Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 Yesterday 18:48:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Covalent Bond
Covalent Bond
Gist
A covalent bond is a chemical bond where atoms share a pair of electrons to achieve a more stable configuration. This sharing creates an attractive force between the nuclei of the atoms and the shared electrons, holding the atoms together in a molecule. Covalent bonds typically form between nonmetals, and the result is either a small molecule with low melting and boiling points or a giant covalent structure with high melting and boiling points.
A covalent bond is a chemical bond formed when two atoms share a pair of electrons to achieve stability. This sharing allows atoms to fill their outer electron shells, and the bond is the force of attraction between the nuclei of both atoms and the shared electrons. Covalent bonds typically form between two nonmetal atoms.
Summary
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding.
Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase covalent bond to 1939, recognizing its first known use. The prefix co- (jointly, partnered) indicates that "co-valent" bonds involve shared "valence", as detailed in valence bond theory.
In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding. Covalency is greatest between atoms that have similar electronegativities, regardless of whether the elements are the same as each other. Covalent bonding that entails the sharing of electrons over more than two atoms is said to be delocalized.
Details
A covalent bond, in chemistry, is the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same electrons. A covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms.
A brief treatment of covalent bonds follows. For full treatment, see chemical bonding: Covalent bonds.
Molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (H2, N2, Cl2, H2O, NH3) together with all organic compounds.
A single line indicates a bond between two atoms (i.e., involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e., involving two electron pairs), and triple lines (≡) represent a triple bond, as found, for example, in carbon monoxide (C≡O). Single bonds consist of one sigma (σ) bond, double bonds have one σ and one pi (π) bond, and triple bonds have one σ and two π bonds.
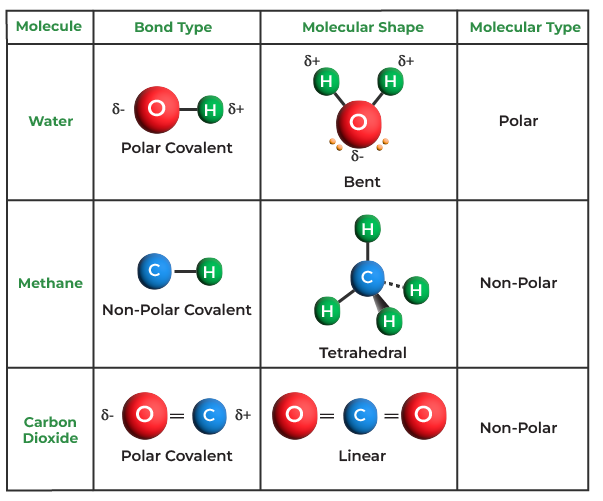
Covalent bonds are directional, meaning that atoms so bonded prefer specific orientations relative to one another; this in turn gives molecules definite shapes, as in the angular (bent) structure of the H2O molecule. Covalent bonds between identical atoms (as in H2) are nonpolar—i.e., electrically uniform—while those between unlike atoms are polar—i.e., one atom is slightly negatively charged and the other is slightly positively charged. This partial ionic character of covalent bonds increases with the difference in the electronegativities of the two atoms. See also ionic bond.
When none of the elements in a compound is a metal, no atoms in the compound have an ionization energy low enough for electron loss to be likely. In such a case, covalence prevails. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Molecules of identical atoms, such as H2 and buckminsterfullerene (C60), are also held together by covalent bonds.
Lewis formulation of a covalent bond
The idea that two electrons can be shared between two atoms and serve as the link between them was first introduced in 1916 by the American chemist G.N. Lewis, who described the formation of such bonds as resulting from the tendencies of certain atoms to combine with one another in order for both to have the electronic structure of a corresponding noble-gas atom.
In Lewis terms a covalent bond is a shared electron pair.
In a Lewis structure of a covalent compound, the shared electron pair between the hydrogen and chlorine ions is represented by a line. The electron pair is called a bonding pair; the three other pairs of electrons on the chlorine atom are called lone pairs and play no direct role in holding the two atoms together.
Each atom in the hydrogen chloride molecule attains a closed-shell octet of electrons by sharing and hence achieves a maximum lowering of energy. In general, an incomplete shell means that some attracting power of a nucleus may be wasted, and adding electrons beyond a closed shell would entail the energetic disadvantage of beginning the next shell of the atom concerned. Lewis’s octet rule is again applicable and is seen to represent the extreme means of achieving lower energy rather than being a goal in itself.
A covalent bond forms if the bonded atoms have a lower total energy than the widely separated atoms. The simplest interpretation of the decrease in energy that occurs when electrons are shared is that both electrons lie between two attracting centres (the nuclei of the two atoms linked by the bond) and hence lie lower in energy than when they experience the attraction of a single centre.
Lewis structures of more complex molecules can be constructed quite simply by extending the process that has been described for hydrogen chloride.
In some older formulations of Lewis structures, a distinction was made between bonds formed by electrons that have been supplied by both atoms (as in H―Cl, where one shared electron can be regarded as supplied by the hydrogen atom and the other by the chlorine atom) and covalent bonds formed when both electrons can be regarded as supplied by one atom, as in the formation of OH− from O2− and H+. Such a bond was called a coordinate covalent bond or a dative bond and symbolized O → H−. However, the difficulties encountered in the attempt to keep track of the origin of bonding electrons and the suggestion that a coordinate covalent bond differs somehow from a covalent bond (it does not) have led to this usage falling into disfavour.
Resonance
The blending together of these structures is actually a quantum mechanical phenomenon called resonance. At this stage, resonance can be regarded as a blending process that spreads double-bond character evenly over the atoms that participate in it. In ozone, for instance, each oxygen-oxygen bond is rendered equivalent by resonance, and each one has a mixture of single-bond and double-bond character (as indicated by its length and strength).
Hypervalence
Lewis structures and the octet rule jointly offer a succinct indication of the type of bonding that occurs in molecules and show the pattern of single and multiple bonds between the atoms. There are many compounds, however, that do not conform to the octet rule. The most common exceptions to the octet rule are the so-called hypervalent compounds. These are species in which there are more atoms attached to a central atom than can be accommodated by an octet of electrons.
In Lewis terms, hypervalence requires the expansion of the octet to 10, 12, and even in some cases 16 electrons. Hypervalent compounds are very common and in general are no less stable than compounds that conform to the octet rule.
The existence of hypervalent compounds would appear to deal a severe blow to the validity of the octet rule and Lewis’s approach to covalent bonding if the expansion of the octet could not be rationalized or its occurrence predicted. Fortunately, it can be rationalized, and the occurrence of hypervalence can be anticipated. In simple terms, experience has shown that hypervalence is rare in periods 1 and 2 of the periodic table (through neon) but is common in and after period 3. Thus, the octet rule can be used with confidence for carbon, nitrogen, oxygen, and fluorine, but hypervalence must be anticipated thereafter. The conventional explanation of this distinction takes note of the fact that in period-3 elements the valence shell has n = 3, and this is the first shell in which d orbitals are available. (These orbitals are occupied after the 4s orbitals have been filled and account for the occurrence of the transition metals in period 4.) It is therefore argued that atoms of this and subsequent periods can use the empty d orbitals to accommodate electrons beyond an octet and hence permit the formation of hypervalent species.
In chemistry, however, it is important not to allow mere correlations to masquerade as explanations. Although it is true that d orbitals are energetically accessible in elements that display hypervalence, it does not follow that they are responsible for it. Indeed, quantum mechanical theories of the chemical bond do not need to invoke d-orbital involvement. These theories suggest that hypervalence is probably no more than a consequence of the greater radii of the atoms of period-3 elements compared with those of period 2, with the result that a central atom can pack more atoms around itself. Thus, hypervalence is more a steric (geometric) problem than an outcome of d-orbital availability.
Additional Information:
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. By sharing their outer most (valence) electrons, atoms can fill up their outer electron shell and gain stability. Nonmetals will readily form covalent bonds with other nonmetals in order to obtain stability, and can form anywhere between one to three covalent bonds with other nonmetals depending on how many valence electrons they posses. Although it is said that atoms share electrons when they form covalent bonds, they do not usually share the electrons equally.
Introduction
Only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher electronegativity resulting in a polar covalent bond. When compared to ionic compounds, covalent compounds usually have a lower melting and boiling point, and have less of a tendency to dissolve in water. Covalent compounds can be in a gas, liquid, or solid state and do not conduct electricity or heat well. The types of covalent bonds can be distinguished by looking at the Lewis dot structure of the molecule. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. A pair of electrons that is shared between two atoms is called a bond pair. A pair of electrons that is not shared between two atoms is called a lone pair.
Octet Rule
The Octet Rule requires all atoms in a molecule to have 8 valence electrons--either by sharing, losing or gaining electrons--to become stable. For Covalent bonds, atoms tend to share their electrons with each other to satisfy the Octet Rule. It requires 8 electrons because that is the amount of electrons needed to fill a s- and p- orbital (electron configuration); also known as a noble gas configuration. Each atom wants to become as stable as the noble gases that have their outer valence shell filled because noble gases have a charge of 0. Although it is important to remember the "magic number", 8, note that there are many Octet rule exceptions.
Single Bonds
A single bond is when two electrons--one pair of electrons--are shared between two atoms. It is depicted by a single line between the two atoms. Although this form of bond is weaker and has a smaller density than a double bond and a triple bond, it is the most stable because it has a lower level of reactivity meaning less vulnerability in losing electrons to atoms that want to steal electrons.
Double Bonds
A Double bond is when two atoms share two pairs of electrons with each other. It is depicted by two horizontal lines between two atoms in a molecule. This type of bond is much stronger than a single bond, but less stable; this is due to its greater amount of reactivity compared to a single bond.
Triple Bond
A Triple bond is when three pairs of electrons are shared between two atoms in a molecule. It is the least stable out of the three general types of covalent bonds. It is very vulnerable to electron thieves!
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1