Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-09-24 17:19:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,128
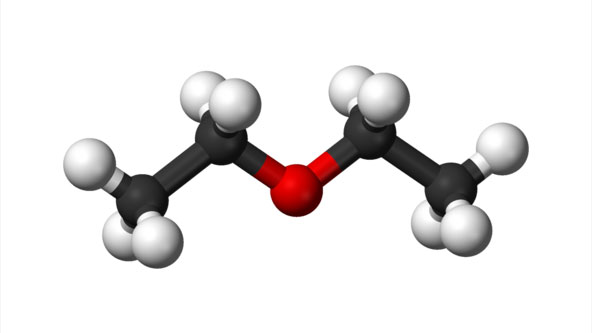
Diethyl Ether
Diethyl Ether
Gist
Diethyl ether is a colorless, volatile, sweet-smelling organic solvent with the chemical formula {(C2H5)}2{O} or C2H5OC2H5, known for its use as a laboratory solvent, starting fluid for engines, and a historical anesthetic. It is highly flammable, can decompose into explosive peroxides over time in the presence of light and air, and its vapors can cause unconsciousness. Key applications include liquid-liquid extraction and as a solvent for oils, fats, resins, and plastics in various industries.
Diethyl ether is used as a laboratory solvent, especially for Grignard reactions and extractions due to its ability to dissolve many organic substances like oils and fats. It was historically used as an inhalation anesthetic in surgery but has largely been replaced by modern, less flammable alternatives. Additionally, it functions as a volatile starting fluid for gasoline and diesel engines, a refrigerant, and an extractant for various organic compounds.
Summary
Diethyl ether, or simply ether (abbreviated eth.), is an organic compound with the chemical formula (CH3CH2)2O, sometimes abbreviated as Et2O. It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs to the ether class of organic compounds. It is a common solvent and was formerly used as a general anesthetic.
Production
Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises: Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%.
Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis.
Uses
The dominant use of diethyl ether is as a solvent. One particular application is in the production of cellulose plastics such as cellulose acetate.
Laboratory solvent
It is a common solvent for the Grignard reaction in addition to other reactions involving organometallic reagents. These uses exploit its basicity. Diethyl ether is a popular non-polar solvent in liquid-liquid extraction. As an extractant, it is immiscible with and less dense than water.
Although immiscible, it has significant solubility in water (6.05 g/(100 ml) at 25 °C) and dissolves 1.5 g/(100 g) (1.0 g/(100 ml)) water at 25 °C.
Fuel
Diethyl ether has a high cetane number of 85–96 and, in combination with petroleum distillates for gasoline and diesel engines, is used as a starting fluid because of its high volatility and low flash point. Ether starting fluid is sold and used in countries with cold climates, as it can help with cold starting an engine at sub-zero temperatures. For the same reason it is also used as a component of the fuel mixture for carbureted compression ignition model engines.
Details
Diethyl ether (C4H10O) is a flammable, volatile, and colorless liquid with a sweet taste and characteristic odor. It is soluble in alcohol, acetone, benzene, and chloroform. Its boiling point is 94 °F (34.5 °C). When exposed to fire or heat, ether releases carbon monoxide (CO); exposure to light causes ether to break down into flammable peroxides. Ether, with a BGPC of 12, is more soluble in blood than either halothane or nitrous oxide. The MAC of ether is 2.0%. Because of the explosive nature of ether, the National Fire Protection Association has given it a flammability rating of 4, corresponding to an extreme fire hazard. Although ether works well as an anesthetic, its propensity to explode prompted anesthetists to find alternative inhaled agents such as chloroform, cyclopropane, and halothane.
Anesthesia induction occurs at a concentration of 100,000 to 150,000 ppm and is maintained with 50,000 ppm. Very small doses to eyes or skin can cause corneal injury and burns. Toxic exposures to ethers (as with other anesthetics) can occur through inhalation, eye or skin contact, and ingestion. The effect of ether is dose-dependent. Symptoms consist of skin, eye, and mucosal irritation leading to an increase in bronchial secretions. Dizziness, drowsiness, bradycardia, hypothermia, or acute excitement may also occur. Laryngospasm, loss of consciousness, and death may result. The aftereffects of emergence from ether-induced anesthesia include nausea, vomiting, and headache.
Additional Information
Ethyl ether, well-known anesthetic, commonly called simply ether, is an organic compound belonging to a large group of compounds called ethers; its molecular structure consists of two ethyl groups linked through an oxygen atom, as in C2H5OC2H5.
Ethyl ether is a colourless, volatile, highly flammable liquid (boiling point 34.5° C [94.1° F]) with a powerful, characteristic odour and a hot, sweetish taste. It is a widely used solvent for bromine, iodine, most fatty and resinous substances, volatile oils, pure rubber, and certain vegetable alkaloids.
Ethyl ether is manufactured by the distillation of ethyl alcohol with sulfuric acid. Pure ether (absolute ether), required for medical purposes and in the preparation of Grignard reagents, is prepared by washing the crude ether with a saturated aqueous solution of calcium chloride, then treating with sodium.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1