Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » This is Cool
- » Ozone
Pages: 1
#1 2025-09-02 23:09:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,634
Ozone
Ozone
Gist
Ozone (O3) is an unstable, blue gas composed of three oxygen atoms that can be beneficial or harmful depending on its location in the atmosphere. In the stratosphere (high atmosphere), the ozone layer naturally shields Earth from harmful ultraviolet (UV) radiation, while at ground level in the troposphere, it's a pollutant formed from nitrogen oxides (NOx) and volatile organic compounds (VOCs), posing health risks and contributing to smog.
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere : (the stratosphere) and lower atmosphere (the troposphere).
Summary
Ozone, also called trioxygen, is an inorganic molecule with the chemical formula O3. It is a pale-blue gas with a distinctively pungent odor. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O2, breaking down in the lower atmosphere to O2 (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.
Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as 0.1 ppm in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black solid. Ozone's instability with regard to more common dioxygen is such that both concentrated gas and liquid ozone may decompose explosively at elevated temperatures, physical shock, or fast warming to the boiling point. It is therefore used commercially only in low concentrations.
Ozone is a powerful oxidizing agent (far more so than dioxygen) and has many industrial and consumer applications related to oxidation. This same high oxidizing potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about 0.1 ppm. While this makes ozone a potent respiratory hazard and pollutant near ground level, a higher concentration in the ozone layer (from two to eight ppm) is beneficial, preventing damaging UV light from reaching the Earth's surface.
Details
Ozone, (O3), is triatomic allotrope of oxygen (a form of oxygen in which the molecule contains three atoms instead of two as in the common form) that accounts for the distinctive odor of the air after a thunderstorm or around electrical equipment. The odor of ozone around electrical machines was reported as early as 1785; ozone’s chemical constitution was established in 1872. Ozone is an irritating pale blue gas that is explosive and toxic, even at low concentrations. It occurs naturally in small amounts in Earth’s stratosphere, where it absorbs solar ultraviolet radiation, which otherwise could cause severe damage to living organisms on Earth’s surface. Under certain conditions, photochemical reactions between nitrogen oxides and hydrocarbons in the lower atmosphere can produce ozone in concentrations high enough to cause irritation of the eyes and mucous membranes. Such ground-level ozone is considered a major air pollutant.
Ozone usually is manufactured by passing an electric discharge through a current of oxygen or dry air. The resulting mixtures of ozone and original gases are suitable for most industrial purposes, although purer ozone may be obtained from them by various methods; for example, upon liquefaction, an oxygen-ozone mixture separates into two layers, of which the denser one contains about 75 percent ozone. The extreme instability and reactivity of concentrated ozone makes its preparation both difficult and hazardous.
Ozone is 1.5 times as dense as oxygen; at −112 °C (−170 °F) it condenses to a dark blue liquid, which freezes at −251.4 °C (−420 °F). The gas decomposes rapidly at temperatures above 100 °C (212 °F) or, in the presence of certain catalysts, at room temperatures. Although it resembles oxygen in many respects, ozone is much more reactive; hence, it is an extremely powerful oxidizing agent, particularly useful in converting olefins into aldehydes, ketones, or carboxylic acids. Because it can decolorize many substances, it is used commercially as a bleaching agent for organic compounds; as a strong germicide it is used to sterilize drinking water as well as to remove objectionable odors and flavors.
Additional Information:
What is ozone and where is it in the atmosphere?
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere ozone molecule (the stratosphere) and lower atmosphere (the troposphere). Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways.
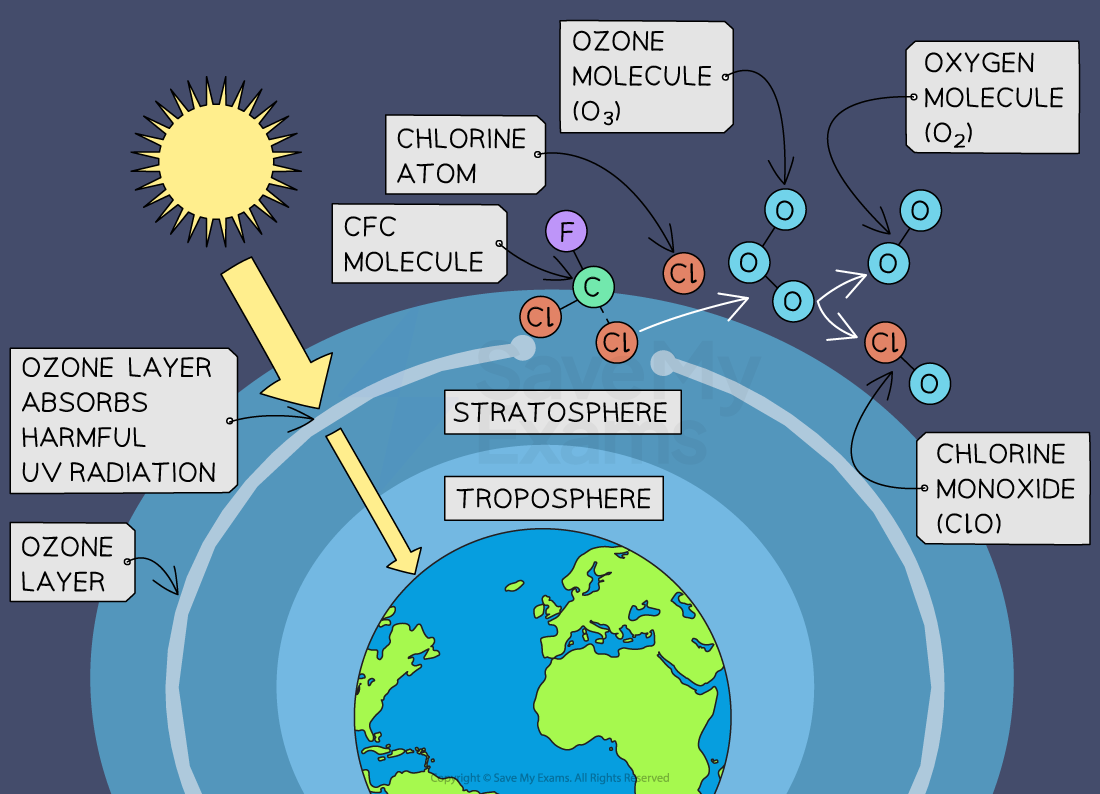
Stratospheric ozone is formed naturally through the interaction of solar ultraviolet (UV) radiation with molecular oxygen (O2). The "ozone layer," approximately 6 through 30 miles above the Earth's surface, reduces the amount of harmful UV radiation reaching the Earth's surface.
Tropospheric or ground-level ozone – what we breathe – is formed primarily from photochemical reactions between two major classes of air pollutants, volatile organic compounds (VOC) and nitrogen oxides (NOx). These reactions have traditionally been viewed as depending upon the presence of heat and sunlight, resulting in higher ambient ozone concentrations in summer months. Within the last decade, however, high ozone concentrations have also been observed under specific circumstances in cold months, where a few high elevation areas in the Western U.S. with high levels of local VOC and NOx emissions have formed ozone when snow is on the ground and temperatures are near or below freezing. Ozone contributes to what we typically experience as "smog" or haze, which still occurs most frequently in the summertime, but can occur throughout the year in some southern and mountain regions.
Although some stratospheric ozone is transported into the troposphere, and some VOC and NOx occur naturally, the majority of ground-level ozone is the result of reactions of man-made VOC and NOx. Significant sources of VOC are chemical plants, gasoline pumps, oil-based paints, autobody shops, and print shops. Nitrogen oxides result primarily from high temperature combustion. Significant sources are power plants, industrial furnaces and boilers, and motor vehicles.
How does atmospheric ozone affect human health?
Ozone has two properties of interest to human health. First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin cancer and cataracts. Second, when inhaled, it reacts chemically with many biological molecules in the respiratory tract, leading to a number of adverse health effects.
Meet the Ozone Molecule
The ozone molecule (O3) is formed in the Earth’s atmosphere primarily through a series of photochemical reactions involving oxygen molecules (O2) and ultraviolet (UV) radiation from the Sun.
i) Ozone is a gas made up of three (3) atoms of oxygen:
O + O + O = O3
ii) Oxygen (we breathe) is made up of two (2) atoms of oxygen:
O + O = O2
The atoms in O2 are stable – each atom “holds on” to the other.
The atoms in O3 consist of a stable pair (O2) and a third, unstable atom.
It is the unstable atom that gives ozone its power! Ozone is generated when energy “splits” the stable O2 bond.
Ozone Can be Generated in Three Ways:
Ozone, a molecule composed of three oxygen atoms (O3), can be generated through various methods. Here are three common ways ozone can be produced:
i) Ultraviolet (UV) Radiation: Ozone can be generated naturally in the Earth’s stratosphere through the interaction of UV radiation from the sun and oxygen molecules (O2). In this process, high-energy UV radiation breaks apart oxygen molecules, forming oxygen radicals (O). These radicals then react with other oxygen molecules to form ozone (O3).
ii) Corona Discharge (Electric Discharge): This method involves passing a high-voltage electric discharge through oxygen gas (O2) or dry air containing oxygen. The electric discharge creates a plasma, which leads to the dissociation of oxygen molecules into oxygen atoms. These oxygen atoms then combine with other oxygen molecules to form ozone.
iii) Cold Plasma (Dielectric Barrier Discharge): In this method, oxygen gas (O2) or dry air containing oxygen is passed through a dielectric barrier discharge (DBD), which is essentially a gap between two electrodes with a dielectric material in between. When a high-voltage alternating current is applied to the electrodes, it creates a plasma that facilitates the dissociation of oxygen molecules into oxygen atoms. These oxygen atoms then combine with other oxygen molecules to produce ozone.
These methods are commonly employed in various industries and applications, such as water treatment, air purification, and industrial processes. Each method has its advantages and limitations depending on the specific requirements of the application.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » This is Cool
- » Ozone