washed fabrics and enameled surfaces. Water hardness that is caused by calcium bicarbonate is known as temporary, because boiling converts the bicarbonate to the insoluble carbonate; hardness from the other salts is called permanent. Calcium and magnesium ions in hard water react with the higher fatty acids of soap to form an insoluble gelatinous curd, thereby causing a waste of the soap. This objectionable reaction does not take place with modern detergents.
In boilers, the calcium and magnesium in hard waters form a hard, adherent scale on the plates. As a result of the poor heat conductivity of the scale, fuel consumption is increased, and the boiler deteriorates rapidly through the external overheating of the plates. Sodium carbonate, if present, hydrolyzes to produce free alkali that causes caustic embrittlement and failure of the boiler plates. Water is softened on a small scale by the addition of ammonia, borax, or trisodium phosphate, together with sodium carbonate (washing soda). The latter precipitates the calcium as carbonate and the magnesium as hydroxide. Water is softened on a large scale by the addition of just enough lime to precipitate the calcium as carbonate and the magnesium as hydroxide, whereupon sodium carbonate is added to remove the remaining calcium salts. In areas where the water is hard, home water softeners are used, making use of the properties of natural or artificial zeolite minerals.
Soft water, water that is free from dissolved salts of such metals as calcium, iron, or magnesium, which form insoluble deposits such as appear as scale in boilers or soap curds in bathtubs and laundry equipment.
Hard water... is water that contains an appreciable quantity of dissolved minerals (like calcium and magnesium).
Soft water... is treated water in which the only ion is sodium.
Hard vs Soft
As rainwater falls, it is naturally soft. However, as water makes its way through the ground and into our waterways, it picks up minerals like chalk, lime and mostly calcium and magnesium and becomes hard water. Since hard water contains essential minerals, it is sometimes the preferred drinking water. Not only because of the health benefits, but also the flavor. On the other hand, soft water tastes salty and is sometimes not suitable for drinking. So why, then, do we soften our water?
The major difference between hard and soft water can best be seen while doing daily housework. Hard water is to blame for dingy looking clothes, dishes with spots and residue, and bathtubs with lots of film and soap scum. Soap is less effective due to its reaction to the magnesium and calcium that lather is not as rich and bubbly. Even hair washed in hard water may feel sticky and look dull. Already relating ourselves with these inconveniences? But we are not finished, hard water can take a toll on household appliances as well as use up more energy. It would also cause serious damage by scale accumulating inside water pipes, water heater, etc. These scale buildup is also known as mineral deposits or scale deposits. When water evaporates by increasing in temperature, the minerals that are suspend in precipitate causing solidified scale.
On the other hand, house workers will love using soft water, as tasks can actually be performed more efficiently with it. Soap will lather better and items will be left cleaner. Glasses will sparkle and hair will look healthy. The shower curtain will be scum-free. Clothes and skin are left softer. In addition to time, soft water can also save money, as less soap and detergents will be used. Since appliances have to work less hard, soft water can also prolong the life of washing machines, dishwaters and water heaters. Energy bills are noticeably lower when in households with water softeners. In a time when energy costs rise higher and higher, this is something for you to consider. Not to mention the benefit of reduced scale buildup.
Soft water is not, however, suggested for those with heart or circulatory problems, or others who may be on a low sodium diet. In the softening process, as minerals are removed, sodium content increases. Research shows that cardiovascular disease has the lowest risk in areas where water has the most mineral content.
There are ways to combat the sodium in soft water, which will allow households to enjoy better tasting water, as well as have the best available water for cleaning needs. They are reverse osmosis, distillation and deionization.
What type is your water? The Water Quality Association of the United States defines hard water as having dissolved mineral hardness of 1 GPG (grain per gallon) or more. Here is a helpful table to show the hardness of water:
Soft Water- less than 1 gpg
Slightly Hard- 1-3.5 gpg
Moderately Hard- 3.5-7 gpg
Very Hard- 7-10 gpg
Extremely Hard- over 10 gpg.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1079 2021-07-13 00:08:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1057) Fission
What is Fission?
When an atom splits into two parts, either through natural decay or when instigated within a lab, it releases energy. This process is known as fission. It has great potential as a source of power, but is also has a number of safety, environmental, and political concerns attached to it that can hinder its use.
Fission definition
An atom contains protons and neutrons in its central nucleus. In fission, the nucleus splits, either through radioactive decay or because it has been bombarded by other subatomic particles known as neutrinos. The resulting pieces have less combined mass than the original nucleus, with the missing mass converted into nuclear energy.
Controlled fission occurs when a very light neutrino bombards the nucleus of an atom, breaking it into two smaller, similarly-sized nuclei. The destruction releases a significant amount of energy — as much as 200 times that of the neutron that started the procedure — as well as releasing at least two more neutrinos.
Controlled reactions of this sort are used to release energy within nuclear power plants. Uncontrolled reactions can fuel nuclear weapons.
Radioactive fission, where the center of a heavy element spontaneously emits a charged particle as it breaks down into a smaller nucleus, does not occur often, and happens only with the heavier elements.
Fission is different from the process of fusion, when two nuclei join together rather than split apart.
Discovering atomic energy
In 1938, German physicists Otto Hahn and Fritz Strassman bombarded a uranium atom with neutrons in an attempt to make heavy elements. In a surprising twist, they wound up splitting the atom into the elements of barium and krypton, both significantly smaller than the uranium that the pair started out with. Previous efforts by physicists had resulted in only very small slivers being cut off of an atom, so the pair was puzzled by the unexpected results.
Austrian-born physicist Lise Meitner, who had fled to Sweden following Hitler's invasion of her country, realized that the split had also released energy. Working on the problem, she established that fission yielded a minimum of two neutrons for each neutron that sparked a collision. Ultimately, other physicists realized that each newly freed neutron could go on to cause two separate reactions, each of which could cause at least two more. A single impact could jumpstart a chain reaction, driving the release of still more energy.
Energy and destruction
In an intellectual chain reaction, scientists began to realize the possibilities incumbent in the new discovery. A letter to U.S. President Franklin Roosevelt at the start of World War II, drafted by Hungarian physicist Leo Szilard and signed by Albert Einstein, noted that such research could be used to create a bomb of epic proportions, and addressed the idea that the Germans could feasibly deliver such a weapon to the American doorstep. Roosevelt allocated money toward American research, and in 1941, the Office of Scientific Research and Development was formed with the aim of applying the research toward national defense.
In 1943, the Army Corp of Engineers took over the research for making a nuclear weapon. Known as the "Manhattan Project," the top-secret endeavor resulted in the formation of the first atomic bomb in July 1945. Two subsequent atomic weapons were used as part of a military strike on the cities of Hiroshima and Nagasaki in Japan.
Since then, nuclear research has been considered extremely sensitive. The knowledge itself is not overly complex, but the materials that fund the process are significantly more difficult to obtain.
More commonly, fission is used to generate energy within a nuclear power plant. However, the process creates a significant amount of nuclear waste that can be hazardous to both people and the environment. At the same time, people often fear the dangers that could come with nuclear plants and do not want them in their area. Such issues mean that nuclear energy is not as popular as more conventional methods of obtaining energy, such as the use of fossil fuels.
In the 1960s, the U.S. government explored the possibility of using fission as a method of rocket propulsion. However, the signing of the Limited (Nuclear) Test Ban Treaty in 1963 put an end to the aboveground explosion of all nuclear weapons, closing the door at least temporarily on the testing of fission-powered rockets.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1080 2021-07-14 01:00:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1058) Radioactivity
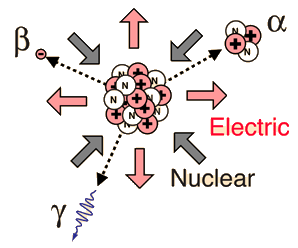
Radioactivity is the physical phenomenon of certain elements - such as uranium - of emitting energy in the form of radiation. This energy comes from the decay of an unstable nucleus. Any nuclear species (particular configuration of protons, neutrons and energy) that exhibit radioactivity are known as radioactive nuclei. Additionally, radioactivity or simply activity can be used as a measurement to describe how many decays a radioactive atom goes through in a period of time. These decays result in an ejection of energy and particles from the nucleus. Radioactivity can also be referred to as radioactive decay or nuclear decay.
The most common forms of radiation include alpha, beta, and gamma radiation, but other types of radioactive decay exist such as proton emission or neutron emission, or spontaneous fission of large nuclei.
Radioactivity has a number of different applications in medicine and industry. Radioactivity is even used in smoke alarms. Additionally, these radioactive elements act as the fuel in nuclear power plants to generate electricity. As well, the radiation from these elements can be used to irradiate foods and keep them from spoiling. To learn more about uses for radioactivity of elements, see isotopes for society.
What Causes Radioactivity?
The stability of a nuclear species (also called a nuclide) is determined by forces within the nucleus. These forces determine its nuclear stability. Unstable nuclear isotopes emit radiation as a result of the conflict in the strength of the repulsive Coulomb force between protons in the nucleus and the attractive strong nuclear force between nucleons. If the Coulomb force and strong nuclear force do not balance, the nuclide in question lays outside the belt of stability and is radioactive. A number known as the neutron-to-proton ratio or N/Z ratio can be used to quickly see if the Coulomb force and strong nuclear force remain fairly balanced or out of balance. For smaller elements near the top of the periodic table, the ratio for stability is nearly 1:1. As the nuclei get larger, the N/Z ratio increases slightly for stability. If a nucleus has too many protons or neutrons, it will likely undergo some sort of transmutation to reach a more stable state (where it changes to some new nuclide with a "better" N/Z ratio).
There are many complex factors that determine whether or not a nuclide be radioactive. For example, if a nuclide has either an odd number of protons or an odd number of neutrons, it is more likely to be radioactive. If both are odd, the nuclide is almost certainly radioactive! This greater instability comes from a desire for protons and neutrons like to "pair up" with particles of the same type, boosting stability. (Of the thousands of nuclides that have been investigated only 4 stable odd-odd nuclei have been found.) Some numbers of protons or neutrons in a nucleus that promote stability. These numbers are known as magic numbers.
Most large nuclides tend to be radioactive, and the last completely stable nuclide is bismuth (which has 83 protons). These large radioactive elements often undergo alpha decay as it quickly lowers the number of protons and neutrons in the nucleus.[5] Most nuclides found in nature are not radioactive, because all of the short-lived radioactive nuclei have already decayed, leaving a vast majority of stable nuclei. There are only 50 naturally occurring nuclides that exhibit radioactivity while there are around 270 stable nuclides. Thousands of short-lived nuclides have been created in laboratories and particle accelerators.
Measuring Radioactivity
Radioactivity can also be used to describe how much ionizing radiation is released by a radioactive material. The SI unit of radioactivity is the becquerel (Bq), equal to one decay per second. The curie (Ci) was the original unit for radioactivity and is equal to 3.7×1010 Bq. Geiger counters can be used to measure the radioactivity of a substance and these devices are widely known for the "clicking" noise they make when they detect a decay producing ionizing radiation.
Another way to measure how radioactive something is is to investigate its half life, since the half life of a nuclide is related to its radiation risk.
Safety
One common misconception about radioactivity is that any radioactive object is harmful to human health. This is not the case, however, as small doses of radiation have not been proven to be harmful to humans. In fact, there are many radioactive products that can be purchased and pose no health threats to humans. Bananas, smoke detectors, some ceramic dishware, cat litter, beer, and brazil nuts are all radioactive.
However, in larger doses radiation does have negative effects on health. When radioactive materials decay, they produce ionizing radiation. Simply put, this type of radiation can strip electrons away from atoms or break chemical bonds (to make ions). This causes damage to living tissues that cannot always be repaired. Chronic exposure to radiation can lead to cancer (as a result of damage at the cellular or molecular level) or other mutations that can be harmful to fetuses. Effects from acute exposure to radiation appear quickly, and include burns and radiation poisoning. The symptoms of radiation poisoning include nausea, weakness, hair loss, and diminished organ function and this radiation sickness can result in death if the dose is high enough.
As well, some radiation is only harmful in certain circumstances. For example, smoke detectors generally contain a source of alpha particles known as americium (which is radioactive). The americium is used to detect the smoke. In the smoke detector itself, this source is radioactive but not harmful. However, because of the nature of alpha particles, the Americium is very dangerous if ingested. Smoke detectors save many lives every year, and changing the batteries once a year is a good idea. Dissecting smoke detectors however can be dangerous.
.
Radioactivity refers to the particles which are emitted from nuclei as a result of nuclear instability. Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. The most common types of radiation are called alpha, beta, and gamma radiation, but there are several other varieties of radioactive decay.
Radioactive decay rates are normally stated in terms of their half-lives, and the half-life of a given nuclear species is related to its radiation risk. The different types of radioactivity lead to different decay paths which transmute the nuclei into other chemical elements. Examining the amounts of the decay products makes possible radioactive dating.
Radiation from nuclear sources is distributed equally in all directions, obeying the inverse square law.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1081 2021-07-15 00:30:13
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1059) Radium
Radium (Ra), radioactive chemical element, the heaviest of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Radium is a silvery white metal that does not occur free in nature.
Element Properties
atomic number : 88
stablest isotope : 226
melting point : about 700 °C (1,300 °F)
boiling point : not well established (about 1,100–1,700 °C [2,000–3,100 °F])
specific gravity : about 5
oxidation state : +2
Occurrence, Properties, And Uses
Radium was discovered (1898) by Pierre Curie, Marie Curie, and an assistant, G. Bémont, after Marie Curie observed that the radioactivity of pitchblende was four or five times greater than that of the uranium it contained and was not fully explained on the basis of radioactive polonium, which she had just discovered in pitchblende residues. The new, powerfully radioactive substance could be concentrated with barium, but, because its chloride was slightly more insoluble, it could be precipitated by fractional crystallization. The separation was followed by the increase in intensity of new lines in the ultraviolet spectrum and by a steady increase in the apparent atomic weight of the material until a value of 225.2 was obtained, remarkably close to the currently accepted value of 226.03. By 1902, 0.1 gram of pure radium chloride was prepared by refining several tons of pitchblende residues, and by 1910 Marie Curie and André-Louis Debierne had isolated the metal itself.
Thirty-four isotopes of radium, all radioactive, are known; their half-lives, except for radium-226 (1,600 years) and radium-228 (5.75 years), are less than a few weeks. The long-lived radium-226 is found in nature as a result of its continuous formation from uranium-238 decay. Radium thus occurs in all uranium ores, but it is more widely distributed because it forms water-soluble compounds; Earth’s surface contains an estimated 1.8 × 10^{13} grams (2 × 10^7 tons) of radium.
Since all the isotopes of radium are radioactive and short-lived on the geological time scale, any primeval radium would have disappeared long ago. Therefore, radium occurs naturally only as a disintegration product in the three natural radioactive decay series (thorium, uranium, and actinium series). Radium-226 is a member of the uranium-decay series. Its parent is thorium-230 and its daughter radon-222. The further decay products, formerly called radium A, B, C, C′, C″, D, and so on, are isotopes of polonium, lead, bismuth, and thallium.
Compounds
The chemistry of radium is what would be expected of the heaviest of the alkaline earths, but the intense radioactivity is its most characteristic property. Its compounds display a faint bluish glow in the dark, a result of their radioactivity in which emitted alpha particles excite electrons in the other elements in the compound and the electrons release their energy as light when they are de-excited. One gram of radium-226 undergoes 3.7 × 10^{10} disintegrations per second, a level of activity that defined the curie (Ci), an early unit of radioactivity. This is an energy release equivalent to about 6.8 × 10^{−3} calorie per second, sufficient to raise the temperature of a well-insulated 25-gram sample of water at the rate of 1 °C every hour. The practical energy release is even greater than this (by four to five times), because of the production of a large number of short-lived radioactive decay products. The alpha particles emitted by radium may be used to initiate nuclear reactions.
Radium’s uses all stem from its radioactivity. The most important use of radium was formerly in medicine, principally for the treatment of cancer by subjecting tumours to the gamma radiation of its daughter isotopes. Radium-223, an alpha emitter with a half-life of 11.43 days, has been studied for use in cell-directed cancer therapy, in which a monoclonal antibody or related targeting protein with high specificity is attached to the radium. In most therapeutic applications, however, radium has been superseded by the less costly and more powerful artificial radioisotopes cobalt-60 and cesium-137. An intimate mixture of radium and beryllium is a moderately intense source of neutrons and has been used for scientific research and for well logging in geophysical prospecting for petroleum. For these uses, however, substitutes have become available. One of the products of radium decay is radon, the heaviest noble gas; this decay process is the chief source of that element. A gram of radium-226 will emit 1 × 10^{−4} millilitre of radon per day.
When a radium salt is mixed with a paste of zinc sulfide, the alpha radiation causes the zinc sulfide to glow, yielding a self-luminescent paint for watch, clock, and instrument dials. From about 1913 up until the 1970s, several million radium dials, coated with a mixture of radium-226 and zinc sulfide, were manufactured. By the early 1930s it was found, however, that exposure to radium posed a serious hazard to health: a number of women who had worked with the radium-containing luminescent paint during the 1910s and ’20s subsequently died. They had ingested considerable amounts of radium through the technique called “lip-pointing,” which meant using their lips and tongues to shape their paintbrushes to a fine tip. Like calcium and strontium, radium tends to concentrate in bones, where its alpha radiation interferes with red corpuscle production, and some of those women developed anemia and bone cancer. The practice of employing radium in luminescent coatings was curtailed in the early 1960s after the high toxicity of the material was recognized. Phosphorescent paints that absorb light and later release it have replaced radium. (The detection of exhaled radon provides a very sensitive test for radium absorption.)
Radium metal may be prepared by electrolytic reduction of its salts, and it displays high chemical reactivity. It is attacked by water with vigorous evolution of hydrogen and by air with the formation of the nitride. It occurs exclusively as the Ra2+ ion in all its compounds. The sulfate, RaSO4, is the most insoluble sulfate known, and the hydroxide, Ra(OH)2, is the most soluble of the alkaline-earth hydroxides. The gradual buildup of helium within crystals of radium bromide, RaBr2, weakens them, and they occasionally explode. In general, the compounds of radium are very similar to their barium counterparts, making separation of the two elements difficult.
In modern technology, radium is separated from barium by fractional crystallization of the bromides, followed by purification through ion-exchange techniques for removal of the last 10 percent of the barium.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1082 2021-07-16 00:44:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1060) Actinium
Actinium (Ac), radioactive chemical element, in Group 3 (IIIb) of the periodic table, atomic number 89. Actinium was discovered (1899) by French chemist André-Louis Debierne in pitchblende residues left after French physicists Pierre and Marie Curie had extracted radium from them, and it was also discovered (1902) independently by German chemist Friedrich Oskar Giesel. Debierne named the element after the Greek word aktinos (“ray”). A ton of pitchblende ore contains about 0.15 mg of actinium. The rare silvery-white metal is highly radioactive, glowing blue in the dark.
The most common isotope of actinium is actinium-227; the others, natural and artificial, are too short-lived to accumulate in macroscopic quantity. Actinium-227, which is one of the decay products of uranium-235, has a 21.8-year half-life and in turn decays almost entirely to thorium-227, but about 1 percent decays to francium-223. This whole disintegration chain with its branches is called the actinium series.
Actinium-225 has a 10-day half-life, decaying by the emission of alpha particles. Its short-lived daughter isotopes emit only alpha and beta particles with no high-energy gamma rays. This isotope can thus deliver high-energy radiation to a tumour without greatly affecting the surrounding tissue. Complexes of actinium-225 have been studied for their use in nuclear medicine.
Actinium, the ions of which in solution are colourless, exhibits an oxidation state of +3, closely resembling the rare-earth lanthanoid elements in its chemical properties. Actinium is the prototype of a second rare-earth-like series, the actinoid elements.
Element Properties
atomic number : 89
stablest isotope : 227
oxidation state : +3
.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1083 2021-07-17 00:31:22
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1061) Molybdenum
Molybdenum (Mo), chemical element, silver-gray refractory metal of Group 6 (VIb) of the periodic table, used to impart superior strength to steel and other alloys at high temperature.
The Swedish chemist Carl Wilhelm Scheele had demonstrated (c. 1778) that the mineral molybdaina (now molybdenite), for a long time thought to be a lead ore or graphite, certainly contains sulfur and possibly a previously unknown metal. At Scheele’s suggestion, Peter Jacob Hjelm, another Swedish chemist, successfully isolated the metal (1782) and named it molybdenum, from the Greek molybdos, “lead.”
Molybdenum is not found free in nature. A relatively rare element, it is about as abundant as tungsten, which it resembles. For molybdenum the chief ore is molybdenite—molybdenum disulfide, MoS2—but molybdates such as lead molybdate, PbMoO4 (wulfenite), and MgMoO4 are also found. Most commercial production is from ores containing the mineral molybdenite. The concentrated mineral is usually roasted in an excess of air to yield molybdenum trioxide (MoO3), also called technical molybdic oxide, which, after purification, can be reduced with hydrogen to the metal. Subsequent treatment depends on the ultimate use of molybdenum. Molybdenum may be added to steel in the furnace in the form of either technical oxide or ferromolybdenum. Ferromolybdenum (containing at least 60 percent molybdenum) is produced by igniting a mixture of technical oxide and iron oxide. Molybdenum metal is produced in the form of a powder by hydrogen reduction of chemically pure molybdic oxide or ammonium molybdate, (NH4)2MoO4. The powder is converted to massive metal by the powder-metallurgy process or by the arc-casting process.
Molybdenum-base alloys and the metal itself have useful strength at temperatures above which most other metals and alloys are molten. The major use of molybdenum, however, is as an alloying agent in the production of ferrous and nonferrous alloys, to which it uniquely contributes hot strength and corrosion resistance, e.g., in jet engines, combustion liners, and afterburner parts. It is one of the most effective elements for increasing hardenability of iron and steel, and it also contributes to the toughness of quenched and tempered steels. The high corrosion resistance needed in the stainless steels used for processing pharmaceuticals and in the chromium steels for automotive trim is uniquely enhanced by small additions of molybdenum. Metallic molybdenum has been used for such electric and electronic parts as filament supports, anodes, and grids. Rod or wire is used for heating elements in electric furnaces operating up to 1,700 °C (3,092 °F). Coatings of molybdenum adhere firmly to steel, iron, aluminum, and other metals and show excellent resistance to wear.
Molybdenum is rather resistant to attack by acids, except for mixtures of concentrated nitric and hydrofluoric acids, and it can be attacked rapidly by alkaline oxidizing melts, such as fused mixtures of potassium nitrate and sodium hydroxide or sodium peroxide; aqueous alkalies, however, are without effect. It is inert to oxygen at normal temperature but combines with it readily at red heat, to give the trioxides, and is attacked by fluorine at room temperature, to give the hexafluorides.
Natural molybdenum is a mixture of seven stable isotopes: molybdenum-92 (15.84 percent), molybdenum-94 (9.04 percent), molybdenum-95 (15.72 percent), molybdenum-96 (16.53 percent), molybdenum-97 (9.46 percent), molybdenum-98 (23.78 percent), and molybdenum-100 (9.13 percent). Molybdenum exhibits oxidation states of +2 to +6 and is considered to display the zero oxidation state in the carbonyl Mo(CO)6. Molybdenum(+6) appears in the trioxide, the most important compound, from which most of its other compounds are prepared, and in the molybdates, used to produce pigments and dyes. Molybdenum disulfide (MoS2), which resembles graphite, is used as a solid lubricant or as an additive to greases and oils. Molybdenum forms hard, refractory, and chemically inert interstitial compounds with boron, carbon, nitrogen, and silicon upon direct reaction with those elements at high temperatures.
Molybdenum is an essential trace element in plants; in legumes as a catalyst it assists bacteria in fixing nitrogen. Molybdenum trioxide and sodium molybdate (Na2MoO4) have been used as micronutrients.
The largest producers of molybdenum are China, the United States, Chile, Peru, Mexico, and Canada.
Element Properties
atomic number : 42
atomic weight : 95.94
melting point : 2,610 °C (4,730 °F)
boiling point : 5,560 °C (10,040 °F)
specific gravity : 10.2 at 20 °C (68 °F)
oxidation states : 0, +2, +3, +4, +5, +6

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1084 2021-07-18 00:24:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1062) Astatine
Astatine (At), radioactive chemical element and the heaviest member of the halogen elements, or Group 17 (VIIa) of the periodic table. Astatine, which has no stable isotopes, was first synthetically produced (1940) at the University of California by American physicists Dale R. Corson, Kenneth R. MacKenzie, and Emilio Segrè, who bombarded bismuth with accelerated alpha particles (helium nuclei) to yield astatine-211 and neutrons. Naturally occurring astatine isotopes have subsequently been found in minute amounts in the three natural radioactive decay series, in which they occur by minor branching (astatine-218 in the uranium series, astatine-216 in the thorium series, and astatine-215 and astatine-219 in the actinium series). Thirty-two isotopes are known; astatine-210, with a half-life of 8.1 hours, is the longest lived. Because astatine has no stable or long-lived isotopes, it was given its name from the Greek word astatos, meaning “unstable.”
Element Properties
atomic number : 85
stablest isotope : 210
oxidation states : −1, +1, +3(?), +5, +7(?)
Production And Use
The only practical way of obtaining astatine is by synthesizing it through nuclear reactions.
It indicates that bismuth-209 takes up one alpha particle and emits x neutrons to form an isotope of astatine, whose atomic weight depends on the number of neutrons lost. Metallic bismuth may be used as a target material. From this, astatine may readily be removed by distillation in air from a stainless-steel tube. The free element begins to distill at 271 °C (520 °F, or the melting point of bismuth), but the operation is best carried out at 800 °C (1,500 °F) with subsequent redistillation. If an aqueous solution of astatine is desired, the element may be separated by washing with an appropriate aqueous solution. Alternatively, the halogen may be removed from the target by chemical methods, such as dissolving in nitric acid, with the latter being removed by boiling.
Another procedure involves the use of a metallic thorium target, which—after bombardment—is dissolved in concentrated hydrochloric acid containing hydrogen fluoride and chlorine.
Analysis
Because of the short half-lives of astatine isotopes, only very small quantities have been available for study. With the exception of a few spectrometric and mass-spectrometric studies, most investigations of astatine chemistry have used tracer techniques, which involve using chemical reactions in a solution with similarly reacting elements as carriers. The amount of astatine is then calculated from the measured radioactivity of the reaction products. However, the rarity of astatine means that these solutions are extremely dilute, with concentrations around or below {10}^{−10} molarity (the number of moles per litre of solution). At such concentrations, the effects of impurities can be very serious, especially for a halogen such as astatine, which exists in several oxidation states and can form many organic compounds. Iodine has been used as a carrier in most experiments. Techniques applied include coprecipitation, solvent extraction, ion exchange, and other forms of chromatography (separation by adsorption differences), electrodeposition (deposition by an electric current), electromigration (movement in an electric field), and diffusion. A direct identification of some astatine compounds has been made by mass spectrometry.
Except for nuclear properties, the only physical property of astatine to be measured directly is the spectrum of atomic astatine. Other physical properties have been predicted from theory and by extrapolation from the properties of other elements.
Chemical Properties
Some of the chemical properties of the element have been established. It generally resembles iodine. Thus, like iodine, it concentrates in the thyroid gland of higher animals. A substantial portion, however, is distributed throughout the body and acts as an internal radiation source.
The astatide ion, At−, is quantitatively coprecipitated with insoluble iodides, such as silver iodide or thallium iodide. The diffusion coefficient of the iodide ion is 1.42 times that of the astatide ion, which moves more slowly toward the anode than the former under given conditions. The ion is formed by reduction of the element, using zinc or sulfur dioxide. It is oxidized to the zero valence state by the ferric ion, Fe3+, iodine (I2), and dilute nitric acid. Thus, the astatide ion is a stronger reducing agent than the iodide ion, and free iodine is a stronger oxidizing agent than astatine.
Free astatine is characterized by volatility from solution and by extractability into organic solvents. It undergoes disproportionation in alkaline media. Astatine is coprecipitated with cesium iodide and thus appears to form polyhalide anions. Astatine extracted into chloroform has been shown to coprecipitate homogeneously with iodine when a portion of the latter is crystallized. Astatine seems to be present as the iodide, which appears to be more polar (i.e., showing separation of electric charge) in character than iodine bromide. It is somewhat soluble in water and much more soluble in benzene and carbon tetrachloride.
Astatine is known to occur in positive oxidation numbers. The astatate ion, (AtO3)−, is coprecipitated with insoluble iodates, such as silver iodate (AgIO3), and is obtained by the oxidation of lower oxidation states with hypochlorite, periodate, or persulfate. So far no evidence for perastatate has been found, but this may be because the ion, (AtO6)5−, may show little tendency to coprecipitate with potassium iodate (KIO4).
Astatine in the +1 state is stabilized by complexation, and complexes formulated as dipyridine astatine perchlorate [At(py)2] [ClO4] and dipyridine astatine nitrate [At(py)2] [NO3] have been prepared. Compounds with the formulas (C6H5)AtCl2, (C6H5)2AtCl, and (C6H5)AtO2 have also been obtained. A variety of methods may be used to synthesize astatobenzene, C6H5At.
.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1085 2021-07-19 00:08:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1063) Rubidium
Rubidium (Rb), chemical element of Group 1 (Ia) in the periodic table, the alkali metal group. Rubidium is the second most reactive metal and is very soft, with a silvery-white lustre.
Rubidium was discovered (1861) spectroscopically by German scientists Robert Bunsen and Gustav Kirchhoff and named after the two prominent red lines of its spectrum. Rubidium and cesium often occur together in nature. Rubidium, however, is more widely scattered and seldom forms a natural mineral; it is found only as an impurity in other minerals, ranging in content up to 5 percent in such minerals as lepidolite, pollucite, and carnallite. Brine samples have also been analyzed that contain up to 6 parts per million of rubidium.
In the principal commercial process of rubidium production, small amounts of rubidium are obtained from the mixture of alkali metal carbonates remaining after lithium salts are extracted from lepidolite. Primarily a potassium carbonate, this by-product also contains approximately 23 percent rubidium and 3 percent cesium carbonates.
The primary difficulty associated with the production of pure rubidium is that it is always found together with cesium in nature and is also mixed with other alkali metals. Because these elements are very similar chemically, their separation presented numerous problems before the advent of ion-exchange methods and ion-specific complexing agents such as crown ethers. Once pure salts have been prepared, it is a straightforward task to convert them to the free metal. This can be done by electrolysis of the fused cyanide or by reduction with calcium or sodium followed by fractional distillation.
Rubidium is difficult to handle because it ignites spontaneously in air, and it reacts violently with water to yield a solution of rubidium hydroxide (RbOH) and hydrogen, which bursts into flames; rubidium is therefore kept in dry mineral oil or an atmosphere of hydrogen. If a metal sample has a large enough surface area, it can burn to form superoxides. Rubidium superoxide (RbO2) is a yellow powder. Rubidium peroxides (Rb2O2) can be formed by oxidation of the metal with the required amount of oxygen. Rubidium forms two other oxides (Rb2O and Rb2O3).
It is used in photoelectric cells and as a “getter” in electron tubes to scavenge the traces of sealed-in gases. Rubidium atomic clocks, or frequency standards, have been constructed, but they are not as precise as cesium atomic clocks. However, aside from these applications, rubidium metal has few commercial uses and is of very minor economic significance. High prices and an uncertain and limited supply discourage the development of commercial uses.
Natural rubidium makes up about 0.01 percent of Earth’s crust; it exists as a mixture of two isotopes: rubidium-85 (72.15 percent) and the radioactive rubidium-87 (27.85 percent), which emits beta rays with a half-life of about 6 × 1011 years. A large number of radioactive isotopes have been artificially prepared, from rubidium-79 to rubidium-95. One estimate of the age of the solar system as 4.6 billion years is based on the ratio of rubidium-87 to strontium-87 in a stony meteorite. Rubidium easily loses its single valence electron but no others, accounting for its oxidation number of +1, although several compounds that contain the anion, Rb-, have been synthesized.
Rubidium and cesium are miscible in all proportions and have complete solid solubility; a melting-point minimum of 9 °C (48 °F) is reached. Rubidium forms a number of mercury amalgams. Because of the increased specific volume of rubidium, as compared with the lighter alkali metals, there is a lesser tendency for it to form alloy systems with other metals.
Element Properties
atomic number : 37
atomic weight : 85.47
melting point : 38.9 °C (102 °F)
boiling point : 688 °C (1,270 °F)
specific gravity : 1.53 (at 20 °C, or 68 °F)
oxidation states : +1, -1 (rare)
.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1086 2021-07-20 00:23:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
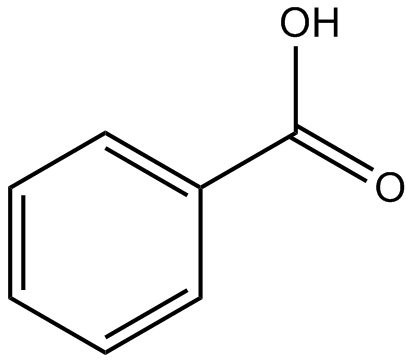
1064) Benzoic acid
Benzoic acid, a white, crystalline organic compound belonging to the family of carboxylic acids, widely used as a food preservative and in the manufacture of various cosmetics, dyes, plastics, and insect repellents.
First described in the 16th century, benzoic acid exists in many plants; it makes up about 20 percent of gum benzoin, a vegetable resin. It was first prepared synthetically about 1860 from compounds derived from coal tar. It is commercially manufactured by the chemical reaction of toluene (a hydrocarbon obtained from petroleum) with oxygen at temperatures around 200° C (about 400° F) in the presence of cobalt and manganese salts as catalysts. Pure benzoic acid melts at 122° C (252° F) and is very slightly soluble in water.
Among the derivatives of benzoic acid are sodium benzoate, a salt used as a food preservative; benzyl benzoate, an ester used as a miticide; and benzoyl peroxide, used in bleaching flour and in initiating chemical reactions for preparing certain plastics.
.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1087 2021-07-21 00:28:24
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1065) Tartaric acid
Tartaric acid, also called dihydroxybutanedioic acid, a dicarboxylic acid, one of the most widely distributed of plant acids, with a number of food and industrial uses. Along with several of its salts, cream of tartar (potassium hydrogen tartrate) and Rochelle salt (potassium sodium tartrate), it is obtained from by-products of wine fermentation. In a partially purified form, tartar was known to the ancient Greeks and Romans; the free acid was first isolated in 1769 by Swedish chemist Carl Wilhelm Scheele. The lees, or sediments, and other waste products from fermentation are heated and neutralized with calcium hydroxide; the precipitated calcium tartrate is then treated with sulfuric acid to produce free tartaric acid. Rochelle salt is prepared from the crude crystalline potassium acid salt, called argol, by neutralization with sodium carbonate. Purified cream of tartar comes chiefly from the filtrates from production of the acid and Rochelle salt. A third salt, tartar emetic (antimony potassium tartrate), is made from the potassium acid salt and antimony oxide.
Three stereoisomeric forms of tartaric acid exist: (1) dextrorotatory tartaric acid (D-tartaric acid) found in grapes and several other fruits, (2) levorotatory tartaric acid (L-tartaric acid) obtained chiefly by resolution of racemic tartaric acid, and (3) a meso or achiral form. Racemic tartaric acid (an equal mixture of D- and L-tartaric acid) is prepared commercially by the molybdenum- or tungsten-catalyzed oxidation of maleic anhydride with hydrogen peroxide.
Study of the crystallographic, chemical, and optical properties of the tartaric acids by French chemist and microbiologist Louis Pasteur laid the basis for modern ideas of stereoisomerism.
The various tartaric acids and the common tartrate salts are all colourless, crystalline solids readily soluble in water. Tartaric acid is widely used as an acidulant in carbonated drinks, effervescent tablets, gelatin desserts, and fruit jellies. It has many industrial applications—e.g., in cleaning and polishing metals, in calico printing, in wool dyeing, and in certain photographic printing and development processes. Rochelle salt is used in silvering mirrors, in processing cheese, and in compounding mild cathartics. Cream of tartar is incorporated into baking powders, hard candies, and taffies; and it is employed in the cleaning of brass, the electrolytic tinning of iron and steel, and the coating of other metals with gold and silver. Tartar emetic is used as an insecticide and a dyeing mordant.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1088 2021-07-22 00:45:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1066) Lactic acid
Lactic acid, also called α-hydroxypropionic acid, or 2-hydroxypropanoic acid, an organic compound belonging to the family of carboxylic acids, present in certain plant juices, in the blood and muscles of animals, and in the soil. It is the commonest acidic constituent of fermented milk products such as sour milk, cheese, and buttermilk.
First isolated in 1780 by a Swedish chemist, Carl Wilhelm Scheele, lactic acid is manufactured by the fermentation of molasses, starch, or whey in the presence of alkaline substances such as lime or calcium carbonate; it is available as aqueous solutions of various concentrations, usually 22–85 percent, and degrees of purity. Lactic acid is used in tanning leather and dyeing wool; as a flavouring agent and preservative in processed cheese, salad dressings, pickles, and carbonated beverages; and as a raw material or a catalyst in numerous chemical processes. Pure lactic acid, rarely prepared, is a colourless, crystalline substance that melts at 18° C (64° F); it rapidly absorbs moisture from the atmosphere.
Lactic acid occurs in the blood (in the form of its salts, called lactates) when glycogen is broken down in muscle and can be converted back to glycogen in the liver. Lactates are also the products of fermentation (q.v.) in certain bacteria..

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1089 2021-07-23 00:13:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1067) Bromine
Bromine (Br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or Group 17 (Group VIIa) of the periodic table.
Element Properties
atomic number : 35
atomic weight : 79.909
melting point : −7.2 °C (19 °F)
boiling point : 59 °C (138 °F)
specific gravity : 3.12 at 20 °C (68 °F)
oxidation states : −1, +1, +3, +5, +7
History
Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard in the residues (bitterns) from the manufacture of sea salt at Montpellier. He liberated the element by passing chlorine through an aqueous solution of the residues, which contained magnesium bromide. Distillation of the material with manganese dioxide and sulfuric acid produced red vapours, which condensed to a dark liquid. The similarity of this procedure to that for making chlorine suggested to Balard that he had obtained a new element similar to chlorine. (The German chemist Justus von Liebig appears to have obtained the element before Balard, but he wrongly considered it to be iodine chloride.) Because of the bad odour of the element, the French Academy of Sciences suggested the name bromine, from the Greek word bromos, meaning “bad smell” or “stench.”
Occurrence And Distribution
A rare element, bromine is found in nature dispersed throughout Earth’s crust only in compounds as soluble and insoluble bromides. Some enrichment occurs in ocean water (65 parts per million by weight), in the Dead Sea (approximately 5 grams per litre [0.7 ounce per gallon]), in some thermal springs, and in rare insoluble silver bromide minerals (such as bromyrite, found in Mexico and Chile). Natural salt deposits and brines are the main sources of bromine and its compounds. Jordan, Israel, China, and the United States led the world in bromine production in the early 21st century; other important bromine-producing countries during that period include Japan, Ukraine, and India.
Natural bromine is a mixture of two stable isotopes: bromine-79 (50.54 percent) and bromine-81 (49.46 percent). Of the 17 known radioactive isotopes of the element, bromine-77 has the longest half-life (57 hours).
Physical And Chemical Properties
Free bromine is a reddish brown liquid with an appreciable vapour pressure at room temperature. Bromine vapour is amber in colour. Bromine has a pungent odour and is irritating to the skin, eyes, and respiratory system. Exposure to concentrated bromine vapour, even for a short time, may be fatal. Like the other halogens, bromine exists as diatomic molecules in all aggregation states.
About 3.41 grams (0.12 ounce) of bromine dissolve in 100 millilitres (0.1 quart) of water at room temperature. The solution is known as bromine water. Like chlorine water, it is a good oxidizing agent, and it is more useful because it does not decompose so readily. It liberates free iodine from iodide-containing solutions and sulfur from hydrogen sulfide. Sulfurous acid is oxidized by bromine water to sulfuric acid. In sunlight bromine water decomposes, with release of oxygen.
From bromine water a hydrate (a clathrate) can be isolated that contains 172 water molecules and 20 cavities capable of accommodating the bromine molecules. Bromine dissolves in aqueous alkali hydroxide solutions, giving bromides, hypobromites, or bromates, depending on the temperature. Bromine is readily extracted from water by organic solvents such as carbon tetrachloride, chloroform, or carbon disulfide, in which it is very soluble. In the organic solvents it gives an orange solution.
The electron affinity of bromine is high and is similar to that of chlorine. It is, however, a less powerful oxidizing agent, chiefly because of the weaker hydration of the bromide ion as compared with the chloride ion. Similarly, a metal-bromine bond is weaker than the corresponding metal-chlorine bond, and this difference is reflected in the chemical reactivity of bromine, which lies between that of chlorine and that of iodine. An organic bromo compound resembles the corresponding chloro derivative but is usually more dense, less volatile, less combustible, and less stable.
Bromine combines violently with the alkali metals and with phosphorus, As, aluminum, and antimony but less violently with certain other metals. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does.
The most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, Br2), +1 (hypobromite, BrO−), +3 (bromite, BrO−2), +5 (bromate, BrO−3), and +7 (perbromate, BrO−4) are also known. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds.
Production And Use
The chief commercial source of bromine is ocean water, from which the element is extracted by means of chemical displacement (oxidation) by chlorine in the presence of sulfuric acid.
The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The free bromine is then mixed with sulfur dioxide, and the mixed gases are passed up a tower down which water is trickling.
Resulting in a mixture of acids that is much richer in bromide ion than seawater. A second treatment with chlorine liberates bromine, which is freed from chlorine and purified by passage over moist iron filings.
Commercial bromine generally contains up to 0.3 percent chlorine. It is usually stored in glass bottles or in barrels coated with lead or Monel metal.
The industrial usage of bromine had been dominated by the compound ethylene bromide (C2H4Br2), which once was added to gasoline with tetraethyl lead to prevent deposition of lead in the engine. Since the renunciation of leaded gasoline, bromine compounds have mainly been used in flame retardants, but ethylene bromide is still an important compound because of its use to destroy nematodes and other pests in soils. Bromine is also used in the production of catalysts, such as aluminum bromide.
Bromine has other uses, as in making various dyes and the compounds tetrabromoethane (C2H2Br4) and bromoform (CHBr3), which are used as liquids in gauges because of their high specific gravity. Until the development of barbiturates in the early 20th century, bromides of potassium, sodium, calcium, strontium, lithium, and ammonium were used widely in medicine because of their sedative action. Silver bromide (AgBr), an important component of photographic film, is, like silver chloride and iodide, light sensitive. Traces of potassium bromate (KBrO3) are added to wheat flour to improve baking. Other bromine compounds of significance include hydrogen bromide (HBr), a colourless gas used as a reducing agent and a catalyst in organic reactions. A solution of the gas in water is called hydrobromic acid, a strong acid that resembles hydrochloric acid in its activity toward metals and their oxides and hydroxides.
Analysis
A sensitive test for bromine is the reaction with fluorescein to give a deep red colour caused by bromination of the organic molecule, or by its reaction with fuchsine dyes in the presence of sulfurous acid, to give a deep blue colour. A more common test involves heating the sample with dilute sulfuric acid in the presence of potassium dichromate; the bromine is then extracted with chloroform, and, upon addition of potassium iodide, the pink colour of iodine appears. The presence of bromine may also be recognized by the evolution of hydrogen bromide containing some brown bromine vapour when a solid sample is treated with concentrated sulfuric acid. Alternatively, chlorine may be added to an aqueous solution of a sample containing bromide, causing development of a brown colour (free bromine).
For the quantitative determination of bromine, the following methods are recommended:
1. Free bromine is titrated with sodium thiosulfate in the presence of potassium iodide.
2. Bromides may be determined either gravimetrically (by weight analysis) or by titration with silver nitrate.
3. In the presence of chloride and iodide, the potentiometric method may be used (as with chlorine).
4. In the absence of iodide, bromide may be oxidized to bromine, which is then determined in the distillate. Alternatively, bromide may be oxidized to bromate by hypochlorous acid. The excess of the oxidizing agent is destroyed by sodium formate, and iodine is liberated by addition of potassium iodide and acid, with the free iodine being titrated by thiosulfate.
5. For the determination of bromine in an organic compound, the latter is oxidized by nitric acid, and the bromine is determined as silver bromide.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1090 2021-07-24 00:14:13
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1068) Virology
Virology, branch of microbiology that deals with the study of viruses.
Although diseases caused by viruses have been known since the 1700s and cures for many were (somewhat later) effected, the causative agent was not closely examined until 1892, when a Russian bacteriologist, D. Ivanovski, observed that the causative agent (later proved to be a virus) of tobacco mosaic disease could pass through a porcelain filter impermeable to bacteria. Modern virology began when two bacteriologists, Frederick William Twort in 1915 and Félix d’Hérelle in 1917, independently discovered the existence of bacteriophages (viruses that infect bacteria).
Direct visualization of viruses became possible after the electron microscope was introduced about 1940. In 1935 tobacco mosaic virus became the first virus to be crystallized; in 1955 the poliomyelitis virus was crystallized. (A virus “crystal” consists of several thousand viruses and, because of its purity, is well suited for chemical studies.) Virology is a discipline of immediate interest because many human diseases, including smallpox, influenza, the common cold, and AIDS, as well as a host of economically important plant and animal diseases, are caused by viruses.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1091 2021-07-25 00:15:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1069) Lagoon
Lagoon, area of relatively shallow, quiet water situated in a coastal environment and having access to the sea but separated from the open marine conditions by a barrier. The barrier may be either a sandy or shingly wave-built feature (such as a sandbar or a barrier island), or it may be a coral reef. Thus, there are two main types of lagoons: (1) elongated or irregular stretches of water that lie between coastal barrier islands and the shoreline and (2) circular or irregular stretches of water surrounded by coral atoll reefs or protected by barrier coral reefs from direct wave action. Lagoon depths are maintained at a moderate level by sedimentation, and this compensates for the subsidence that commonly attends reef formation. Because the reef is an organic structure, the lagoonal sediments contain much calcareous material. The sheltered waters support highly productive ecosystems made up of a distinctive flora and fauna.
Lagoon Types
Barrier island lagoons
Barrier island, or coastal, lagoons are characterized by quiet water conditions, fine-grained sedimentation, and, in many cases, brackish marshes. Water movements are related to discharge of river flow through the lagoon and to the regular influx and egress of tidal waters through the inlets that normally separate the barrier islands. Coastal lagoons are generally characteristic of coasts of low or moderate energy, occurring especially on the east coasts of continents where the swells are less violent and in high latitudes where offshore ice provides some protection. They also are associated with low coasts and rarely occur where high cliffs form the coast. They can form only where there is abundant sediment for construction of the protective barrier islands. Too much sediment from the mainland, however, can lead to delta formation rather than lagoons, although lagoons frequently occur along the outer delta margin and between delta distributaries
Coastal lagoons are widely distributed throughout the world and have been estimated to constitute about 13 percent of the total world coastline. Lagoons are more common on coasts with moderate to low tidal ranges; for example, they occur widely on low coasts of the southern Baltic, the southeast North Sea, the Black Sea, the Caspian Sea, and the Mediterranean Sea, as well as on low coasts of the southeastern United States and the Gulf of Mexico. Lagoon coasts also occur along southern Brazil, the east coast of Madagascar, northeastern Russia, Japan, and isolated parts of Africa, India, Australia, and New Zealand.
Coral lagoons
Coral lagoons are restricted to tropical open seas that provide the conditions necessary for coral growth. They are best exemplified by the roughly circular quiet waters that are surrounded by warm-water coral atoll reefs. Coral lagoons occur widely in the western Pacific, in parts of the Indian Ocean, and in isolated places in the Caribbean, mainly within 25° latitude of the Equator. Coral lagoons are of great importance to many island communities in the Pacific, particularly where they provide the only quiet water for use as harbours, although the passage through the reef into the lagoon is often perilous.
Coral-reef lagoons also occur on marginal reefs such as the Great Barrier Reef of Australia, but the most spectacular examples are the atolls of the Pacific Ocean, some of which are more than 50 km (30 miles) across. Some atolls consist only of a lagoon, often with a fairly uniform depth, surrounded by a low-lying coral reef; some include one or more high, rocky volcanic islands, and others are complex, with small reefs surrounded by lagoons within a larger reef. All are thought to have been built by the upward growth of coral during a relative rise in sea level due to subsidence and eustatic (global) change.
Nature Of The Lagoon Environment
Dimensions
Coral lagoon dimensions range from small atolls to those so wide that the coral reefs on the far side cannot be seen across the lagoon. Atoll widths range from about 2.5 to nearly 100 km (1.5 to nearly 62 miles), but the mean value is about 20 km (about 12 miles). Depths rarely exceed 60 metres (about 200 feet) and many are shallower, usually less than 20 metres (about 65 feet) deep. The lagoon of Mayotte island in the Comoro archipelago in the Indian Ocean attains a maximum depth of about 92 metres (about 300 feet), but it is generally shallower. That lagoon is about 16 km (about 10 miles) in width at its widest point and lies behind a barrier reef that encircles the island, forming an atoll about 55 km (about 34 miles) in diameter.
Barrier island lagoons are usually elongated, though irregular ones may occur where river estuaries flood behind barriers. This occurs on the east coast of the United States, where lagoons extend intermittently for nearly 1,500 km (about 900 miles) along the coast. The Gippsland Lakes in Victoria, Australia, exemplify a complex lagoon system formed behind a 149-km (93-mile) beach. Elongated lagoons up to 64 km (about 40 miles) in length lie behind the beach barrier, and larger lagoons, such as Lake Wellington, lie behind the southwestern end. Postglacial subsidence has flooded the lowland in this area. The lagoons are shallow: Lake Wellington is less than 3.5 metres (11.5 feet) in depth, and much of Lake King is less than 6 metres (about 20 feet) deep. Scour holes as deep as 16.5 metres (about 54 feet) do occur, however. The elongated lagoon behind the barrier is only 1 to 1.5 metres (3 to 5 feet) deep, typical of barrier island lagoons.
Water circulation
The degree of water circulation depends on the width of the tidal inlets, the tidal range, and the amount of runoff from adjacent land areas. Maximum velocities are attained at the points where the water passes through the barriers. In the entrance to the Gippsland lagoons, for example, tidal currents reach 5.6 km (3.5 miles) per hour, but river floods that escape to the ocean can raise the velocity to 13 km (8 miles) per hour. Water may be blown into the lagoon by strong winds; the increased level results in an outflowing current when the wind drops. Seiches—that is, rhythmic oscillations of water in enclosed or partially enclosed water bodies—can be created in this way. Small waves can be generated within lagoons when the wind blows along their maximum dimension. These may reach 1.25 metres (about 4 feet) in height and 1.5 to 9 metres (about 5 to 30 feet) in length in the Gippsland lagoons. In coral atoll lagoons there is little or no runoff, and seawater moves in and out through the passes, where tidal currents reach their maxima. Velocities of 19 to 22 km (12 to 14 miles) per hour have been recorded in the Hao Channel of the Tuamotu Archipelago in French Polynesia.
A lagoon into which a major river flows is known as an estuarine lagoon and may be regarded as a special kind of estuary.
Water temperature and salinity
In the Mayotte lagoon the water has the same properties as the upper layers of the open ocean. The salinity is close to 35 parts per thousand (ppt), and the temperature varies between 27 and 24 °C (81 and 75 °F). This is typical of coral lagoons, but the temperature and salinity of barrier island lagoons are more variable because of their wider climatic range. Because they are shallow, lagoon waters approximate the air temperature: colder than the open ocean in winter, warmer in summer.
Salinities decrease as a function of the amount of runoff entering the lagoon in relation to the tidal influx. Lagoons may be considered brackish, marine, or hypersaline. Brackish lagoons receive much runoff, and salinity increases toward the tidal inlets. The Gippsland lagoons exemplify this type. The salinity at the inner end varies from 0.5 to 5 ppt according to season, and central values vary between 5 and 20 ppt.
In hot arid regions lagoons lose more water by evaporation than they receive from land drainage. This causes surface waters to become more dense than seawater and to sink to the bottom. Seawater flows in at the surface to replace that lost by evaporation, creating a circulation the reverse of that found in estuaries. If exchange with the open sea is limited, the lagoon may become much more saline than the open sea. Consequently, various species of plants and animals become adapted to life in high salinities. Laguna Madre in Texas and Syvash Sound in the Black Sea are examples of hypersaline lagoons. (They have salinities of 65 and 132 ppt, respectively.) Salt deposits may form in these conditions. The denser saline water tends to lie beneath the fresher water, where mixing is not severe.
Equilibrium bottom profiles
Lagoons behind coastal barriers normally are zones of fine sedimentation. Their bottom profiles, therefore, build up gradually with time. Typical depths of the Texas lagoons vary between 1.25 and 3.5 metres (4 and 11 feet), and their floors are flat. Early theories that attempted to relate the form of the offshore and lagoon profile are no longer held; and, because the lagoon profile changes with continued deposition, it cannot be used to establish the process of lagoon formation. The profile is usually gently undulating, but it may contain deeper channels, especially near the tidal inlets. Profiles across coral lagoons either are smooth and flat from calcareous sedimentation or contain knolls of growing or dead coral. There are 2,300 such knobs in the Enewetak Lagoon in the Marshall Islands.
Factors Involved In Lagoon Formation
The essential feature that causes the lagoon to exist is the barrier that separates it from the ocean. In the coral lagoon the formation of the reef depends on the existence of suitable conditions for reef growth.
Barrier bars and sediment sources
The barrier island lagoons, on the other hand, depend not on organic processes but on waves, which act in a suitable environment on an adequate supply of bottom material, most commonly sand. Barrier islands are formed in those areas where the coastal slope is flatter than the equilibrium slope required by the long constructive swells—i.e., the waves that build up the foreshore in front of their breakpoint. They are, therefore, found on low coasts. They may occur in areas of subsidence, stability, or emergence, wherever sufficient sand exists.
The barrier islands that form the lagoons are made of sand, but the sediments of the lagoon are usually finer, as conditions are quieter. The lagoonal muds differ from shelf muds. Glauconite is rare in lagoon muds, but oyster reefs may occur as along the Texas coast. The muds are found away from the channels, in which only coarse sediment can rest, owing to strong currents. Flocculation in the saline lagoon water expedites clay deposition. The source of the fine sediment is from inland areas, and transport is by rivers. The details of lagoon sedimentation vary with the nature of the river load. Sedimentation rates are much greater in the lagoon than the adjacent open ocean, because deposition is continuous over much of the lagoon and is often aided by flocculation and vegetation. In the Texas lagoons from 1875 to 1936, 20 cm (about 8 inches) of deposition occurred in spite of 30 cm (about 12 inches) of subsidence; the sedimentation rate, therefore, was about one metre in 100 years.
Waves, tides, and surf
The barrier islands are formed by the waves, which build up their equilibrium profile by deposition on a gradient that is too flat. The level of the growing accumulation may be raised by the wind, forming dunes. Where the land behind the growing barrier is low, it will become flooded to form a lagoon if sea level rises slowly. Such a rise of sea level has occurred during the past 20,000 years. As long as the barrier island can maintain its level above the sea, as sea level rises, the lagoon will exist until it is filled with sediment. Not all lagoons and barrier island complexes have formed during rising sea-level conditions, but, where there is evidence that no open-sea foreshore sediments lie on the land side of the barrier, this hypothesis seems likely. In some barriers, however, outbuilding of material by glacial outwash streams or rivers may provide a suitably low gradient and enough sediment to form a barrier, as along the south coast of Iceland. In other areas material carried alongshore to form a spit may develop into a bay-mouth barrier, enclosing a lagoon. Such features can be of sand or shingle (pebbles). The Fleet, a brackish body of water behind Chesil Beach in southern England, is an example of the latter type.
Waves within the lagoon may develop cuspate (pointed) spits along the land side of the barrier and the inner edge of the lagoon. These features may eventually break the lagoon into almost circular or oval water bodies. Examples occur in the Chukchi Sea lagoons in northeastern Russia and elsewhere where vegetation does not form marshland.
Storms and catastrophic events
Storms and tsunamis exert an effect on lagoons when they breach or overtop the barriers around a lagoon by raising the water level temporarily. Major changes in configuration can occur in a short time. Hurricanes, for instance, can cause serious effects on the coast of Texas, along which lagoons are common. Padre Island was lowered to below mean low tide at its southern end by one storm in 1919, and several washover channels were cut. (Washover channels are inlets of water excavated by the wave action produced by a severe storm.) The mainland shore also suffered erosion.
Deposition may also occur; saline marls have been laid down on freshwater marsh, and small beach ridges may be built inside the lagoon where the high water level drives sand inland over mud. Coral reefs are more resistant to storms than are mobile sandy barriers.
The effect of time
Lagoons of both types change with time. In both, a relative rise of sea level with time is important in the development of the lagoon. In coral atolls there is evidence from deep boring that English naturalist Charles Darwin’s original subsidence hypothesis of atoll formation, via barrier reefs from fringing reefs around a subsiding volcanic peak, is substantially correct in many cases. As long as the coral can maintain its growth at a suitable level as its foundation subsides, the atoll will continue to enclose a lagoon, which is floored by coral or calcareous sediment derived from the reef and which maintains its depth by growth or deposition.
The postglacial rise of sea level also has influenced barrier island formation in many instances. When sea level rises too fast, a barrier may be drowned, and its lagoon will cease to exist.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1092 2021-07-26 00:44:26
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1070) Tributary
A tributary is a freshwater stream that feeds into a larger stream, river or other body of water. The larger, or parent, river is called the mainstem.
A tributary is a freshwater stream that feeds into a larger stream or river. The larger, or parent, river is called the mainstem. The point where a tributary meets the mainstem is called the confluence. Tributaries, also called affluents, do not flow directly into the ocean.
Most large rivers are formed from many tributaries. Each tributary drains a different watershed, carrying runoff and snowmelt from that area. Each tributary's watershed makes up the larger watershed of the mainstem.
The Missouri River's massive watershed, for example, is created by the watersheds of dozens of tributaries extending from the provinces of Alberta and Saskatchewan, Canada, through seven states in the Upper Midwest of the U.S. The Missouri, in turn, is the largest tributary of the Mississippi River, which it meets at a confluence in St. Louis, Missouri. The Mississippi River watershed is the fourth-largest in the world.
A "left-bank tributary" or "right-bank tributary" indicates the side of the river a tributary enters. When identifying a left-bank or right-bank tributary, a geographer looks downstream (the direction the river is flowing).
The Euphrates River, the longest river in southwestern Asia, stretches 2,700 kilometers (1,678 miles). The tiny streams that feed the Euphrates originate in the mountains of eastern Turkey. These streams become the Balikh and the Sajur Rivers, which join the Euphrates at different confluences in Syria. The Balikh is a left-bank tributary of the Euphrates, while the Sajur is a right-bank tributary.
Sometimes, tributaries have the same name as the river into which they drain. These tributaries are called forks. Different forks are usually identified by the direction in which they flow into the mainstem.
The Shenandoah River, for example, flows through the U.S. states of West Virginia and Virginia. It has two long tributaries, the North Fork and South Fork, which meet at a confluence in Harpers Ferry, West Virginia.
The opposite of a tributary is a distributary. A distributary is a stream that branches off and flows apart from the mainstem of a stream or river. The process is called river bifurcation.
At the Continental Divide in the U.S. state of Wyoming, the small North Two Ocean Creek bifurcates into Pacific Creek and Atlantic Creek. The water from each of these distributaries flows into the ocean for which it is named.
Classifying Tributaries
There are two leading methods geographers and potamologists (people who study rivers) use to classify tributaries.
The first method lists a river's tributaries starting with those closest to the source, or headwaters, of the river. The earliest tributaries of the Rhine River, for example, include the Thur River of Switzerland and the Ill River of Austria. The Rhine, one of the longest rivers in Europe, has its source in the Alps and its mouth in the North Sea.
The second method lists a river's tributaries by their flow. Small streams are identified with low numbers, while larger tributaries have higher numbers. The Tshuapa and Kasai Rivers are both left-bank tributaries of the Congo River, the deepest river in the world. The Tshuapa is a smaller river, and has a lower tributary ranking, than the Kasai.
People and Tributaries
Human activity in tributaries is often responsible for polluting the mainstem. The river carries all the runoff and pollution from all its tributaries.
Rivers with tributaries that drain land that is not used for agriculture or development are usually less polluted than rivers with tributaries in agricultural or urban areas.
Development, not size, determines the pollution of rivers. The Amazon River, with the largest drainage basin in the world, is much cleaner than the Hudson River, for instance. Tributaries to the Amazon flow through undeveloped regions of the Andes Mountains and rain forests in Brazil, Venezuela, Colombia, Peru, and Bolivia. The Hudson River flows through one of the largest urban areas on Earth, New York City.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1093 2021-07-27 01:33:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1071) Atoll
Atoll, coral reef enclosing a lagoon. Atolls consist of ribbons of reef that may not always be circular but whose broad configuration is a closed shape up to dozens of kilometres across, enclosing a lagoon that may be approximately 50 metres (160 feet) deep or more.
Most of the reef itself is a submarine feature, rising from the abyssal floors of the ocean to just beneath high-tide level. Around the rim along the top of the reef there are usually low, flat islands or more continuous strips of low, flat land. Some of these islands have been settled by oceanic peoples like the Maldivians, Polynesians, and Micronesians for many centuries.
The origin of atolls has always fascinated sailors and naturalists, who early appreciated that, although reef-building organisms inhabit only the shallowest depths of the sea (about 100 metres [330 feet]), the reefs rose from much deeper. The modern explanation of atolls incorporates the theory of Charles Darwin, who suggested that atolls represented the final stage of a continuing upgrowth of reef around a sinking extinct volcanic island that had long since disappeared from view.
Reefs tend to grow outward from a fringing-reef stage toward the better conditions of open water and also grow upward if the foundations beneath are sinking. After thousands of years the actively growing reef structure becomes separated from the volcanic shoreline by an intervening stretch of lagoon water. This is the barrier-reef stage. The volcanic island eventually subsides from view, leaving a reef whose uppermost part is like a saucer whose rim reaches up to sea level and whose deeper central area is a lagoon.
Different kinds of reefs and volcanic islands are found together in the tropical oceans, related to each other in such a way that they can be interpreted as representing the progressive stages postulated by the subsidence theory. Stronger direct evidence for subsidence has come from geologic drilling of atolls (first at Enewetak atoll in 1952), which revealed the presence of volcanic rock about 1,400 metres (4,600 feet) below the modern reef top. Changes in sea level complicate the subsidence model. These have been relatively frequent during the last 2,000,000 years or more and mostly result from cycles of glaciation.
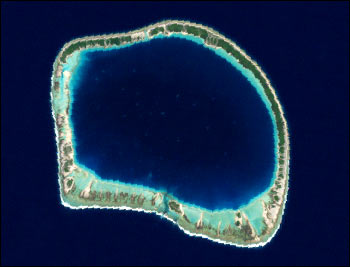
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1094 2021-07-28 00:26:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1072) Storm
Storm, violent atmospheric disturbance, characterized by low barometric pressure, cloud cover, precipitation, strong winds, and possibly lightning and thunder.
Storm is a generic term, popularly used to describe a large variety of atmospheric disturbances, ranging from ordinary rain showers and snowstorms to thunderstorms, wind and wind-related disturbances, such as gales, tornadoes, tropical cyclones, and sandstorms.
In meteorological terminology storm is restricted to a cyclone with a strong low pressure centre, strong winds, ranging from 103–117 kilometres per hour (64–73 miles per hour), accompanied by heavy precipitation, and at times, lightning and thunder.
A storm is any disturbed state of an environment or in an astronomical body's atmosphere especially affecting its surface, and strongly implying severe weather. It may be marked by significant disruptions to normal conditions such as strong wind, tornadoes, hail, thunder and lightning (a thunderstorm), heavy precipitation (snowstorm, rainstorm), heavy freezing rain (ice storm), strong winds (tropical cyclone, windstorm), or wind transporting some substance through the atmosphere as in a dust storm, blizzard, sandstorm, etc.
Storms have the potential to harm lives and property via storm surge, heavy rain or snow causing flooding or road impassibility, lightning, wildfires, and vertical wind shear. Systems with significant rainfall and duration help alleviate drought in places they move through. Heavy snowfall can allow special recreational activities to take place which would not be possible otherwise, such as skiing and snowmobiling.
The English word comes from Proto-Germanic *sturmaz meaning "noise, tumult".
Storms are created when a center of low pressure develops with the system of high pressure surrounding it. This combination of opposing forces can create winds and result in the formation of storm clouds such as cumulonimbus. Small localized areas of low pressure can form from hot air rising off hot ground, resulting in smaller disturbances such as dust devils and whirlwinds.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1095 2021-07-29 00:25:26
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1073) Gale
Gale, wind that is stronger than a breeze; specifically a wind of 28–55 knots (50–102 km per hour) corresponding to force numbers 7 to 10 on the Beaufort scale. As issued by weather service forecasters, gale warnings occur when forecasted winds range from 34 to 47 knots (63 to 87 km per hour).
A gale is a strong wind, typically used as a descriptor in nautical contexts. The U.S. National Weather Service defines a gale as 34–47 knots (63–87 km/h, 17.5–24.2 m/s or 39–54 miles/hour) of sustained surface winds. Forecasters typically issue gale warnings when winds of this strength are expected. In the United States, a gale warning is specifically a maritime warning; the land-based equivalent in National Weather Service warning products is a wind advisory.
Other sources use minima as low as 28 knots (52 km/h; 14 m/s; 32 mph), and maxima as high as 90 knots (170 km/h; 46 m/s; 100 mph). Through 1986, the National Hurricane Center used the term gale to refer to winds of tropical force for coastal areas, between 33 knots (61 km/h; 17 m/s; 38 mph) and 63 knots (117 km/h; 72 mph; 32 m/s). The 90 knots (170 km/h; 46 m/s; 100 mph) definition is very non-standard. A common alternative definition of the maximum is 55 knots (102 km/h; 63 mph; 28 m/s).
The most common way of measuring wind force is with the Beaufort scale which defines a gale as wind from 50 kilometres per hour (14 m/s) to 102 kilometres per hour (28 m/s). It is an empirical measure for describing wind speed based mainly on observed sea conditions. On the original 1810 Beaufort wind force scale, there were four different "gale" designations whereas generally today there are two gale forces, 8 and 9, and a near gale 7.
The word gale is derived from the older gail, but its origin is uncertain.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1096 2021-07-30 00:55:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1074) Geiger Counter
Geiger counter is a device which is used to detect and measure particles in the ionized gases. It is widely used in applications like radiological protection, radiation dosimetry, and experimental physics. It is made up of the metallic tube, filled with gas and a high voltage range of multiples of 100V is applied to this gas. It detects alpha, beta, and gamma particles.
When radioactive isotopes are used in medical research work on humans, it is important to make sure that the amount of radioactive material administered to human subjects is as little as possible. In order to achieve this, a very sensitive instrument is necessary to measure the radioactivity of materials. A ‘particle detector’ to measure the ionizing radiation was developed by Geiger and Muller in the year 1928 and they called it a ‘Geiger Muller Counter’ which in short is known as the ‘GM counter.’
In the large and dominant use as a hand-held radiation survey instrument, it would be one of the planet’s renowned radiation detection instruments.
Principle of Geiger Counter
The Geiger counter would contain Geiger-Müller tube, the element of sense that detects the radiation and the electronics that processes that would provide the result.
The Geiger-Müller tube is filled with a gas such as helium, neon, or argon at the pressure being the lowest, where there is an application of high voltage. There would be the conduction of the electrical charge on the tube when a particle or photon of incident radiation would turn the gas conductive by the means of ionization.
Types of Geiger Counter
The Geiger counter is dictated entirely by the design of the tube, can be generally categorised into two types:
• End Window
• Windowless
End Window
This style of the tube would have a small window at one of its ends. This window would be helpful in ionizing particles that could travel easily.
Windowless
As the name suggests, this type of tube would not have any windows and the thickness would be in the range of one to two mm. This type of tube is used for detecting high penetrating radiations.
Geiger Counter Units
The measurement of particles would be in different units, the widely used one of them is the Counts Per Minute (CPM). The measurement of radioactivity would be in micro-(µSv/hr) – Sieverts per hour and (mR/hr)milli-Roentgens per hour.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1097 2021-07-31 00:34:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1075) Split-brain syndrome
Split-brain syndrome, also called callosal disconnection syndrome, condition characterized by a cluster of neurological abnormalities arising from the partial or complete severing or lesioning of the corpus callosum, the bundle of nerves that connects the right and left hemispheres of the brain.
Although it is not fully understood whether the processing of specific tasks is dependent on both hemispheres of the brain, the two hemispheres appear to each have some control over certain tasks. The left hemisphere, for example, is generally responsible for analytical tasks, such as calculating and reading. In many individuals, it is also the dominant centre for speech and language (though the right hemisphere is involved in language processing to a minor extent). In general, the right hemisphere is more efficient at dealing with spatial tasks, such as navigating a maze or reading a map, than the left hemisphere. The two hemispheres, however, routinely communicate with one another through the corpus callosum. This connection further serves as the conduit through which certain sensory signals are transmitted from one side of the body to the contralateral (opposite) side of the brain and through which motor control is effected in the reverse direction (i.e., the right hemisphere controls the left side of the body, and vice versa).
Among the first to characterize split-brain syndrome was American neurobiologist Roger Wolcott Sperry, who in the 1960s studied human split-brain subjects and contributed to the discovery that the left and right hemispheres of the brain carry out specialized duties. For this work, Sperry received a share of the 1981 Nobel Prize for Physiology or Medicine.
Causes Of Split-Brain Syndrome
The primary cause of split-brain syndrome is intentional severing of the corpus callosum, partially or completely, through a surgical procedure known as corpus callosotomy. Rarely performed in the 21st century (having been replaced largely by drug treatments and other procedures), this operation is reserved as a last measure of treatment for extreme and uncontrollable forms of epilepsy in which violent seizures spread from one side of the brain to the other. By preventing the propagation of seizure activity across the hemispheres, corpus callosotomy can greatly improve the patient’s quality of life. However, following the operation, patients develop acute hemispheric disconnection symptoms that last for days or weeks and chronic symptoms that often are permanent.
Less-common causes of split-brain syndrome include stroke, infectious lesion, tumour, or ruptured artery. Many of these events result in varying degrees of spontaneous damage to the corpus callosum. The syndrome can also be caused by multiple sclerosis and in rare instances by agenesis of the corpus callosum, in which the connection fails to develop or develops incompletely. (Lesions in the corpus callosum also occur in patients with Marchiafava-Bignami disease, a rare alcoholism-related condition, but the more global brain damage associated with this disease leads to stupor, seizures, and coma, rather than the features typical of split-brain syndrome.)
Symptoms Of Split-Brain Syndrome
Many patients with split-brain syndrome retain intact memory and social skills. Split-brain patients also maintain motor skills that were learned before the onset of their condition and require both sides of the body; examples include walking, swimming, and biking. They can also learn new tasks that involve either parallel or mirrored movements of their fingers or hands. They cannot, however, learn to perform new tasks that require interdependent movement of each hand, such as learning to play the piano, where both hands must work together to produce the desired music. Eye movements also remain coordinated.
Since information cannot be directly shared between the two hemispheres, split-brain patients display unusual behaviours, particularly concerning speech and object recognition. For instance, when blindfolded a split-brain patient may not be able to name a familiar object that is held in the left hand, because information for the sense of touch is relayed from the left side of the body to the right hemisphere, which typically has a weak language centre. Without an intact corpus callosum, a person cannot access verbal information in the left hemisphere as long as the object remains in the left hand. For the same reason, the patient may have difficulty using the left hand to execute verbal commands; the inability to respond to commands with motor activity is a form of apraxia. To compensate for deficiencies in touch recognition by the left hand and left-hand apraxia, the patient (still blindfolded) may hold the object in the right hand, which relays information to the left hemisphere, providing access to the patient’s dominant verbal bank and enabling him to speak the name of the object. Upon hearing the name of a given object, the patient may also use the left hand to retrieve it; this presumably is because auditory information is processed by both hemispheres. The diffuse nature by which sounds and smells are processed across the brain appears to underlie other problems experienced by split-brain patients. For example, patients are unable to name odours presented to the right nostril, though the left hand can point out the source. Some symptoms of chronic disconnection can improve with time.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1098 2021-08-01 02:18:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1076) Meningitis
Meningitis, inflammation of the meninges, the membranes covering the brain and spinal cord. Meningitis can be caused by various infectious agents, including viruses, fungi, and protozoans, but bacteria produce the most life-threatening forms. The patient usually experiences fever, headache, vomiting, irritability, anorexia, and stiffness in the neck.
Meningococcal Meningitis
The term meningitis is often applied to meningococcal meningitis, which is caused by Neisseria meningitidis, known commonly as meningococcus. Meningococcal meningitis is worldwide in distribution. It is primarily a disease of youth and especially of children under age 10, though all ages may be affected.
Epidemics of meningococcal meningitis took place at irregular intervals, with death occurring in 40–50 percent of cases, until the use of antibiotic drugs greatly reduced both mortality rates and the incidence of the disease in countries worldwide. However, severe epidemics still affect parts of Africa, particularly across north-central sub-Saharan Africa, a region that has become known as the meningitis belt. An epidemic that struck Africa in 1996–97 caused illness in more then 250,000 people and led to some 25,000 deaths. In the early part of the 21st century, devastating outbreaks continued in Africa’s meningitis belt, leading to campaigns for the development of new and effective vaccines and treatments.
There are multiple types, or serogroups, of N. meningitidis that can cause meningococcal meningitis. Serogroups A, B, C, Y, and W-135 are among the most infectious, producing the majority of cases of the disease worldwide. In Africa, serogroup A is responsible for an estimated 90 percent of meningitis outbreaks.
Meningococcal meningitis is usually acquired by nasal droplet transmission. Certain genetic variations, however, have been found to increase susceptibility to the disease. Genes that have been implicated include those designated CFH (complement factor H) and CFHR3 (complement factor H-related 3), which encode proteins that normally are involved in the immune recognition and destruction of invading bacteria. When mutated, however, N. meningitidis escapes recognition by the proteins and thereby is able to cause disease.
Other Bacterial Causes Of Meningitis
Meningitis caused by H. influenzae occurs most often in infants and young children and only rarely in older persons. Its course and symptoms resemble those of N. meningitidis. The bacterium Streptococcus pneumoniae is a common cause of meningitis in adults. In many developing countries, tuberculous meningitis is common.
Various other strains of streptococci, as well as strains of pneumococci and staphylococci, can also cause meningitis. A bacterial infection elsewhere in the body may be carried to the meninges through the bloodstream itself or from an adjacent infected organ, such as the middle ear or the nasal sinuses. The infectious agents multiply in the meninges, where they produce a pus that thickens the cerebrospinal fluid, thereby causing various symptoms and complications such as seizures, deafness, blindness, paralysis, and various degrees of impairment of the intellect.
Course Of The Disease
Bacterial meningitis usually has three main stages. At first, the bacteria multiply in the nasal passages and throat, often causing no painful symptoms. Next, they invade the blood, introducing toxic substances into the circulation and causing fever; if the infection is caused by N. meningitidis, a rash may appear and develop into hemorrhagic spots (petechiae and purpura) in severe cases. In the third stage, the bacteria multiply in the meninges, where they produce intense inflammatory changes and an exudate of pus.
A characteristic of meningitis is the rapid onset of symptoms, which may result in death within only a few hours. The first symptom of meningitis is usually vomiting. A severe bursting headache develops when the meninges have become inflamed and the pressure of the cerebrospinal fluid has increased. Stiffness of the neck then develops, owing to irritation of the spinal nerves supplying those muscles. Deep tendon reflexes are exaggerated, and convulsions may occur in infants and small children. In more severe cases, the cerebrospinal fluid becomes so thickened by pus that the passages between the ventricles (cavities) of the brain and the spaces in the spinal meninges become blocked, causing fluid to accumulate. The accumulation of fluid in the ventricles may in turn result in hydrocephalus, which causes coma and death unless relieved.
Diagnosis And Treatment
Diagnosis of meningitis is made by examination and confirmed by the performance of a test called a spinal tap (or lumbar puncture). In this test a needle is inserted into the lower part of the patient’s back between two vertebrae (bones of the spinal column) and a small sample of cerebrospinal fluid is removed. If a bacterial, tuberculous, or fungal infection is found, patients will need intensive medical care; appropriate antibiotics must be administered as soon as meningitis is suspected. The mortality and morbidity of the bacterial disease are substantial, even with the prompt use of appropriate antibiotic therapy.
The early diagnosis and prompt treatment of meningitis are particularly important in preventing possible permanent damage to the brain, especially in affected children. Meningococcal meningitis is best treated with penicillin. Cases caused by H. influenzae are treated with ampicillin or chloramphenicol. These drugs have reduced mortality rates from bacterial meningitis to less than 5 percent in some areas.
Meningitis Vaccines
Vaccines against some types of N. meningitidis are available. These include serogroup-specific polysaccharide vaccines that may be given in bivalent (A and C), trivalent (A, C, and W-135), or tetravalent (A, C, W-135, and Y) form and that are made from purified complex carbohydrates associated with the outer surface of the bacteria. Because these vaccines do not work in young children, scientists developed meningococcal conjugate vaccines, in which the polysaccharide is attached to a protein to which the developing immune system can respond, resulting in the generation of antibodies against the polysaccharide. Conjugate vaccines that have been developed include a serogroup C conjugate and a tetravalent (A, C, W-135, and Y) conjugate. In 2010 the first conjugate vaccine designed to provide sustained immunity against N. meningitidis serogroup A was developed and made available to people living in Africa’s meningitis belt.
Serogroup B meningococcal vaccines have been tested clinically. These vaccines have been much more difficult to develop, in part because the antigenic carbohydrate is nearly identical to a carbohydrate found in human neurological tissue. In addition, there exists vast genetic variability among the different strains of virulent serogroup B bacteria, and hence no single vaccine is capable of effectively protecting against all potential epidemic strains.
A vaccine that gives protection against the type b strain of H. influenzae became commercially available in the 1980s and has proved effective in safeguarding infants and children from the disease. To control the spread of meningitis caused by H. influenzae or N. meningitis, the antibiotic derivative rifampin should be administered to any who have come in contact with the disease.
Other Forms Of Meningitis
Various other forms of meningitis are caused by viruses and ordinarily have a short, uncomplicated, self-limited course that does not require specific therapy. Patients usually recover in three to five days, typically without any serious result.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1099 2021-08-02 00:18:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1077) Essential tremor
Essential tremor, disorder of the nervous system characterized by involuntary oscillating movements that typically affect the muscles of the arms, hands, face, head, and neck. These involuntary movements often make daily tasks, such as writing, eating, or dressing, difficult. The disorder also may affect the voice and, in rare cases, the legs, sometimes causing difficulty with walking. The tremor is usually absent at rest and is not associated with any other primary symptoms, which distinguishes it from the type of tremor that occurs in Parkinson disease.
Essential tremor is common and affects both men and women. Onset most often occurs in people over age 65, although the disorder can appear in people of all ages. The cause of essential tremor is unknown; however, the disorder does tend to run in families. There are several genetic variations that have been identified in association with essential tremor. The best-characterized variation occurs in a gene known as DRD3 (dopamine receptor 3; formerly designated ETM1, or essential tremor 1). The DRD3 gene encodes a protein called dopamine receptor D3. This receptor binds dopamine, a neurotransmitter that normally inhibits the transmission of nerve impulses in the brain, thereby mediating physical movements. However, the variant DRD3 gene encodes a receptor molecule that alters neuronal response to dopamine, presumably giving rise to the involuntary movements of essential tremor. Variations in a gene called HS1BP3 (HCLS1 binding protein 3) have been identified in association with essential tremor, although the mechanisms by which these variations give rise to the disorder are unclear.
There is no cure for essential tremor; however, there exist a variety of treatments that can be effective in reducing the severity of involuntary movements. In some patients the symptoms of tremors can be controlled through lifestyle and dietary modifications to eliminate stress and the intake of stimulants such as caffeine. Physical therapy can improve muscle control and coordination in the arms and legs of some patients, and speech therapy can alleviate symptoms of mild voice tremor. In cases in which tremor interrupts daily tasks and affects quality of life, drugs or surgery may be necessary to control symptoms. Agents known as beta-blockers (e.g., propanolol), which act on the nervous system to reduce neuronal excitation, are effective treatments, particularly for reducing tremors that affect the hands and voice. In addition, the antiepileptic agent primidone, which reduces neuronal excitation in the brain, is effective in suppressing most symptoms of essential tremor. Patients with essential tremor that does not respond to drug therapy may need surgery, such as deep brain stimulation, to relieve debilitating symptoms; however, because there are dangerous risks associated with brain surgery, it is considered a last resort.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1100 2021-08-03 00:03:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,786
Re: Miscellany
1078) Cephalic disorder
Cephalic disorder, any of several conditions affecting the structure and function of the human brain and central nervous system that are caused by either abnormalities in fetal development or trauma to the fetus. Cephalic disorders affect infants and children worldwide. There often is no effective means of prevention or treatment, and thus the disorders typically are associated with poor outcome. There are many different kinds of cephalic disorders; this article discusses the principal types.
Anencephaly
Anencephaly occurs when significant portions of the brain and skull are missing. The condition results from a failure of the upper region of the neural tube to close in early embryonic development, specifically within the first month of pregnancy. (The neural tube is the primitive structure from which develops the central nervous system.) Females are more likely to be affected than males. Insufficient maternal intake of folic acid is thought to increase the likelihood of anencephaly as well as other neural tube defects. The condition can be diagnosed during pregnancy through blood screening tests and ultrasound, and it is immediately apparent at birth. Infants born with anencephaly die at or soon after birth.
Colpocephaly
Colpocephaly is the enlargement of the occipital horns, which are located at the posterior (rear) end of the lateral ventricles and protrude into the occipital lobe at the back of the brain. Their enlargement is due to insufficient development of the posterior cerebrum (the cerebrum is the largest, uppermost portion of the brain). The precise cause of colpocephaly is unknown, though in the first two trimesters of pregnancy, specifically between months two and six, some anomaly within the uterine environment may precipitate the condition. Children who are born with this malformation are affected by microcephaly, intellectual disability, muscle spasms, and seizures. The prognosis with colpocephaly is directly correlated with the degree of abnormality.
Holoprosencephaly
Holoprosencephaly results from the failed development of the prosencephalon (forebrain) in the embryo. The prosencephalon fails to divide and form the two cerebral hemispheres, resulting in defects in both the brain and the face. About one-half of holoprosencephaly cases are caused by chromosomal defects, such as trisomy 13 (Patau syndrome) and trisomy 18 (Edwards syndrome). The clinical presentation of this disorder falls along a broad spectrum, with defects as minor as a cleft lip or as severe as cyclopia (one eye). One risk factor for the development of holoprosencephaly is birth to a diabetic mother.
Hydranencephaly
Hydranencephaly is a form of porencephaly in which the brain lacks cerebral hemispheres and instead is occupied by cerebrospinal fluid (CSF)-filled sacs. The condition develops after the 12th week of pregnancy and is caused by a stroke or other vascular event, by an injury, or by an infection. Although the infant may appear normal at birth, after a few weeks he or she demonstrates irritability and hypertonia (excessive muscle tone). Seizures and hydrocephalus, in which CFS accumulates in the brain, leading to enlargement of the head, develop after several months. Death typically occurs during the first year of life, although reports exist of patients surviving to age 10 or even 20.
Iniencephaly
Iniencephaly is evident when the head is tilted back, in an extreme retroflexed position, such that an infant is born with the face looking upward; in most cases, the neck is apparently absent. The disorder is accompanied by severe spinal defects and often brain and face malformations, such as anencephaly and cyclopia. Iniencephaly is a neural tube defect. The skin of the face and chest is continuous, as is the skin of the scalp and back. Females are affected more than males. These infants tend to survive for only a few hours after birth.
Lissencephaly
Lissencephaly means “smooth brain.” The normal brain surface has many folds and grooves (gyri and sulci), but a brain affected by lissencephaly does not; the folds may be incomplete or entirely absent. Lissencephaly is further characterized by microcephaly (reduced head size) and by symptoms such as muscle spasms, seizures, abnormal facial expressions, failure to thrive, and deformation of the hands, fingers, or toes. Lissencephaly is caused by abnormalities in the migration of neurons in embryonic development, which in turn may be caused by genetic mutations, viral infection of the fetus or uterus, or insufficient delivery of blood to the embryonic brain. A number of different forms of lissencephaly have been recognized, which cover a spectrum of severity. Examples of several types include Miller-Dieker syndrome, isolated lissencephaly sequence, and Walker-Warburg syndrome. With intensive treatment, some affected infants can survive well into childhood; however, many die before age 10.
Megalencephaly
Megalencephaly, or macrencephaly, is characterized by a large and heavy brain, abnormally so for the child’s sex and weight for age (usually a brain weight greater than 2.5 standard deviations over the mean). The condition appears to be associated with defects in the mechanisms that regulate the growth and production of cells in the brain, resulting in increased cell size and cell number. The head may be enlarged at birth, or it may become enlarged as the child’s development progresses. In the latter case, megalencephaly may occur as part of another disorder, such as leukodystrophy or neurofibromatosis. Children with megalencephaly experience brain malfunction, and they typically are affected by developmental delays and seizures. The disorder is three to four times more common in males than females.
Microcephaly
Microcephaly means “small head”; it is defined formally as a head circumference that is smaller than two standard deviations from the average for sex and age. It can be caused by a variety of factors that affect the mother during pregnancy, including exposure to chemicals or radiation, infections, diabetes, vitamin and nutrient deficiency, or drug or alcohol abuse. The most frequent causes, however, are chromosomal abnormalities that result in an inappropriately developing brain. Along with a small head circumference, patients with microcephaly can experience developmental delay as well as a variety of neurological disorders, including seizures and problems with balance and coordination. Some affected children experience only mild symptoms and otherwise develop normally.
Porencephaly
Porencephaly occurs when CSF-filled cysts or cavities form in the cerebral hemispheres. The condition is very rare and can manifest before birth, when caused by inherited defects in brain development in the prenatal period, or after birth, by destruction of brain tissue by stroke or infection in infancy. Signs and symptoms of porencephaly include developmental delay, including delayed growth and speech development; seizures; incomplete paralysis; hypotonia (reduced muscle tone) with spastic muscle contraction; and hydrocephalus, microcephaly, or macrocephaly (an enlarged head). Severely affected individuals often do not survive to adulthood.
Schizencephaly
Schizencephaly is a type of porencephaly in which slits (clefts) develop in the cerebral hemispheres. Genetic abnormalities appear to play a role in at least one form of the disorder. Maternal factors, such as the use of certain medications or contact with certain toxins while pregnant, may also cause schizencephaly. The underlying cellular defects appear to be related to improper neuronal migration during early prenatal development. Patients with schizencephaly that affects the entire brain (with bilateral slits) typically suffer from developmental delay, whereas patients who are affected on only one side of the brain may have unilateral (one-sided) weakness but potentially normal intelligence.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
