Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Thallium
Pages: 1
#1 Today 13:41:24
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,669
Thallium
Thallium
Gist
Thallium (Tl) is a highly toxic, silvery-white post-transition metal, atomic number 81, found in trace amounts in the Earth's crust and sulfide ores. Although once used in rodenticides and some medical applications, its use has been largely banned due to severe poisoning risks, mimicking symptoms of other diseases, and potential for criminal misuse. Thallium poisoning can be treated with Prussian Blue, and the element is still used in some specialized electronic and optical applications.
Thallium blood concentration levels are normal below 2 µg/L, and toxic at concentrations greater than 200 µg/L. Long-term effects of thallium exposure can include difficulty walking, various involuntary movement disorders, and impairment of thought and mood. Neurological damage resolves slowly and may be permanent.
Summary
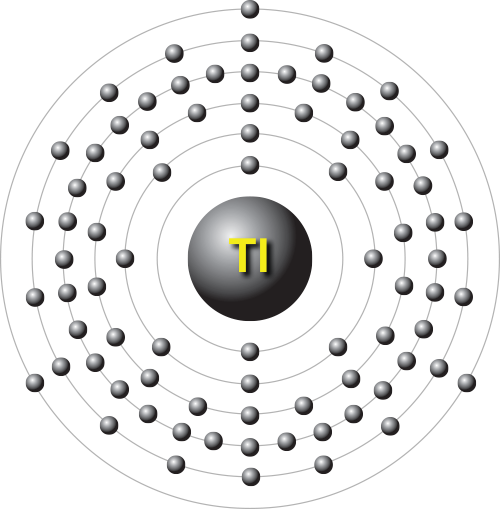
Thallium is a chemical element; it has symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently, in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862, Lamy by electrolysis and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the International Exhibition, which opened on 1 May that year.
Thallium tends to form the +3 and +1 oxidation states. The +3 state resembles that of the other elements in group 13 (boron, aluminium, gallium, indium). However, the +1 state, which is far more prominent in thallium than the elements above it, recalls the chemistry of alkali metals and thallium(I) ions are found geologically mostly in potassium-based ores and (when ingested) are handled in many ways like potassium ions (K+) by ion pumps in living cells.
Commercially, thallium is produced not from potassium ores, but as a byproduct from refining of heavy-metal sulfide ores. Approximately 65% of thallium production is used in the electronics industry and the remainder is used in the pharmaceutical industry and in glass manufacturing. It is also used in infrared detectors. The radioisotope thallium-201 (as the soluble chloride TlCl) is used in small amounts as an agent in a nuclear medicine scan, during one type of nuclear cardiac stress test.
Soluble thallium salts (many of which are nearly tasteless) are highly toxic and they were historically used in rat poisons and insecticides. Because of their nonselective toxicity, use of these compounds has been restricted or banned in many countries. Thallium poisoning usually results in hair loss.
Details
Thallium (Tl) is a chemical element, metal of main Group 13 (IIIa, or boron group) of the periodic table, poisonous and of limited commercial value. Like lead, thallium is a soft, low-melting element of low tensile strength. Freshly cut thallium has a metallic lustre that dulls to bluish gray upon exposure to air. The metal continues to oxidize upon prolonged contact with air, generating a heavy nonprotective oxide crust. Thallium dissolves slowly in hydrochloric acid and dilute sulfuric acid and rapidly in nitric acid.
Rarer than tin, thallium is concentrated in only a few minerals that have no commercial value. Trace amounts of thallium are present in sulfide ores of zinc and lead; in the roasting of these ores, the thallium becomes concentrated in the flue dusts, from which it is recovered.
British chemist Sir William Crookes discovered (1861) thallium by observing the prominent green spectral line generated by selenium-bearing pyrites that had been used in the manufacture of sulfuric acid. Crookes and French chemist Claude-Auguste Lamy independently isolated (1862) thallium, showing it to be a metal.
Two crystalline forms of the element are known: close-packed hexagonal below about 230 °C (450 °F) and body-centred cubic above. Natural thallium, the heaviest of the boron group elements, consists almost entirely of a mixture of two stable isotopes: thallium-203 (29.5 percent) and thallium-205 (70.5 percent). Traces of several short-lived isotopes occur as decay products in the three natural radioactive disintegration series: thallium-206 and thallium-210 (uranium series), thallium-208 (thorium series), and thallium-207 (actinium series).
Thallium metal has no commercial use, and thallium compounds have no major commercial application, since thallous sulfate was largely replaced in the 1960s as a rodenticide and insecticide. Thallous compounds have a few limited uses. For example, mixed bromide-iodide crystals (TlBr and TlI) that transmit infrared light have been fabricated into lenses, windows, and prisms for infrared optical systems. The sulfide (Tl2S) has been employed as the essential component in a highly sensitive photoelectric cell and the oxysulfide in an infrared-sensitive photocell (thallofide cell). Thallium forms its oxides in two different oxidation states, +1 (Tl2O) and +3 (Tl2O3). Tl2O has been used as an ingredient in highly refractive optical glasses and as a colouring agent in artificial gems; Tl2O3 is an n-type semiconductor. Alkali halide crystals, such as sodium iodide, have been doped or activated by thallium compounds to produce inorganic phosphors for use in scintillation counters to detect radiation.
Thallium imparts a brilliant green coloration to a bunsen flame. Thallous chromate, formula Tl2CrO4, is best used in the quantitative analysis of thallium, after any thallic ion, Tl3+, present in the sample has been reduced to the thallous state, Tl+.
Thallium is typical of the Group 13 elements in having an s2p1 outer electron configuration. Promoting an electron from an s to a p orbital allows the element to be three or four covalent. With thallium, however, the energy required for s → p promotion is high relative to the Tl–X covalent bond energy that is regained on formation of TlX3; hence, a derivative with a +3 oxidation state is not a very energetically favoured reaction product. Thus, thallium, unlike the other boron group elements, predominantly forms singly charged thallium salts having thallium in the +1 rather than the +3 oxidation state (the 6s2 electrons remain unused). It is the only element to form a stable singly charged cation with the outer electron configuration (n-1)d10ns2, which is, unusually enough, not an inert gas configuration. In water the colourless, more stable thallous ion, Tl+, resembles the heavier alkali metal ions and silver; the compounds of thallium in its +3 state are easily reduced to compounds of the metal in its +1 state.
In its oxidation state of +3, thallium resembles aluminum, although the ion Tl3+ appears to be too large to form alums. The very close similarity in size of the singly charged thallium ion, Tl+, and the rubidium ion, Rb+, makes many Tl+ salts, such as the chromate, sulfate, nitrate, and halides, isomorphous (i.e., have an identical crystal structure) to the corresponding rubidium salts; also, the ion Tl+ is able to replace the ion Rb+ in the alums. Thus, thallium does form an alum, but in doing so it replaces the M+ ion, rather than the expected metal atom M3+, in M+M3+(SO4)2∙12H2O.
Soluble thallium compounds are toxic. The metal itself is changed to such compounds by contact with moist air or skin. Thallium poisoning, which may be fatal, causes nervous and gastrointestinal disorders and rapid loss of hair.
Element Properties
atomic number : 81
atomic weight : 204.37
melting point : 303.5 °C (578.3 °F)
boiling point : 1,457 °C (2,655 °F)
specific gravity : 11.85 (at 20 °C [68 °F])
oxidation states : +1, +3.
Additional Information:
Appearance
A soft, silvery-white metal that tarnishes easily.
Uses
The use of thallium is limited as it is a toxic element. Thallium sulfate was employed as a rodent killer – it is odourless and tasteless – but household use of this poison has been prohibited in most developed countries.
Most thallium is used by the electronics industry in photoelectric cells. Thallium oxide is used to produce special glass with a high index of refraction, and also low melting glass that becomes fluid at about 125K.
An alloy of mercury containing 8% thallium has a melting point 20°C lower than mercury alone. This can be used in low temperature thermometers and switches.
Biological role
Thallium has no known biological role. It is very toxic and there is evidence that the vapour is both teratogenic (disturbs the development of an embryo or foetus) and carcinogenic. It can displace potassium around the body affecting the central nervous system.
Natural abundance
Thallium is found in several ores. One of these is pyrites, which is used to produce sulfuric acid. Some thallium is obtained from pyrites, but it is mainly obtained as a by-product of copper, zinc and lead refining.
Thallium is also present in manganese nodules found on the ocean floor.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Thallium