Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-10-01 19:38:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,197
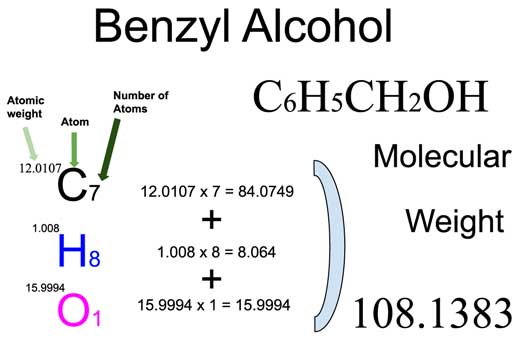
Benzyl Alcohol
Benzyl Alcohol
Gist
Benzyl alcohol, or phenylmethanol (C6H5CH2OH), is a colorless liquid with a mild, aromatic odor, naturally occurring in plants and used in cosmetics and pharmaceuticals as a solvent, preservative, and viscosity-reducing agent. While it has low toxicity in small amounts and is approved for food contact plastics, high concentrations can cause skin and eye irritation. In neonates, however, it can lead to a severe and potentially fatal "gasping baby syndrome" if administered in high doses.
Benzyl alcohol is a versatile chemical used as a preservative in cosmetics and pharmaceuticals to prevent contamination, a solvent in paints and inks, a local anesthetic for pain relief, and an active ingredient in lice treatments by suffocating lice. It also functions as a flavoring agent in some foods and a precursor in industrial applications for creating ethers and esters.
Summary
Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.
Natural occurrences
Benzyl alcohol is produced naturally by many plants and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth and ylang-ylang. It is also found in castoreum from the castor sacs of beavers. Benzyl esters also occur naturally.
Preparation
Benzyl alcohol is produced industrially from toluene via benzyl chloride, which is hydrolyzed.
Another route entails hydrogenation of benzaldehyde, a by-product of the oxidation of toluene to benzoic acid.
For laboratory use, Grignard reaction of phenylmagnesium bromide (C6H5MgBr) with formaldehyde and the Cannizzaro reaction of benzaldehyde also give benzyl alcohol. The latter also gives benzoic acid, an example of an organic disproportionation reaction.
Details
Although available for some years as an over-the-counter health product, benzyl alcohol was approved in 2003 by the Food and Drug Administration (FDA) as a new prescription drug for the treatment of head lice. Unlike typical pediculicides such as permethrin and lindane which act through a neurotoxic mode of action, benzyl alcohol is thought to operate via a unique mechanism involving physical pulmonary asphyxiation. The presence of benzyl alcohol in such a wide range of consumer products is explained by its bacteriostatic and antiseptic properties in conjunction with its comparatively modest toxicity.
Outside of its natural occurrence in foods (apricots, cranberries, and cocoa), manufactured benzyl alcohol is used as a flavor-enhancing solvent, and can be used in baked goods, liqueurs and wines. In commercially manufactured cosmetics and skin products, benzyl alcohol is frequently used as a preservative due to its ability to kill microbes – especially parasites. In 1998, the FDA reported benzyl alcohol to be present in 322 cosmetic formulations belonging to 43 cosmetic-product categories.
Additionally, benzyl alcohol is used in photographic development. Aside from developing color movie films, as a solvent, benzyl alcohol is a component of inks, paints and epoxy resin coatings. It is an indirect food additive for use as a component of resinous and polymeric coatings.
Additional Information
Benzyl alcohol is an organic compound, of molecular formula C6H5CH2OH, that occurs combined with carboxylic acids (as esters) in balsams and oils of jasmine and other flowers. Several of its natural and synthetic esters have long been used in perfumery; the alcohol itself has become important in the second half of the 20th century as a developer booster in the processing of colour motion-picture film and as a dyeing assistant for filament nylons. Benzyl alcohol is manufactured by the hydrolysis of benzyl chloride in the presence of soda ash.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
Pages: 1