Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-09-25 17:22:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,128
Acetic Acid
Acetic Acid
Gist
Acetic acid is an organic compound, ethanoic acid, with the chemical formula CH3COOH that is known for its pungent odor and sour taste. It is a weak acid and a natural body metabolite found in fruits and vegetables. Commonly known as the active ingredient in vinegar, it is used in food as a preservative, an acidulant, and a flavoring agent. Other applications include cleaning, as a herbicide, in manufacturing, and as a solvent.
While many fruits contain organic acids, they primarily feature citric, malic, and other acids for their tartness, not acetic acid. Acetic acid is naturally present in fermented products like apple cider vinegar and fruit vinegar, not raw fruits themselves. Fruits like apples and grapes contain other acids, such as malic acid, and can be used to create vinegar, a significant source of acetic acid.
Acetic acid is used in the manufacture of acetic anhydride, cellulose acetate, vinyl acetate monomer, acetic esters, chloracetic acid, plastics, dyes, insecticides, photographic chemicals, and rubber.
Summary
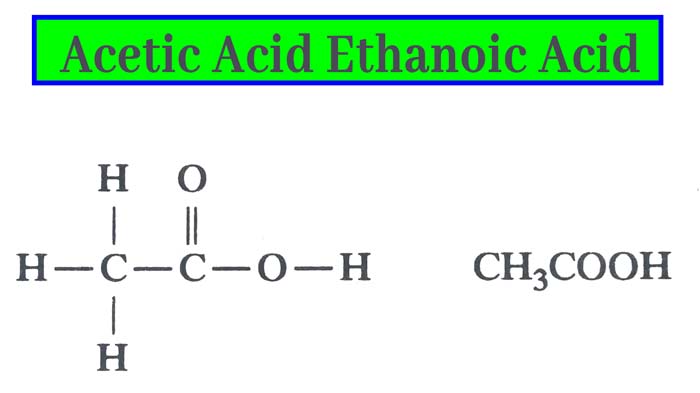
Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Acetic acid is the active component of vinegar. Historically, vinegar was produced from the third century BC, making acetic acid likely the first acid to be produced in large quantities.
Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats.
The global demand for acetic acid as of 2023 is about 17.88 million metric tonnes per year (t/a). Most of the world's acetic acid is produced via the carbonylation of methanol. Its production and subsequent industrial use poses health hazards to workers, including incidental skin damage and chronic respiratory injuries from inhalation.
Details
Acetic acid (CH3COOH) is the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and oxidation of natural carbohydrates is called vinegar; a salt, ester, or acylal of acetic acid is called acetate. Industrially, acetic acid is used in the preparation of metal acetates, used in some printing processes; vinyl acetate, employed in the production of plastics; cellulose acetate, used in making photographic films and textiles; and volatile organic esters (such as ethyl and butyl acetates), widely used as solvents for resins, paints, and lacquers. Biologically, acetic acid is an important metabolic intermediate, and it occurs naturally in body fluids and in plant juices.
Acetic acid has been prepared on an industrial scale by air oxidation of acetaldehyde, by oxidation of ethanol (ethyl alcohol), and by oxidation of butane and butene. Today acetic acid is manufactured by a process developed by the chemical company Monsanto in the 1960s; it involves a rhodium-iodine catalyzed carbonylation of methanol (methyl alcohol).
Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °C [244.2 °F]; melting point 16.6 °C [61.9 °F]) that is completely miscible with water.
(Vinegar is a sour liquid that is made by the fermentation of any of numerous dilute alcoholic liquids into a liquid containing acetic acid. Vinegar may be produced from a variety of materials: apples or grapes (wine or cider vinegar); malted barley or oats (malt vinegar); and industrial alcohol (distilled white vinegar). There are also vinegars made from beer, sugars, rice, and other substances. As a commercial product, however, vinegar was probably first made from wine (French vin, “wine”; aigre, “sour”).
Vinegar can be made from any liquid that is capable of being converted into alcohol in a two-step process. The fruit juice or other liquid contains sugar, which is converted into alcohol and carbon dioxide gas by the actions of yeast enzymes. The alcohol thus formed combines with atmospheric oxygen by the action of Acetobacter bacteria, forming acetic acid and water. Organic acids and esters derived from the fruit or other source material are also present and are responsible for the flavour and aroma variations of vinegar. Table vinegar contains approximately 4 percent acetic acid.
In 1864 the French chemist and bacteriologist Louis Pasteur showed that it is Acetobacter bacteria that cause the conversion of alcohol to acetic acid. These bacteria work together symbiotically, producing enough acetic acid to prevent invasion by other organisms.
Despite its ancient origin, the technology of vinegar production advanced slowly, improvements consisting principally of better methods of aeration. The Orleans process, best-known of the old methods, used a barrel of about 50 gallons (200 l) capacity. A mash consisting of wine or other alcoholic liquid was poured into the barrel, and a small amount of vinegar containing a mass of vinegar bacteria, called mother of vinegar, was added to start the reaction. One or two small air holes drilled above the liquid level exposed the surface to aeration. The finished vinegar was drawn off through a wooden spigot near the bottom. Care was taken in refilling the barrel with the new charge of raw ingredients to avoid breaking up the surface film of bacteria.
Early in the 18th century, a Dutch technologist, Hermann Boerhaave, found that the rate of acid production in the vinegar process was directly proportional to the amount of surface exposed to air. Thus, subsequent methods attempted to introduce more air into the casks. In the 20th century, continuous aeration—air bubbles pumped through the mash—was developed.
Vinegar’s principal uses are the flavouring of foods and the preservation, or pickling, of meat products, fish, fruit, and vegetables. For use as a condiment, vinegar is often flavoured with garlic, onions, tarragon, or other herbs and spices. Mixed with oil and seasonings it becomes a classic cold sauce—vinaigrette—used as a dressing on vegetable salads and served as a sauce with cold cooked vegetables, meats, and fish. Vinegar is also a common ingredient in marinades and is widely used in the pickling of cucumbers and other vegetables.)
Additional Information
One of the most common ways consumers may come into contact with acetic acid is in the form of household vinegar, which generally contains about 5 percent acetic acid and 95 percent water.
When acetic acid is at 99.5 percent concentration, it is referred to a glacial acetic acid, which can be used as raw material and solvent in the production of other chemical products.
Industrial applications of glacial acetic acid include producing vinyl acetate, as solvent to dissolve oils, sulfur and iodine; acidizing oil and gas; manufacturing pharmaceuticals and vitamins, and food processing.
Occupational exposure to glacial acetic acid can occur through inhalation and skin or eye contact. OSHA has set standards for exposure to acetic acid.
Uses & Benefits
One of the most common ways consumers may come into contact with acetic acid is in the form of household vinegar, which is naturally made from fermentable sources such as wine, potatoes, apples, grapes, berries and grains. Vinegar is a clear solution generally containing about 5 percent acetic acid and 95 percent water. Vinegar is used as a food ingredient and can also be an ingredient in personal care products, household cleaners, pet shampoos and many other products for the home:
Food Preparation: Vinegar is a common food ingredient, often used as a brine in pickling liquids, vinaigrettes, marinades and other salad dressings. Vinegar also can be used in food preparation to help control Salmonella contamination in meat and poultry products.
Cleaning: Vinegar can be used throughout the home as a window cleaner, to clean automatic coffee makers and dishes, as a rinsing agent for dishwashers, and to clean bathroom tile and grout. Vinegar can also be used to clean food-related tools and equipment because it generally does not leave behind a harmful residue and requires less rinsing.
Gardening: In concentrations of 10 to 20 percent, acetic acid can be used as a weed killer on gardens and lawns. When used as an herbicide, the acetic acid can kill weeds that have emerged from the soil, but does not affect the roots of the weed, so they can regrow.
When acetic acid is at 99.5 percent concentration, it is referred to as glacial acetic acid. Glacial acetic acid has a variety of uses, including as a raw material and solvent in the production of other chemical products.
Industrial applications for glacial acetic acid include:
* Vinyl Acetate, cellulose fibers and plastics: Acetic acid is used to make many chemicals, including vinyl acetate, acetic anhydride and acetate esters.
** Vinyl acetate is used to make polyvinyl acetate, a polymer used in paints, adhesives, plastics and textile finishes.
** Acetic anhydride is used in the manufacture of cellulose acetate fibers and plastics used for photographic film, clothing and coatings.
** Acetic acid is also used in the chemical reaction to produce purified terephthalic acid (PTA), which is used to manufacture the PET plastic resin used in synthetic fibers, food containers, beverage bottles and plastic films.
* Solvents: Acetic acid is a hydrophilic solvent, similar to ethanol. It dissolves compounds such as oils, sulfur and iodine and mixes with water, chloroform and hexane.
* Acidizing oil and gas: Acetic acid can help reduce metal corrosion and scale build-up in oil and gas well applications. It is also used in oil well stimulation to improve flow and increase production of oil and gas.
* Pharmaceuticals and vitamins: The pharmaceutical industry uses acetic acid in the manufacture of vitamins, antibiotics, hormones and other products.
* Food Processing: Acetic acid is commonly used as a cleaning and disinfecting product in food processing plants.
* Other uses: Salts of acetic acid and various rubber and photographic chemicals are made from acetic acid. Acetic acid and its sodium salt are commonly used as a food preservative.
(Acetic acid in its pure form (99.5 percent concentration) is also known as glacial acetic acid. Glacial acetic acid has numerous industrial uses. Vinegar contains 4 to 8 percent acetic acid, and is made from the fermentation of fruit or grain juices/liquids.)
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1