Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Density
Pages: 1
#1 Yesterday 16:16:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,792
Density
Density
Gist
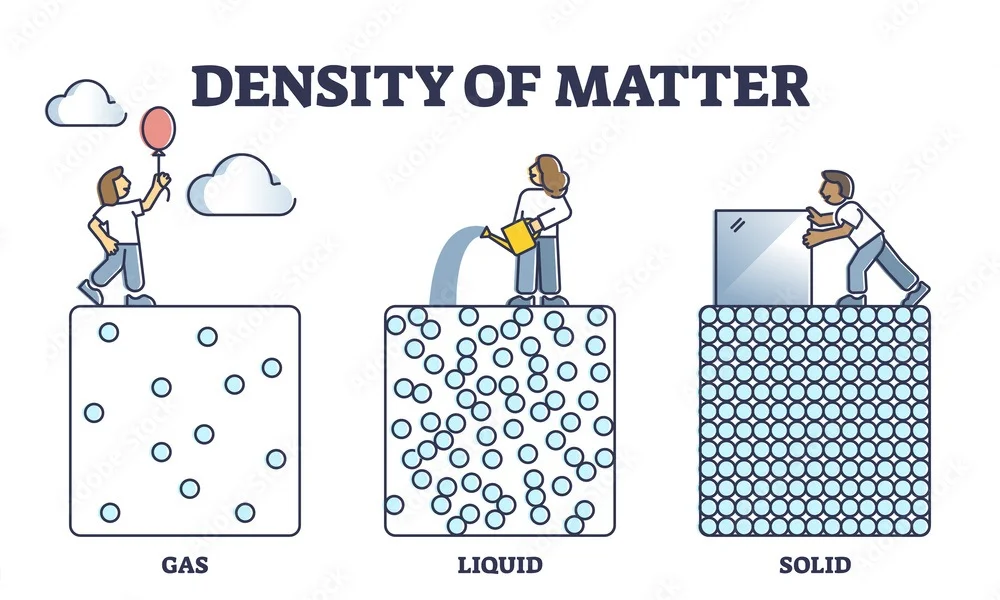
Density is the measure of how much mass is packed into a given volume, calculated by dividing an object's mass by its volume, with common units being kilograms per cubic meter (kg/m^3) or grams per cubic centimeter (g/cm³). It tells you how compact or heavy a substance feels for its size, with denser materials like lead having tightly packed particles, while less dense materials like Styrofoam have sparse particles and can even float on denser liquids, as seen with pumice.
In simple words, density is how much "stuff" (mass) is packed into a certain amount of space (volume). Think of it as how compact or heavy something feels for its size: a brick is dense (lots of mass in a small space), while a sponge is not very dense (less mass in the same space).
Summary
Density is mass of a unit volume of a material substance. The formula for density is d = M/V, where d is density, M is mass, and V is volume. Density is commonly expressed in units of grams per cubic centimetre. For example, the density of water is 1 gram per cubic centimetre, and Earth’s density is 5.51 grams per cubic centimetre. Density can also be expressed as kilograms per cubic metre (in metre-kilogram-second or SI units). For example, the density of air is 1.2 kilograms per cubic metre. The densities of common solids, liquids, and gases are listed in textbooks and handbooks. Density offers a convenient means of obtaining the mass of a body from its volume or vice versa; the mass is equal to the volume multiplied by the density (M = Vd), while the volume is equal to the mass divided by the density (V = M/d). The weight of a body, which is usually of more practical interest than its mass, can be obtained by multiplying the mass by the acceleration of gravity. Tables that list the weight per unit volume of substances are also available; this quantity has various titles, such as weight density, specific weight, or unit weight. See also specific gravity. The expression particle density refers to the number of particles per unit volume, not to the density of a single particle, and it is usually expressed as n.
Density applications are widespread, from making ships float (by controlling overall density with air) and submarines dive (filling ballast tanks with water) to separating oil from water and identifying substances. It's crucial in engineering for building design, determining fluid behavior (buoyancy, aerodynamics), creating life vests (low density), and even in medical diagnostics and quality control for fuels and beverages, helping to determine purity and composition.
Details
Density is mass per unit volume.
For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure.
To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative density" or "specific gravity", i.e. the ratio of the density of the material to that of a standard material, usually water. Thus a relative density less than one relative to water means that the substance floats in water.
The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases. Increasing the pressure on an object decreases the volume of the object and thus increases its density. Increasing the temperature of a substance while maintaining a constant pressure decreases its density by increasing its volume (with a few exceptions). In most fluids, heating the bottom of the fluid results in convection due to the decrease in the density of the heated fluid, which causes it to rise relative to denser unheated material.
The reciprocal of the density of a substance is occasionally called its specific volume, a term sometimes used in thermodynamics. Density is an intensive property in that increasing the amount of a substance does not increase its density; rather it increases its mass.
Other conceptually comparable quantities or ratios include specific density, relative density (specific gravity), and specific weight.
The concept of mass density is generalized in the International System of Quantities to volumic quantities, the quotient of any physical quantity and volume, such as charge density or volumic electric charge.
Additional Information
Density is a measurement that compares the amount of matter an object has to its volume. An object with much matter in a certain volume has high density. An object with little matter in the same amount of volume has a low density. Density is found by dividing the mass of an object by its volume:
rho = m/V
where ρ is the density, m is the mass, and V is the volume.
Changes of density
In general, density can be changed by changing either the pressure or the temperature. Increasing the pressure always increases the density of a material. Increasing the temperature usually lowers the density, but there are exceptions. For example, the density of water increases slightly between its melting point at 0 °C and 4 °C. When water freezes, it expands by about 9% in volume, making ice that is less dense than liquid water. Water expands as it drops below 4 °C.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Density