Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » This is Cool
- » Benzene
Pages: 1
#1 2025-09-29 18:24:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,128
Benzene
Benzene
Gist
Benzene is a clear, sweet-smelling, highly flammable liquid hydrocarbon with the chemical formula C₆H₆, composed of a hexagonal ring of six carbon atoms with one hydrogen atom bonded to each. It's found in crude oil and produced by both natural and man-made processes. While an important industrial solvent and precursor for many chemicals, including plastics, resins, and detergents, benzene is a known human carcinogen that can cause leukemia and other health problems, with exposure primarily occurring through tobacco smoke, vehicle exhaust, and industrial emissions.
Benzene is used as a raw material to make plastics, synthetic fibers, resins, and nylon, and is also used in the production of dyes, detergents, and pesticides. It serves as a solvent in some chemical and pharmaceutical processes and is a component of gasoline, improving fuel efficiency. Major derivatives include styrene (for polystyrene), phenol (for resins), and cyclohexane (for nylon).
Summary
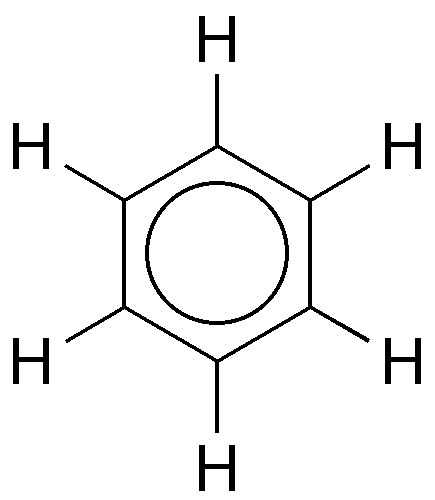
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms and satisfying Hückel's rule, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. Benzene is a volatile organic compound.
Benzene is classified as a carcinogen. Its particular effects on human health, such as the long-term results of accidental exposure, have been reported on by news organizations such as The New York Times. For instance, a 2022 article stated that benzene contamination in the Boston metropolitan area caused hazardous conditions in multiple places, with the publication noting that the compound may eventually cause leukemia in some individuals.
Details
Benzene (C6H6) is the simplest organic, aromatic hydrocarbon and parent compound of numerous important aromatic compounds. Benzene is a colourless liquid with a characteristic odour and is primarily used in the production of polystyrene. It is highly toxic and is a known carcinogen; exposure to it may cause leukemia. As a result, there are strict controls on benzene emissions.
Discovery of benzene
Benzene was first discovered by the English scientist Michael Faraday in 1825 in illuminating gas. In 1834 German chemist Eilhardt Mitscherlich heated benzoic acid with lime and produced benzene. In 1845 German chemist A.W. von Hofmann isolated benzene from coal tar.
The structure of benzene has been of interest since its discovery. German chemists Joseph Loschmidt (in 1861) and August Kekule von Stradonitz (in 1866) independently proposed a cyclic arrangement of six carbons with alternating single and double bonds. Kekule subsequently modified his structural formula to one in which oscillation of the double bonds gave two equivalent structures in rapid equilibrium. In 1931 American chemist Linus Pauling suggested that benzene had a single structure, which was a resonance hybrid of the two Kekule structures.
Characteristics of benzene
Modern bonding models (valence-bond and molecular orbital theories) explain the structure and stability of benzene in terms of delocalization of six of its electrons, where delocalization in this case refers to the attraction of an electron by all six carbons of the ring instead of just one or two of them. This delocalization causes the electrons to be more strongly held, making benzene more stable and less reactive than expected for an unsaturated hydrocarbon. As a result, the hydrogenation of benzene occurs somewhat more slowly than the hydrogenation of alkenes (other organic compounds that contain carbon-carbon double bonds), and benzene is much more difficult to oxidize than alkenes. Most of the reactions of benzene belong to a class called electrophilic aromatic substitution that leave the ring itself intact but replace one of the attached hydrogens. These reactions are versatile and widely used to prepare derivatives of benzene.
Experimental studies, especially those employing X-ray diffraction, show benzene to have a planar structure with each carbon-carbon bond distance equal to 1.40 angstroms (Å). This value is exactly halfway between the C=C distance (1.34 Å) and C—C distance (1.46 Å) of a C=C—C=C unit, suggesting a bond type midway between a double bond and a single bond (all bond angles are 120°). Benzene has a boiling point of 80.1 °C (176.2 °F) and a melting point of 5.5 °C (41.9 °F), and it is freely soluble in organic solvents, but only slightly soluble in water.
Uses of benzene
At one time, benzene was obtained almost entirely from coal tar; however, since about 1950, these methods have been replaced by petroleum-based processes. More than half of the benzene produced each year is converted to ethylbenzene, then to styrene, and then to polystyrene. The next largest use of benzene is in the preparation of phenol. Other uses include the preparation of aniline (for dyes) and dodecylbenzene (for detergents).
Additional Information
Benzene is the cornerstone of aromatic organic molecules. It has a long and checkered history—much too long to cover here—so the focus will be on the very old and very new.
Legendary British scientist Michael Faraday is primarily known for his discoveries in electricity and electromagnetism; but it was he who first isolated benzene in 1825 from coal-derived “illuminating gas”, which was used for lighting in the early 19th century.
Chemists soon determined that the benzene molecule contains six carbon and six hydrogen atoms; but for decades, they struggled to determine its precise structure. In 1865, German chemist Friedrich August Kekulé published a paper in which he described benzene as consisting of a ring of six carbon atoms, each bonded to a hydrogen atom. The story goes that Kekulé dreamt of a snake biting its tail, which inspired him to conceive of the structure.
Kekulé’s original concept was that the carbon atoms are attached to each other by alternating single and double bonds. But in the years following his original paper, his experiments showed that all six bonds are equivalent, which meant that each one oscillates between single and double bonding. Eventually, researchers, including Linus Pauling, concluded that this phenomenon is the basis of aromaticity, in which the carbon atoms are connected to each other via σ- and π-bonding rather than discrete double bonds.
In the 20th century, benzene, derived mostly from petroleum, came into widespread production for use as a solvent and as a starting material for manufacturing other organic compounds, especially ethylbenzene, which is converted to styrene. The use of benzene for making a large array of products continues today; but its use as a solvent declined abruptly in the 1980s, when it was established as a human carcinogen.
People nonetheless continue to be exposed to benzene. Gasoline contains 0.5–2.0 vol% benzene, which is one reason that gasoline dispensers now connect tightly to automobiles’ fuel tanks. In the past year, studies have shown that household stoves that use natural gas or propane can expose residents to harmful concentrations of benzene.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » This is Cool
- » Benzene