Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Berkelium
Pages: 1
#1 Yesterday 21:42:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,932
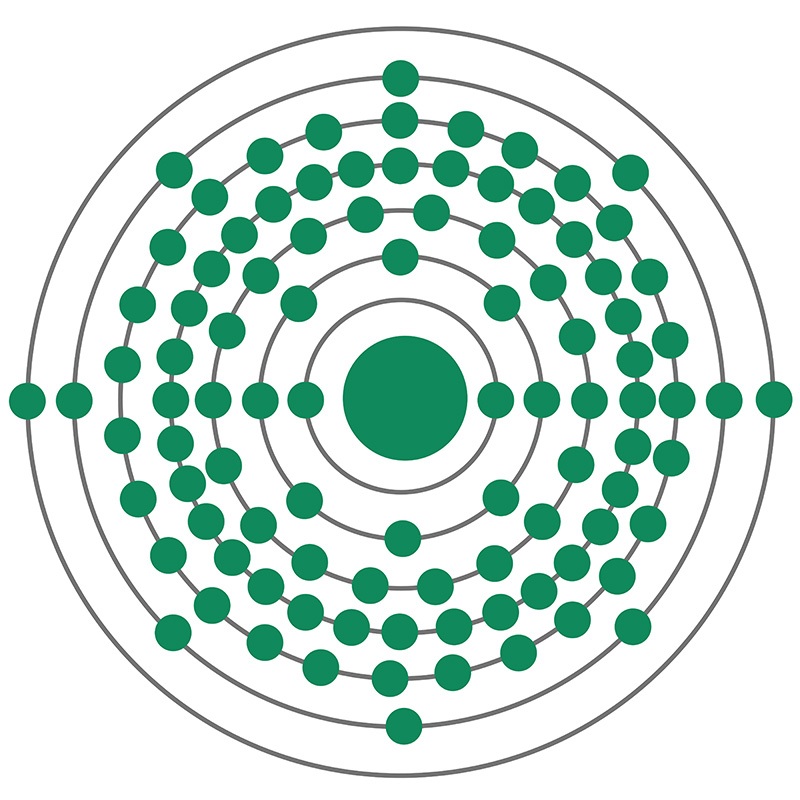
Berkelium
Berkelium
Gist
Berkelium (Bk) is a radioactive, synthetic chemical element with atomic number 97, discovered in 1949 at the Lawrence Berkeley National Laboratory and named after the city of Berkeley, California. It is a member of the actinide series and is produced artificially in nuclear reactors or particle accelerators. While not used for technological or biological purposes, berkelium's isotopes are used for basic scientific research and in the creation of heavier elements, though only very small quantities are available.
Berkelium has no commercial or technological uses due to its extreme rarity, high radioactivity, and toxicity. Its primary and only use is as a target material in basic scientific research to synthesize superheavy elements, such as Tennessine, and for studying the chemistry of heavier transuranium elements.
Summary
Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.
The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The longest-lived and second-most important isotope, 247Bk, can be synthesized via irradiation of 244Cm with high-energy alpha particles.
Just over one gram of berkelium has been produced in the United States since 1967. There is no practical application of berkelium outside scientific research which is mostly directed at the synthesis of heavier transuranium elements and superheavy elements. A 22-milligram batch of berkelium-249 was prepared during a 250-day irradiation period and then purified for a further 90 days at Oak Ridge in 2009. This sample was used to synthesize the new element tennessine for the first time in 2009 at the Joint Institute for Nuclear Research, Russia, after it was bombarded with calcium-48 ions for 150 days. This was the culmination of the Russia–US collaboration on the synthesis of the heaviest elements on the periodic table.
Berkelium is a soft, silvery-white, radioactive metal. The berkelium-249 isotope emits low-energy beta particles and thus is relatively safe to handle. It decays with a half-life of 330 days to californium-249, which is a strong emitter of ionizing alpha particles. This gradual transmutation is an important consideration when studying the properties of elemental berkelium and its chemical compounds, since the formation of californium brings not only chemical contamination, but also free-radical effects and self-heating from the emitted alpha particles.
Details
Berkelium (Bk) is a synthetic chemical element of the actinoid series of the periodic table, atomic number 97. Not occurring in nature, berkelium (as the isotope berkelium-243) was discovered in December 1949 by American chemists Stanley G. Thompson, Albert Ghiorso, and Glenn T. Seaborg at the University of California, Berkeley, as a product resulting from the helium-ion (alpha-particle) bombardment of americium-241 (atomic number 95) in a 152-cm (60-inch) cyclotron. The element was named after the city of Berkeley, where it was discovered.
All berkelium isotopes are radioactive; berkelium-247 is the longest-lived (1,380-year half-life). Berkelium-249 (330-day half-life) has been widely used in the chemical studies of the element because it can be produced in weighable amounts that are isotopically pure by nuclear reactions beginning with curium-244. The only use of berkelium has been in the synthesis of heavier elements such as tennessine. Metallic berkelium has been prepared; it is electropositive, reactive, and silver-coloured like the other actinoid metals, with a specific gravity of 14.8.
Tracer chemical investigations have shown that berkelium exists in aqueous solutions in the +3 and +4 oxidation states, presumably as Bk3+ and Bk4+ ions. The solubility properties of berkelium in its two oxidation states are entirely analogous to those of the other actinoids and to the lanthanoid elements (especially cerium) in the corresponding oxidation states. Solid compounds, including the oxides BkO2 and Bk2O3 and the trihalides such as the trichloride BkCl3, have been synthesized on the submicrogram scale.
Element Properties
atomic number : 97
stablest isotope : 247
oxidation states : +3, +4.
Additional Information:
Appearance
Berkelium is a radioactive, silvery metal.
Uses
Because it is so rare, berkelium has no commercial or technological use at present.
Biological role
Berkelium has no known biological role. It is toxic due to its radioactivity.
Natural abundance
Less than a gram of berkelium is made each year. It is made in nuclear reactors by the neutron bombardment of plutonium-239.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Berkelium