Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Anode
Pages: 1
#1 Yesterday 16:35:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Anode
Anode
Gist
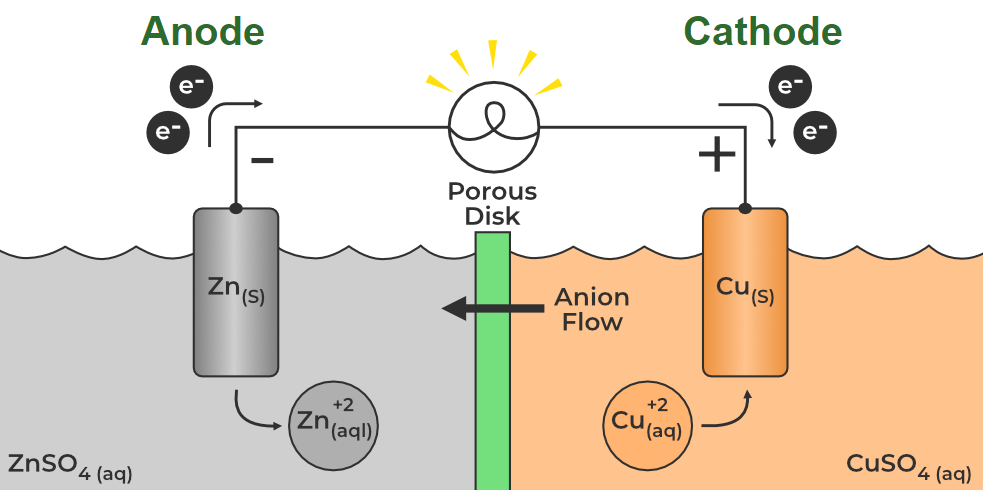
An anode is an electrode where oxidation (loss of electrons) occurs, and it's the point where conventional current enters an electrical device, though its charge can be positive or negative depending on the cell type. In batteries, it's the negative terminal during discharge (releasing electrons) but becomes positive during charging as an external voltage pulls electrons away, forcing oxidation.
Anode Rays are also called a positive ray or a canal ray. Anode rays consist of a positive charge. So, they tilt next to perforated cathode, which consists of negative charge. Moreover, it passes through the anode rays. They pass next by canals or holes which further produce fluorescence.
Summary
An anode is a negative electrode (or negative terminal) and one of the essential parts of a battery. The anode is usually made of a metal that oxidizes and sends electrons to the cathode (the positive electrode). This electrochemical reaction produces electrons (i.e., electricity).
How Does an Anode Work?
An anode is an oxidizing metal, such as zinc or lithium, which means it loses electrons, making it negatively charged. It resides in an electrolyte solvent and slowly erodes as electrons move along a conductor to the cathode.
The conductor (whether a metal wire or tube) is how we access the electricity the anode makes and, ultimately, how a battery powers electronic devices. Once the anode completely erodes, the battery dies (or loses charge).
Common Anode Materials
Household (alkaline) batteries typically have a zinc anode, while lithium-ion batteries usually have a graphite anode. Other metals, including lithium and platinum, are also used as anodes in various battery chemistries. A suitable anode should be an efficient reducing agent, have good conductivity and stability, and have a high coulombic output (the electrical energy output).
Details
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for anode current into device. The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a + is the cathode (while discharging).
In both a galvanic cell and an electrolytic cell, the anode is the electrode at which the oxidation reaction occurs. In a galvanic cell, the anode is the wire or plate having excess negative charge as a result of the oxidation reaction. In an electrolytic cell, the anode is the wire or plate upon which excess positive charge is imposed.[2] As a result of this, anions will tend to move towards the anode, where they will undergo oxidation.
Historically, the anode of a galvanic cell was also known as the zincode because it was usually composed of zinc.
Charge flow
The terms anode and cathode are not defined by the voltage polarity of electrodes, but are usually defined by the direction of current through the electrode. An anode usually is the electrode of a device through which conventional current (positive charge) flows into the device from an external circuit, while a cathode usually is the electrode through which conventional current flows out of the device.
In general, if the current through the electrodes reverses direction, as occurs, for example, in a rechargeable battery when it is being charged, the roles of the electrodes as anode and cathode are reversed. However, the definition of anode and cathode is different for electrical devices such as diodes and vacuum tubes where the electrode naming is fixed and does not depend on the actual charge flow (current). These devices usually allow substantial current flow in one direction but negligible current in the other direction. Therefore, the electrodes are named based on the direction of this forward current. In a diode, the anode is the terminal through which current enters and the cathode is the terminal through which current leaves, when the diode is forward biased. The names of the electrodes do not change in cases where reverse current flows through the device. Similarly, in a vacuum tube, only one electrode can thermionically emit electrons into the evacuated tube, so electrons can only enter the device from the external circuit through the heated electrode. Therefore, this electrode is permanently named the cathode, and the electrode through which the electrons exit the tube is named the anode.
Conventional current depends not only on the direction the charge carriers move, but also the carriers' electric charge. The currents outside the device are usually carried by electrons in a metal conductor. Since electrons have a negative charge, the direction of electron flow is opposite to the direction of conventional current. Consequently, electrons leave the device through the anode and enter the device through the cathode.
Additional Information:
Difference Between Anode and Cathode
Here are some key differences between cathode and anode.
Anode : Cathode
* The anode is the electrode where electricity moves into.
* The cathode is the electrode where electricity is given out or flows out.
* The anode is usually the positive side.
* A cathode is a negative side.
* It acts as an electron donor.
* It acts as an electron acceptor.
* In an electrolytic cell, oxidation reaction takes place at the anode.
* In an electrolytic cell, a reduction reaction takes place at the cathode.
* In galvanic cells, an anode can become a cathode.
* In galvanic cells, a cathode can become an anode.
Frequently Asked Questions on Cathode and Anode
Q1 : What is the charge of an anode and cathode?
A1 : The anode is regarded as negative in a galvanic (voltaic) cell and the cathode is deemed positive. This seems appropriate because the anode is the origin of electrons and where the electrons flow is the cathode.
Q2 : Does oxidation occur at the anode or cathode?
A2 : The anode is where the response to oxidation occurs. That’s where the metal loses electrons, in other words.
Q3 : What is the charge on anode and cathode?
A3 : There is an oxidation response at the anode. The oxidized species would lose electrons, leaving this electrode with an accumulation of electrons. Therefore, the anode is charged negatively. In contrast to the cathode, there is a reduction response where the decreased species would obtain electrons. Therefore, the electrode, i.e. the cathode, lacks electrons and is therefore charged positively.
Q4 : Are cations positive or negative?
A4 : A cation is defined as a positively charged ion or an atom that has lost an electron.
Q5 : What are the materials used for anode and cathode?
A5 : Metals like zinc and lithium are often used as substrates for anodes.
Q6 : What is anode and cathode in corrosion?
A6 : Iron metal functions as the anode in a galvanic cell during the corrosion phase and is oxidized to Fe2+; at the cathode, oxygen is decreased to water.
Q7 : Does reduction always occur at the cathode?
A7 : Reduction at the cathode always happens, and oxidation at the anode always happens. Because decrease is the addition of electrons.
Q8 : Is LED cathode positive or negative?
A8 : LEDs are generally labelled in some way by their cathode. The cathode should be linked to the driving voltage source’s floor or adverse side and the anode to the positive side.
Q9 : Do electrons always flow from an anode to a cathode?
A9 : Yes, electrons always flow from an anode to a cathode or from the oxidation half cell to the reduction half cell.
Q10 : What is the primary goal of a salt bridge?
A 10 : The primary goal of a salt bridge is to maintain the electrical neutrality of the cell and minimise the liquid junction potential.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Anode