Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#101 Re: Exercises » Compute the solution: » 2025-07-07 16:36:06
Hi,
2416.
#102 Re: This is Cool » Miscellany » 2025-07-06 23:36:34
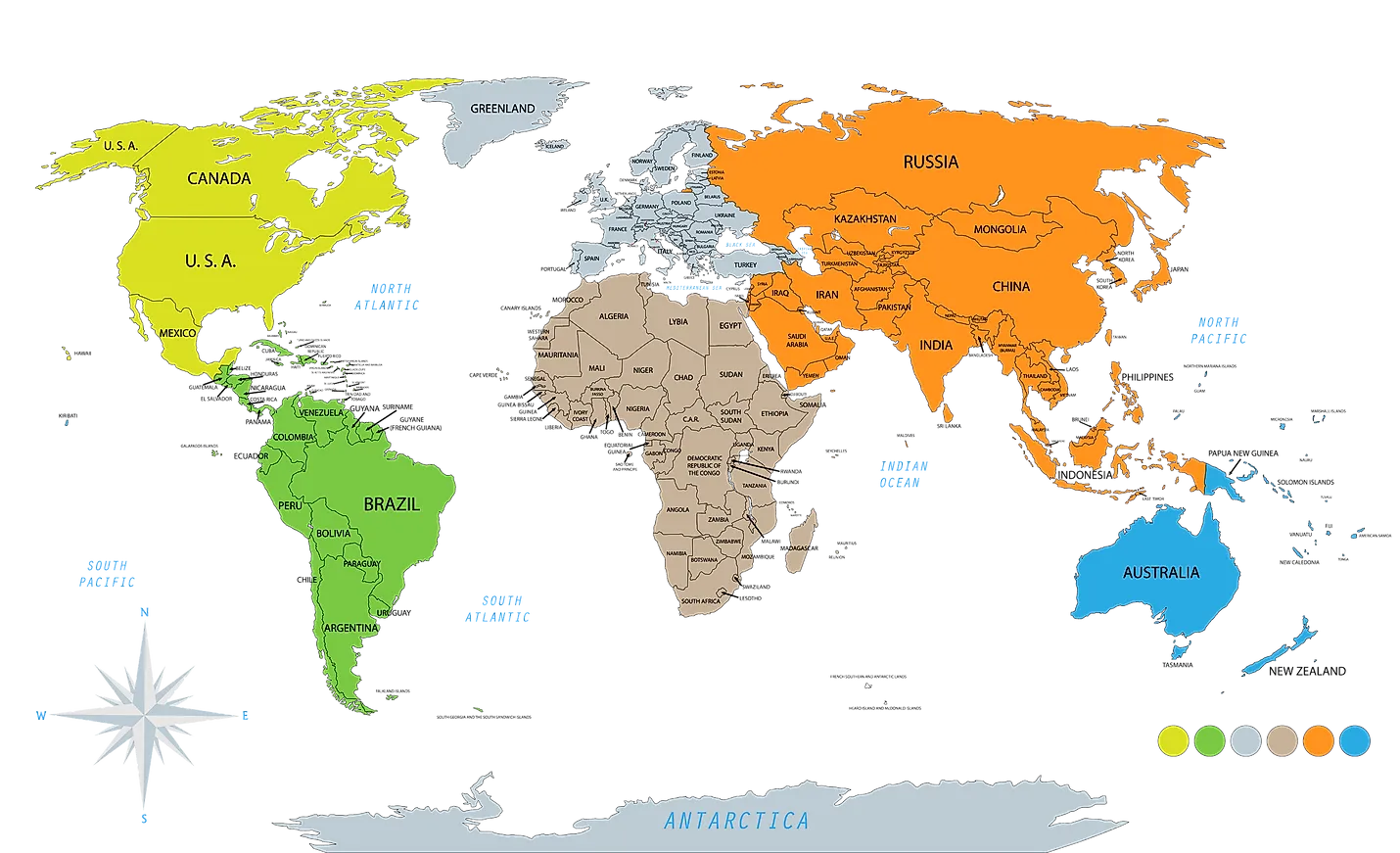
2330) Continent
Gist
A continent is one of the Earth's seven main landmasses. They are, in approximate order of size: Asia, Africa, North America, South America, Antarctica, Europe, and Australia. These are conventionally defined by convention rather than strict criteria.
The seven continents in order from largest to smallest by land area are: Asia, Africa, North America, South America, Antarctica, Europe, and Australia (sometimes called Oceania or Australasia).
What defines a Continent?
By convention, continents "are understood to be large, continuous, discrete masses of land, ideally separated by expanses of water". By this definition, all continents have to be an island of some metric.
Summary
A continent is any of several large geographical regions. Continents are generally identified by convention rather than any strict criteria. A continent could be a single large landmass, a part of a very large landmass, as in the case of Asia or Europe within Eurasia, or a landmass and nearby islands within its continental shelf. Due to these varying definitions, the number of continents varies; up to seven or as few as four geographical regions are commonly regarded as continents. Most English-speaking countries recognize seven regions as continents. In order from largest to smallest in area, these seven regions are Asia, Africa, North America, South America, Antarctica, Europe, and Australia (sometimes called Oceania or Australasia). Different variations with fewer continents merge some of these regions; examples of this are merging Asia and Europe into Eurasia, North America and South America into the Americas (or simply America), and Africa, Asia, and Europe into Afro-Eurasia.
Oceanic islands are occasionally grouped with a nearby continent to divide all the world's land into geographical regions. Under this scheme, most of the island countries and territories in the Pacific Ocean are grouped together with the continent of Australia to form the geographical region of Oceania.
In geology, a continent is defined as "one of Earth's major landmasses, including both dry land and continental shelves". The geological continents correspond to seven large areas of continental crust that are found on the tectonic plates, but exclude small continental fragments such as Madagascar that are generally referred to as microcontinents. Continental crust is only known to exist on Earth.
The idea of continental drift gained recognition in the 20th century. It postulates that the current continents formed from the breaking up of a supercontinent (Pangaea) that formed hundreds of millions of years ago.
Details
When geographers identify a continent, they usually include all the islands associated with it. Japan, for instance, is part of the continent of Asia. Likewise, Greenland and all the islands in the Caribbean Sea are usually considered part of North America.
Together, the continents add up to about 148 million square kilometers (57 million square miles) of land. Continents make up most—but not all—of Earth’s land surface. A very small portion of the total land area is made of islands that are not considered physical parts of continents. The ocean covers almost three-fourths of Earth. The area of the ocean is more than double the area of all the continents combined. All continents border at least one ocean. Asia, the largest continent, has the longest series of coastlines.
Coastlines, however, do not indicate the actual boundaries of the continents. Continents are defined by their continental shelves. A continental shelf is a gently sloping area that extends outward from the beach far into the ocean. A continental shelf is part of the ocean, but also part of the continent.
To human geographers, continents are also culturally distinct. The continents of Europe and Asia, for example, are actually part of a single, enormous piece of land called Eurasia. But historically, the areas of Asia and Europe have been separated because of people’s perceptions about their different cultures. Because of this, most geographers continue to divide Eurasia into Europe and Asia. An imaginary line, running from the northern Ural Mountains in Russia south to the Caspian and Black Seas, separates Europe, to the west, from Asia, to the east.
Building the Continents
Earth formed 4.6 billion years ago from a great, swirling cloud of cosmic dust and gas. The continuous smashing of space debris and the pull of gravity made the inside of Earth heat up. As the heat increased, some of Earth’s rocky materials melted and rose to the surface, where they cooled and formed a crust. Heavier material sank toward Earth’s center. Eventually, Earth came to have three main layers: the core, the mantle and the crust.
The crust and the top portion of the mantle form a rigid shell around Earth that is broken into huge sections called tectonic plates. The heat from inside Earth causes the plates to slide around on the molten mantle. Today, tectonic plates continue to slowly slide around the surface, just as they have for hundreds of millions of years. Geologists believe the interaction of the plates, a process called plate tectonics, contributed to the creation of continents.
Studies of rocks found in ancient areas of North America have revealed that the oldest known pieces of the continents began to form nearly 4 billion years ago, soon after Earth formed. At that time, a primitive ocean covered Earth. Only a small fraction of the crust was made of continental material. Scientists theorize that this material built up along the boundaries of tectonic plates during a process called subduction. During subduction, plates collide and the edge of one plate slides beneath the edge of another.
When heavy oceanic crust subducted toward the mantle, it melted in the mantle’s intense heat. Once it melted, the rock became lighter. Now in the form of magma, it rose through the overlying plate and burst out as lava. When the lava cooled, it hardened into igneous rock.
Gradually, the igneous rock built up into small volcanic islands above the surface of the ocean. Over time, these islands grew bigger, partly as the result of more lava flows and partly from the buildup of material scraped off descending plates. When plates carrying islands subducted, the islands themselves did not descend into the mantle. Their material fused with that of islands on the neighboring plate. This made even larger landmasses in the form of the first continents.
The building of volcanic islands and continental material through plate tectonics is a process that continues today. Continental crust is much lighter than oceanic crust. In subduction zones, where tectonic plates interact with each other, oceanic crust always subducts beneath continental crust. Oceanic crust is constantly being recycled in the mantle. For this reason, continental crust is much, much older than oceanic crust.
Wandering Continents
If you could visit Earth as it was millions of years ago, it would look very different. The continents have not always been where they are today. About 480 million years ago, most continents were scattered chunks of land lying along or below the Equator. Millions of years of continuous tectonic activity changed their positions, and by 240 million years ago, almost all of the world’s land was joined in a single, huge continent.
By about 200 million years ago, the forces that helped form the supercontinent caused it to begin to break apart. The pieces of the supercontinent that began to move apart were the beginnings of the continents that we know today.
A giant landmass that would become Europe, Asia, and North America separated from another mass that would split up into other continents. In time, Antarctica and Australia, still joined together, broke away and drifted south. The small piece of land that would become the peninsula of India broke away and for millions of years moved north as a large island. It eventually collided with Asia. Gradually, the different landmasses moved to their present locations.
The positions of the continents are always changing. North America and Europe are moving away from each other at the rate of about 2.5 centimeters (1 inch) per year. If you could visit the planet in the future, you might find that part of the U.S. state of California had separated from North America and become an island. Africa might have split in two along the Great Rift Valley. It is even possible that another supercontinent may form someday.
Continental Features
The surface of the continents has changed many times because of mountain building, weathering, erosion and buildup of sediment. Continuous, slow movement of tectonic plates also changes surface features.
The rocks that form the continents have been shaped and reshaped many times. Great mountain ranges have risen and then have been worn away. Ocean waters have flooded huge areas and then gradually dried up. Massive ice sheets have come and gone, sculpting the landscape in the process.
Today, all continents have great mountain ranges, vast plains, extensive plateaus, and complex river systems. The landmasses’ average elevation above sea level is about 838 meters (2,750 feet).
Although each is unique, all the continents share two basic features: old, geologically stable regions, and younger, somewhat more active regions. In the younger regions, the process of mountain building has happened recently and often continues to happen.
The power for mountain building, or orogeny, comes from plate tectonics. One way mountains form is through the collision of two tectonic plates. The impact creates wrinkles in the crust, just as a rug wrinkles when you push against one end of it. Such a collision created Asia’s Himalaya mountain range several million years ago. The plate carrying India slowly and forcefully shoved the landmass of India into Asia, which was riding on another plate. The collision continues today, causing the Himalayas to continually grow taller.
Recently formed mountains, called coastal ranges, rise near the western coasts of North and South America. Older, more stable mountain ranges are found in the interior of continents. The Appalachians of North America and the Urals, on the border between Europe and Asia, are older mountain ranges that are not geologically active.
Even older than these ancient, eroded mountain ranges are flatter, more stable areas of the continents called cratons. A craton is an area of ancient crust that formed during the Earth’s early history. Every continent has a craton. Microcontinents, like New Zealand, lack cratons.
Cratons have two forms: shields and platforms. Shields are bare rocks that may be the roots or cores of ancient mountain ranges that have completely eroded away. Platforms are cratons with sediment and sedimentary rock lying on top.
The Canadian Shield makes up about a quarter of North America. For hundreds of thousands of years, sheets of ice up to 3.2 kilometers (2 miles) thick coated the Canadian Shield. The moving ice wore away material on top of ancient rock layers, exposing some of the oldest formations on Earth. When you stand on the oldest part of the Canadian Shield, you stand directly on rocks that formed more than 3.5 billion years ago.
North America
North America, the third-largest continent, extends from the tiny Aleutian Islands in the northwest to the Isthmus of Panama in the south. The continent includes the enormous island of Greenland (an autonomous territory of Denmark) in the northeast. In the far north, the continent stretches halfway around the world, from Greenland to the Aleutians. But at Panama’s narrowest part, the continent is just 50 kilometers (31 miles) across.
Young mountains—including the Rockies, North America’s largest chain—rise in the West. Some of Earth’s youngest mountains are found in the Cascade Range of the U.S. states of Washington, Oregon and California. Some peaks there began to form only about a million years ago—a wink of an eye in Earth’s long history. North America’s older mountain ranges rise near the East Coast of the United States and Canada.
In between the mountain systems lie wide plains that contain deep, rich soil. Much of the soil was formed from material deposited during the most recent glacial period. This ice age reached its peak about 18,000 years ago. As glaciers retreated, streams of melted ice dropped sediment on the land, building layers of fertile soil in the plains region. Grain grown in this region, called the “breadbasket of North America,” feeds a large part of the world.
North America contains a variety of natural wonders. Landforms and all types of vegetation can be found within its boundaries. North America has deep canyons, such as Copper Canyon in the Mexican state of Chihuahua. Yellowstone National Park, in the U.S. state of Wyoming, has some of the world’s most active geysers. Canada’s Bay of Fundy has the greatest variation of tide levels in the world. The Great Lakes form the planet’s largest area of fresh water. In the U.S. state of California, giant sequoias—the largest tree species in the world—grow more than 76 meters (250 feet) tall and nearly 31 meters (100 feet) around.
Greenland, off the east coast of Canada, is the world’s largest island. Despite its name, Greenland is mostly covered with ice. Its ice is a remnant of the great ice sheets that once blanketed much of the North American continent. Greenland is the only place besides Antarctica that still has an ice sheet.
From the freezing Arctic to the tropical jungles of Central America, North America has more climate variation than any other continent. Almost every type of ecosystem is represented somewhere on the continent, from coral reefs in the Caribbean to Greenland’s ice sheet to the Great Plains in the United States and Canada. North America has two overarching types of ecology, both of which support a wide variety of flora and fauna. One is the Nearctic region, which spans Canada, most of the United States and northern Mexico. Animals native to this region include bison (Bison bison), moose (Alces alces) and the California condor (Gymnogyps californianus). The other is the Neotropical region, which covers southern Mexico and extends south. Animals native to this region include llamas (Lama glama), tapirs and vipers.
Today, North America is made of Canada, the United States, Greenland, Mexico, Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Panama, and the island countries and territories that dot the Caribbean Sea and the western North Atlantic. People have migrated to North America for thousands of years and continue to immigrate to North America today. Since the United States became a country in 1783, more than 86 million people have immigrated to the country, adding to the Indigenous people, colonizers and formerly enslaved people (mostly Africans) already in the country. Despite their lives, cultures and customs being threatened or destroyed by European colonizers, more than 500 Indigenous nations continue to live in North America, including the Inuit of Arctic Canada and Alaska, the Iroquois of the United States and the Nahua of Mexico.
Most of North America sits on the North American Plate. Parts of the Canadian province of British Columbia and the U.S. states of Washington, Oregon, and California sit on the tiny Juan de Fuca Plate. Parts of California and the Mexican state of Baja California sit on the enormous Pacific Plate. Parts of Baja California and the Mexican states of Baja California Sur, Sonora, Sinaloa, and Jalisco sit on the Cocos Plate. The Caribbean Plate carries most of the small islands of the Caribbean Sea (south of the island of Cuba) as well as Central America from Honduras to Panama. The Hawaiian Islands, in the middle of the Pacific Ocean on the Pacific Plate, are usually considered part of North America.
South America
South America is connected to North America by the narrow Isthmus of Panama. These two continents were no’t always connected; they came together only 3 million years ago. South America is the fourth-largest continent and extends from the sunny beaches of the Caribbean Sea to the frigid waters near the Antarctic Circle.
South America’s southernmost islands, called Tierra del Fuego, are less than 1,120 kilometers (700 miles) from Antarctica. These islands even host some Antarctic birds, such as penguins, albatrosses and terns. Though its name comes from Spanish colonizers, the islands were home to many Indigenous groups, including the Yaghan. During colonization, many Yaghan died from European diseases and colonial violence, but there remains a sizable population on the islands, which now belong to Argentina and Chile.
The Andes, Earth’s longest terrestrial mountain range, stretch the entire length of South America. Many active volcanoes dot the range. These volcanic areas are fueled by heat generated as a large oceanic plate, called the Nazca Plate, grinds beneath the plate carrying South America.
The central-southern area of South America has pampas, or plains. These rich areas are ideal for agriculture. Growing wheat is a major industry in the pampas. Grazing animals, such as cattle and sheep, are also raised in the pampas region.
In northern South America, the Amazon River and its tributaries flow through the world’s largest tropical rainforest. In volume, the Amazon is the largest river in the world. More water flows from it than from the next six largest rivers combined.
South America is also home to the world’s tallest waterfall, Angel Falls, in the country of Venezuela. Water flows more than 979 meters (3,212 feet)—almost a mile. The falls are so high that most of the water evaporates into mist or is blown away by wind before it reaches the ground.
South American rainforests contain an enormous wealth of animal and plant life. More than 15,000 species of plants and animals are found only in the Amazon Basin, including the Amazon River dolphin (Inia geoffrensis) and the blue-throated macaw (Ara glaucogularis). Many Amazonian plant species are sources of food and medicine for the rest of the world. Scientists are trying to find ways to preserve this precious and fragile environment as people move into the Amazon Basin and clear land for settlements and agriculture.
Twelve independent countries make up South America: Brazil, Colombia, Argentina, Peru, Venezuela, Chile, Ecuador, Bolivia, Paraguay, Uruguay, Guyana, and Suriname. The territories of French Guiana, once colonized by and now an incorporated part of France, and the Falkland Islands, part of the United Kingdom, are also part of South America. The colonization of South America by Spain and Portugal destroyed many Indigenous cultures, but others, such as the Guarani in Brazil, have successfully fought against their oppressors to maintain their ways of life.
Almost all of South America sits on top of the South American Plate.
Europe
Europe, the sixth-largest continent, contains just 7% of the world’s land. In total area, the continent of Europe is only slightly larger than the country of Canada. However, the population of Europe is more than twice that of South America. Europe has more than 40 countries and many well-known cities, including London, England; Paris, France; Berlin, Germany; Rome, Italy; Madrid, Spain; and Moscow, Russia.
Most European countries have access to the ocean. The continent is bordered by the Arctic Ocean in the north, the Atlantic Ocean in the west, the Caspian Sea in the southeast, and the Mediterranean and Black Seas in the south. The nearness of these bodies of water and the navigation of many of Europe’s rivers played a major role in the continent’s history. Early Europeans learned the river systems of the Volga, Danube, Don, Rhine and Po, and could successfully travel the length and width of the small continent for trade, communication or conquest.
Navigation and colonization outside of Europe was an important part of the development of the continent’s economic, social, linguistic and political legacy. Europeans colonized land on every continent except Antarctica, leading to huge, and often catastrophic, changes for the Indigenous people. European countries extracted natural resources from the countries they colonized, leading to increased wealth for the colonizers at the expense of the people in their colonies, who continue to face wealth disparities and political instability to this day.
In the east, the Ural Mountains separate Europe from Asia. The nations of Russia and Kazakhstan straddle both continents. Another range, the Kjølen Mountains, extends along the northern part of the border between Sweden and Norway. To the south, the Alps form an arc stretching from Albania to Austria, then across Switzerland and northern Italy into France. As the youngest and steepest of Europe’s mountains, the Alps geologically resemble the Rockies of North America, another young range.
A large area of gently rolling plains extends from northern France eastward to the Urals. A climate of warm summers, cold winters and plentiful rain helps make much of this European farmland very productive.
Human development in Europe led to the extinction or near extinction of many wild animals indigenous to the continent. Some indigenous animals that have survived include the Eurasian lynx (Lynx lynx), the Golden eagle (Aquila chrysaetos) and Mediterranean tortoises (Testudo graeca).
Almost all of Europe sits on the massive Eurasian Plate.
Africa
Africa, the second-largest continent, covers an area more than three times that of the United States. From north to south, Africa stretches about 8,000 kilometers (5,000 miles). It is connected to Asia by the Isthmus of Suez in Egypt.
The Sahara, which covers much of North Africa, is the world’s largest hot desert. The world’s longest river, the Nile, flows more than 6,560 kilometers (4,100 miles) from its most remote headwaters in Lake Victoria to the Mediterranean Sea in the north. A series of falls and rapids along the southern part of the river makes navigation difficult. The Nile has played an important role in the history of Africa. In ancient Egyptian civilization, it was a source of life for food, water, and transportation.
The top half of Africa is mostly dry, hot desert. The middle area has savannas, or flat, grassy plains. This region is home to wild animals such as lions (Panthera leo), giraffes, elephants, hyenas, cheetahs (Acinonyx jubatus) and wildebeests. The central and southern areas of Africa are dominated by rainforests. Many of these forests thrive around Africa’s other great rivers, the Zambezi, the Congo and the Niger. These rivers were also home to Great Zimbabwe, the Kingdom of Kongo and the Ghana Empire, respectively. However, trees from the rainforests fed by these rivers are being cut down for many of the same reasons deforestation is taking place in the rainforests of South America and Asia: development for businesses, homes and agriculture.
Much of Africa is a high plateau surrounded by narrow strips of coastal lowlands. Hilly uplands and mountains rise in some areas of the interior. Glaciers on Mount Kilimanjaro in Tanzania sit just miles from the tropical jungles below. Even though Kilimanjaro is not far from the Equator, snow covers its summit all year long.
In eastern Africa, a giant depression called the Great Rift Valley runs from the Red Sea to the country of Mozambique. (The rift valley actually starts in southwestern Asia.) The Great Rift Valley is a site of major tectonic activity, where the continent of Africa is splitting into two. Geologists have already named the two parts of the African Plate. The Nubian Plate carries most of the continent to the west of the rift, while the Somali Plate carries the far eastern part of the continent, including the so-called “Horn of Africa.” The Horn of Africa is a peninsula that resembles the upturned horn of a rhinoceros. The countries of Eritrea, Ethiopia, Djibouti and Somalia sit on the Horn of Africa and the Somali Plate.
Africa is home to 56 countries but only 18.3% of the world’s total population. The area of central-eastern Africa is important to scientists who study evolution and the earliest origins of humanity. This area is thought to be the place where hominins began to evolve. During the era of colonization, more than 12.5 million Africans were kidnapped from Africa and enslaved as part of the transatlantic slave trade. Later, nearly all of Africa was colonized by Europe, until the beginning in the mid-20th century when African leaders began to break free. Today, many indigenous groups in Africa continue to fight for their autonomy. The Maasai in Tanzania, for example, who raise cattle as part of their traditional culture, are fighting for land that is being taken away to use for other agricultural purposes or game reserves.
The entire continent of Africa sits on the African Plate.
Asia
Asia, the largest continent, stretches from the eastern Mediterranean Sea to the western Pacific Ocean. There are more than 40 countries in Asia. Some are among the most-populated countries in the world, including China, India and Indonesia. About 60 percent of Earth’s population lives in Asia. More than a third of the world’s people live in China and India alone. Asia has the world’s highest population of Indigenous people of any continent, but some, like the Hmong of Southeast Asia, have faced systemic persecution. Asia includes many islands, some of them countries. The Philippines, Indonesia, Japan and Taiwan are major island nations in Asia.
Most of Asia’s people live in cities or fertile farming areas near river valleys, plains and coasts. The plateaus in Central Asia are largely unsuitable for farming and are thinly populated.
Asia has some of the world’s most desired natural resources, which European countries sought to exploit through colonization. Through the years, European colonizers have gained wealth through the control of spices, opium, rubber and oil. Today, foreign powers compete for political influence and access to natural resources, such as rare earth materials, lithium, timber, oil and natural gas.
Asia accounts for almost a third of the world’s land. The continent has a wide range of climate regions, from polar in the Siberian Arctic to tropical in equatorial Indonesia. Parts of Central Asia, including the Gobi Desert in China and Mongolia, are dry year-round. Southeast Asia, on the other hand, depends on the annual monsoons, which bring rain and make agriculture possible. Asia’s climate and topography vary greatly, providing ecosystems for a wide variety of animals, including King cobras, Asian elephants (Elephas maximus) and Oriental scops owls (Otus sunia).Monsoon rains and snowmelt feed Asian rivers, such as the Ganges, the Yellow, the Mekong, the Indus and the Yangtze. The rich valley between the Tigris and Euphrates Rivers in western Asia is called the “Fertile Crescent” for its place in the development of agriculture and human civilization.Asia is the most mountainous of all the continents. More than 50 of the highest peaks in the world are in Asia. Mount Everest, which reaches more than 8,700 meters (29,000 feet) high in the Himalayan range, is the highest point on Earth. These mountains have become major destination spots for adventurous travelers.Plate tectonics continuously push the mountains higher. As the landmass of India pushes northward into the landmass of Eurasia, parts of the Himalayas rise at a rate of about 2.5 centimeters (1 inch) every five years. Asia contains not only Earth’s highest elevation, but also its lowest place on land: the shores of the Dead Sea in Israel, Jordan and the Palestinian West Bank (under Israeli control). The land there lies more than 390 meters (1,300 feet) below sea level.
Although the Eurasian Plate carries most of Asia, it is not the only one supporting major parts of the large continent. The Arabian Peninsula, in the continent’s southwest, is carried by the Arabian Plate. The Indian Plate supports the Indian peninsula, sometimes called the South Asian subcontinent. The Australian Plate carries some islands in Indonesia. The North American Plate carries eastern Siberia and the northern islands of Japan.
Australia
In addition to being the smallest continent, Australia is the flattest and the second-driest, after Antarctica. The continent is sometimes called Oceania, to include the thousands of tiny islands of the Central and South Pacific, most notably Melanesia, Micronesia and Polynesia (including the U.S. state of Hawai‘i). However, the continent of Australia itself includes only the nation of Australia, the eastern portion of the island of New Guinea (part of the nation of Papua New Guinea) and the island nation of New Zealand.Australia covers just fewer than 8.5 million square kilometers (about 3.5 million square miles). Its population is about 31 million. It is the most sparsely populated continent, after Antarctica.A plateau in the middle of mainland Australia makes up most of the continent’s total area. Rainfall is light on the plateau, and not many people have settled there. The Great Dividing Range, a long mountain range, rises near the east coast and extends from the northern part of the territory of Queensland through the territories of New South Wales and Victoria. Mainland Australia is known for the Outback, a desert area in the interior. This area is so dry, hot, and barren that few people live there.In addition to the hot plateaus and deserts in mainland Australia, the continent also features lush equatorial rain forests on the island of New Guinea, tropical beaches, and high mountain peaks and glaciers in New Zealand. Most of Australia’s people live in cities along the southern and eastern coasts of the mainland. Major cities include Perth, Sydney, Brisbane, Melbourne and Adelaide, all in Australia. The country of Australia has two main Indigenous groups: Aboriginal and Torres Strait Islander. European colonization of Australia threatened and harmed the culture and health of both Indigenous groups, but they were able to resist and protect their way of life, which they continue to do to this day.
Biologists who study animals consider Australia a living laboratory. When the continent began to break away from Antarctica more than 60 million years ago, it carried a cargo of animals with it. Isolated from life on other continents, the animals developed into creatures unique to Australia, such as the koala (Phascolarctos cinereus), the platypus (Ornithorhynchus anatinus) and the Tasmanian devil (Sarcophilus harrisii).The Great Barrier Reef, off mainland Australia’s northeast coast, is another living laboratory. The world’s largest coral reef ecosystem, it is home to thousands of species of fish, sponges, marine mammals, corals, and crustaceans. The reef itself is 1,920 kilometers (1,200 miles) of living coral communities. By some estimates, it is the world’s largest living organism. Most of Australia sits on the Australian Plate. The southern part of the South Island of New Zealand sits on the Pacific Plate.
Antarctica
Antarctica is the windiest, driest and iciest place on Earth. Antarctica is larger than Europe or Australia, but unlike those continents, it has no permanent human population. People who live and work there are scientific researchers and support staff, such as pilots and cooks. The large animals that live in Antarctica, such as penguins, albatrosses and seals, generally rely on the sea to survive. The climate of Antarctica makes it impossible to support agriculture or a permanent civilization. Temperatures in Antarctica, much lower than Arctic temperatures, plunge lower than -73 degrees Celsius (-100 degrees Fahrenheit).Scientific bases and laboratories have been established in Antarctica for studies in fields like geology, oceanography and meteorology. The freezing temperatures of Antarctica make it an excellent place to study the history of Earth’s atmosphere and climate. Ice cores from the massive Antarctic ice sheet have recorded changes in Earth’s temperature and atmospheric gases for thousands of years. Antarctica is also an ideal place for discovering meteorites, or stony objects that have impacted Earth from outer space. The dark meteorites, often made of metals like iron, stand out from the white landscape of most of the continent.Antarctica is almost completely covered with ice, sometimes as thick as 3.2 kilometers (2 miles). In winter, Antarctica’s surface area may double as pack ice builds up in the ocean around the continent. Like all other continents, Antarctica has volcanic activity. The most active volcano is Mount Erebus, which is less than 1,392 kilometers (870 miles) from the South Pole. Evidence of its frequent eruptions can be found in the hot, molten rock beneath the continent’s icy surface.Antarctica does not have any countries. However, scientific groups from different countries inhabit the research stations. A multinational treaty negotiated in 1959 and reviewed in 1991 states that research in Antarctica can only be used for peaceful purposes. McMurdo Station, the largest community in Antarctica, is operated by the United States. Vostok Station, where the coldest temperature on Earth was recorded, is operated by Russia. All of Antarctica sits on the Antarctic Plate.
Additional Information
A continent is one of the larger continuous masses of land, namely, Asia, Africa, North America, South America, Antarctica, Europe, and Australia, listed in order of size. (Europe and Asia are sometimes considered a single continent, Eurasia.)
There is great variation in the sizes of continents; Asia is more than five times as large as Australia. The largest island in the world, Greenland, is only about one-fourth the size of Australia. The continents differ sharply in their degree of compactness. Africa has the most regular coastline and, consequently, the lowest ratio of coastline to total area. Europe is the most irregular and indented and has by far the highest ratio of coastline to total area.
The continents are not distributed evenly over the surface of the globe. If a hemisphere map centred in northwestern Europe is drawn, most of the world’s land area can be seen to lie within that hemisphere. More than two-thirds of the Earth’s land surface lies north of the Equator, and all the continents except Antarctica are wedge shaped, wider in the north than they are in the south.
The distribution of the continental platforms and ocean basins on the surface of the globe and the distribution of the major landform features have long been among the most intriguing problems for scientific investigation and theorizing. Among the many hypotheses that have been offered as explanation are: (1) the tetrahedral (four-faced) theory, in which a cooling earth assumes the shape of a tetrahedron by spherical collapse; (2) the accretion theory, in which younger rocks attached to older shield areas became buckled to form the landforms; (3) the continental-drift theory, in which an ancient floating continent drifted apart; and (4) the convection-current theory, in which convection currents in the Earth’s interior dragged the crust to cause folding and mountain making.
Geological and seismological evidence accumulated in the 20th century indicates that the continental platforms do “float” on a crust of heavier material that forms a layer completely enveloping the Earth. Each continent has one of the so-called shield areas that formed 2 billion to 4 billion years ago and is the core of the continent to which the remainder (most of the continent) has been added. Even the rocks of the extremely old shield areas are older in the centre and younger toward the margins, indicating that this process of accumulation started early. In North America the whole northeast quarter of the continent, called the Canadian, or Laurentian, Shield, is characterized by the ancient rocks of what might be called the original continent. In Europe the shield area underlies the eastern Scandinavian peninsula and Finland. The Guiana Highlands of South America are the core of that continent. Much of eastern Siberia is underlain by the ancient rocks, as are western Australia and southern Africa.
#103 Science HQ » Zinc » 2025-07-06 20:19:02
- Jai Ganesh
- Replies: 0
Zinc
Gist
Zinc is found in cells throughout the body. It is needed for the body's defensive (immune) system to properly work. It plays a role in cell division, cell growth, wound healing, and the breakdown of carbohydrates. Zinc is also needed for the senses of smell and taste.
Summary
The metallic element zinc is commonly used to coat buckets, rainspouts, and other iron or steel objects to prevent rusting. There are, however, many other uses for this bluish white and fairly soft metal. All brass contains zinc. Zinc is an ingredient in some medicines. In the human body, zinc is an essential trace element. In the red blood cells it helps to metabolize carbon dioxide, and the zinc present in the pancreas may aid in the storage of insulin.
Element Properties
Symbol : Zn
Atomic number : 30
Atomic weight : 65.39
Group in periodic table : 12 (IIb)
Boiling point : 1,665 °F (907 °C)
Melting point : 788 °F (420 °C)
Specific gravity : 7.13
The first people to recognize zinc as a distinct metal were apparently the Hindus in about the 13th century. In the West, commercial zinc production got under way in England by the middle of the 18th century.
A large share of the zinc produced today is used for galvanizing iron and steel—that is, coating them with zinc to make them rustproof. Brass making generally ranks second in the use of zinc. The different types of brass contain from 5 to 45 percent zinc. For many purposes, zinc is simply flattened into sheets called rolled zinc. These sheets are used in the manufacture of many roofing products, refrigerator linings, and printing plates.
The compounds of zinc have numerous uses. Because of its high heat conductivity, zinc oxide is used in rubber as a heat dissipater. Zinc sulfate is used in weed killers. Zinc sulfide has been used in X-ray screens and in luminous dials for clocks and watches. Zinc compounds are also used in medicines and toiletries. Metallic zinc is distilled from crushed ore by heating it with carbon (usually coal), and condensing the zinc vapor. Zinc is also separated from the ore by electrolysis.
Details
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity (electrowinning).
Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an important cofactor for many enzymes, and the only metal which appears in all enzyme classes. Zinc is also an essential nutrient element for coral growth.
Zinc deficiency affects about two billion people in the developing world and is associated with many diseases. In children, deficiency causes growth retardation, delayed sexual maturation, infection susceptibility, and diarrhea. Enzymes with a zinc atom in the reactive center are widespread in biochemistry, such as alcohol dehydrogenase in humans. Consumption of excess zinc may cause ataxia, lethargy, and copper deficiency. In marine biomes, notably within polar regions, a deficit of zinc can compromise the vitality of primary algal communities, potentially destabilizing the intricate marine trophic structures and consequently impacting biodiversity.
Brass, an alloy of copper and zinc in various proportions, was used as early as the third millennium BC in the Aegean area and the region which currently includes Iraq, the United Arab Emirates, Kalmykia, Turkmenistan and Georgia. In the second millennium BC it was used in the regions currently including West India, Uzbekistan, Iran, Syria, Iraq, and Israel. Zinc metal was not produced on a large scale until the 12th century in India, though it was known to the ancient Romans and Greeks. The mines of Rajasthan have given definite evidence of zinc production going back to the 6th century BC.[23] The oldest evidence of pure zinc comes from Zawar, in Rajasthan, as early as the 9th century AD when a distillation process was employed to make pure zinc.[24] Alchemists burned zinc in air to form what they called "philosopher's wool" or "white snow".
The element was probably named by the alchemist Paracelsus after the German word Zinke (prong, tooth). German chemist Andreas Sigismund Marggraf is credited with discovering pure metallic zinc in 1746. Work by Luigi Galvani and Alessandro Volta uncovered the electrochemical properties of zinc by 1800.
Corrosion-resistant zinc plating of iron (hot-dip galvanizing) is the major application for zinc. Other applications are in electrical batteries, small non-structural castings, and alloys such as brass. A variety of zinc compounds are commonly used, such as zinc carbonate and zinc gluconate (as dietary supplements), zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and dimethylzinc or diethylzinc in the organic laboratory.
Additional Information:
Occurrence, uses, and properties
A little more abundant than copper, zinc makes up an average of 65 grams (2.3 ounces) of every ton of Earth’s crust. The chief zinc mineral is the sulfide sphalerite (zinc blende), which, together with its oxidation products smithsonite and hemimorphite, constitute nearly all of the world’s zinc ore. Native zinc has been reported from Australia, New Zealand, and the United States, and the leading early 21st-century producers of zinc are China, Australia and Peru. For zinc’s mineralogical properties, see native element.
Zinc is an essential trace element in the human body, where it is found in high concentration in the red blood cells as an essential part of the enzyme carbonic anhydrase, which promotes many reactions relating to carbon dioxide metabolism. The zinc present in the pancreas may aid in the storage of insulin. Zinc is a component of some enzymes that digest protein in the gastrointestinal tract. Zinc deficiency in nut-bearing and fruit trees causes such diseases as pecan rosette, little leaf, and mottle leaf. Zinc functions in the hemosycotypsin of snails’ blood to transport oxygen in a way analogous to iron in the hemoglobin of human blood.
Metallic zinc is produced by roasting the sulfide ores and then either leaching the oxidized product in sulfuric acid or smelting it in a blast furnace. Zinc is won from the leach solution by electrolysis or is condensed from the blast furnace gas and then distilled of impurities.
The major uses of zinc metal are in galvanizing iron and steel against corrosion and in making brasses and alloys for die-casting. Zinc itself forms an impervious coating of its oxide on exposure to the atmosphere, and hence the metal is more resistant to ordinary atmospheres than iron and corrodes at a much lower rate. In addition, because zinc tends to oxidize in preference to iron, some protection is afforded the steel surface even if some of it is exposed through cracks. The zinc coating is formed either by hot-dip galvanizing or electrogalvanizing.
Hot-dip galvanizing is the most common procedure for coating steel with zinc. This may be a batch process known as general galvanizing or a continuous coating of coils of steel strip. In general galvanizing, steel is pickled in acid, treated with fluxing agents, and then dipped in a bath of molten zinc at about 450 °C (840 °F). Layers of iron-zinc alloy are formed on the surface and are topped with an outer layer of zinc. Objects so treated range from small nuts and bolts to steel window frames and large girders used in construction. An ordinary grade of zinc containing up to 1.5 percent lead is normally used in this process.
In electrogalvanizing, zinc is deposited on a steel strop in as many as 20 consecutive electrolytic coating cells. There are several successful cell designs; the simple vertical cell is discussed here to explain the principle. The strip, connected to the negative side of a direct current through large-diameter conductor rolls located above and between two cells, is dipped into a tank of electrolyte by a submerged sink roll. Partially submerged anodes, opposing the strip, are connected to the positive side of the electric current by heavy bus bars. Zinc cations (i.e., positively charged zinc atoms) present in the electrolyte are converted by the current into regular zinc atoms, which deposit on the strip. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously added to the electrolyte. In the latter case the anodes are made of insoluble materials, such as titanium coated with iridium oxide. The electrolyte is an acidic solution of zinc sulfide or zinc chloride with other bath additions to improve the quality of the coating and the current efficiency. Coating thickness is easier to control than in the hot-dip process because of the good relationship between electrical current and deposited zinc.
The negative electrode (outside can) in one common type of electric dry cell is composed of zinc. Another important series of alloys are those formed by the addition of 4 to 5 percent aluminum to zinc; these have a relatively low melting point but possess good mechanical properties and can be cast under pressure in steel dies. Considerable quantities of zinc in the rolled form are used for roofing, particularly in Europe; small additions of copper and titanium improve creep resistance—i.e., resistance to gradual deformation.
Freshly cast zinc has a bluish silver surface but slowly oxidizes in air to form a grayish protective oxide film. Highly pure zinc (99.99 percent) is ductile; the so-called prime western grade (99.8 percent pure) is brittle when cold but above 100 °C (212 °F) can be rolled into sheets that remain flexible. Zinc crystallizes in the hexagonal close-packed structure. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the Zn2+ ion) preferentially because of its higher electrode potential. This so-called sacrificial protection, coupled with the much greater corrosion resistance of zinc under atmospheric conditions, is the basis for galvanizing.
Natural zinc is a mixture of five stable isotopes: 64Zn (48.6 percent), 66Zn (27.9 percent), 67Zn (4.1 percent), 68Zn (18.8 percent), and 70Zn (0.6 percent).
History
Metallic zinc appeared much later in history than the other common metals. Copper, lead, tin, and iron can be obtained as the molten metals by heating their oxide ores with charcoal (carbon), a process called reduction, in shaft furnaces, which were developed quite early in history. Zinc oxide, however, cannot be reduced by carbon until temperatures are reached well above the relatively low boiling point of the metal (907 °C). Thus, the furnaces developed to smelt the other metals could not produce zinc. Small quantities of metallic zinc can sometimes be found in the flues of lead blast furnaces.
There is some evidence that the Greeks knew of the existence of zinc and called it pseudargyras, or “false silver,” but they had no method of producing it in quantity. The Romans as early as 200 bce produced considerable quantities of brass, an alloy of zinc and copper, by heating in crucibles a mixture of zinc oxide and charcoal covered with lumps of metallic copper. The zinc oxide was reduced in the lower part of the crucible. Zinc vapour was formed and dissolved in the copper to form brass. At the end of the process the temperature was raised to melt the brass for casting into ingots. Brass production was the Romans’ only use of zinc.
The realization that to make zinc it was necessary to produce the metal as a vapour and then condense it seems first to have been reached in India in the 13th or 14th century. The metallurgists of China had achieved large-scale production of zinc by the 16th century. In the West this principle was first applied in England in 1743 under the leadership of William Champion. At the end of the 18th century in Belgium and Poland improvements were made in the furnace, and the process remained unchanged until an electrolytic process was developed in 1917. At the end of the 1920s a radical advance was made in the United States by developing a continuous retort process, and during the 1930s an electrothermic process was designed for producing zinc continuously. A development of the 1960s was the zinc-lead blast furnace, in which rapid quenching of the gases is a key principle. Zinc production processes are treated in detail in zinc processing.
Compounds
In chemical compounds, zinc exhibits almost exclusively a +2 oxidation state. A few compounds of zinc in the +1 state have been reported, but never any compounds of zinc in the +3 state or higher.
Zinc oxide, ZnO, is one of the most important zinc compounds. It can be prepared in a state of high purity and in a variety of crystal shapes and sizes by burning zinc vapour in air. Because of its high heat conductivity and capacity, zinc oxide is frequently incorporated into rubber as a heat dissipater. In the crystal of zinc oxide, the lattice (i.e., the orderly structure formed by the ions) is an open one in which the zinc and oxygen ions occupy only 44 percent of the volume. Defects can be created in the lattice by specific treatments such as the introduction of foreign atoms or of zinc atoms in the vacancies of the lattice. Such treatment of zinc oxide crystals produces various electrical, photoelectrical, and catalytic properties. As a result, zinc oxide is used as a semiconductor in the production of phosphors for television tubes and fluorescent lamps. Its effects on the reactivity of many compounds make it useful as a catalyst in such operations as the manufacture of synthetic rubber and methanol. It is also used in paints, cosmetics, plastics, pharmaceuticals, and printing inks. Because under the influence of light the electrical conductivity of zinc oxide can be increased many times, it is employed in certain photocopying processes.
Zinc sulfate, ZnSO4, is an intermediate compound in the production of zinc from its ores by the electrolytic process. It is used as a weed killer, in the manufacture of viscose rayon, and in dyeing, in which it functions as a mordant. Zinc chloride, ZnCl2, can be prepared by a direct reaction or by evaporating the aqueous solution formed in various reactions. It is strongly deliquescent (water-absorbing) and is utilized as a drying agent and as a flux. In aqueous solution it is used as a wood preservative. Zinc sulfide, ZnS, occurs in nature as the mineral sphalerite and may be prepared by treating solutions of zinc salts with hydrogen sulfide. It was long used as a white pigment but has been gradually replaced by titanium dioxide. Zinc sulfide has luminescent properties when activated by the addition of small quantities of copper, manganese, silver, and so has been used in X-ray screens, in luminous dials for clocks and watches, and in fluorescent lights.

#104 Dark Discussions at Cafe Infinity » Classifified Quotes - I » 2025-07-06 18:35:14
- Jai Ganesh
- Replies: 0
Classified Quotes - I
1. There are four ways, and only four ways, in which we have contact with the world. We are evaluated and classified by these four contacts: what we do, how we look, what we say, and how we say it. - Dale Carnegie
2. I had a very strong feeling about the Vietnam War, and I had a strong feeling about participating in it. The military draft was in place, I was summoned for a physical exam, and I was either going to be classified as fit for military service or make my objection to it. So I made my objection to it. - Harrison Ford
3. The United States strongly condemns the illegal disclosure of classified information. It puts people's lives in danger, threatens our national security, and undermines our efforts to work with other countries to solve shared problems. - Hillary Clinton
4. But there are advantages to being elected President. The day after I was elected, I had my high school grades classified Top Secret. - Ronald Reagan
5. Searching for alternative life on Earth might seem misconceived, because there is excellent evidence that every kind of life so far studied evolved from a common ancestor that lived billions of years ago. Yet most of the life that exists on Earth has never been properly classified. - Paul Davies
6. When Ford sells a car, a dealer isn't allowed to take out the engine and put a different one in. When a newsstand sells the Washington Post, no one can go to the newsstand and pay them to rip out the classified section and put their own classified section in - if they could, they would do so. - Bill Gates
7. I'm not a do-gooder. It embarrassed me to be classified as a humanitarian. I simply take part in activities that I believe in. - Gregory Peck
8. I'm a very determined businesswoman... I've got lots of things to do, and I don't have time to be classified as difficult, and I don't have time. - Kim Basinger.
#105 Jokes » Dancer Jokes - IV » 2025-07-06 18:11:07
- Jai Ganesh
- Replies: 0
Q: What do you get if you cross a computer with a ballet dancer?
A: The Netcracker suite.
* * *
Q: Where did the hamburger go to dance?
A: At the Meat ball.
* * *
Q: Where do one-legged dancers go for Breakfast?
A: Ihop.
* * *
Q: What do tired line dancers do?
A: They Line Down.
* * *
Q: What do you call a line dancer on a cruise?
A: An Ocean "Liner".
* * *
#106 Re: Jai Ganesh's Puzzles » General Quiz » 2025-07-06 17:04:18
Hi,
#10437. What does the term in Biology Selective breeding (also called artificial selection) mean?
#10438. What does the term in Biology Asexual reproduction mean?
#107 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-07-06 16:53:53
Hi,
#5627. What does the verb (used with object) paddle mean?
#5628. What does the noun pail mean?
#108 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-07-06 16:30:13
Hi,
#2404. What does the medical term Peritonitis mean?
#109 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-07-06 16:18:22
Hi,
#9669.
#110 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-07-06 16:10:29
Hi,
#6174.
#111 Re: Exercises » Compute the solution: » 2025-07-06 15:33:15
Hi,
2415.
#112 Dark Discussions at Cafe Infinity » Classics Quotes » 2025-07-05 22:05:21
- Jai Ganesh
- Replies: 0
Classics Quotes
1. I don't read a great deal of fiction, to my shame, other than the classics. - Richard Attenborough
2. Whenever I am abroad, I spend hours and hours at video stores. I look for classics from filmmakers from all over the world. - Mani Ratnam
3. The early versions of 'Shell's Wonderful World of Golf' were great. It's sort of interesting: as it progressed, it became worse and worse, but the early versions were really fantastic with Jimmy Demaret and Gene Sarazen. They were classics. - Donald Trump
4. I love the Russian classics very much, the Russian classical literature. But I also read modern literature. As far as Russian literature is concerned, I am very fond of Tolstoy and Chekhov, and I also enjoy reading Gogol very much. - Vladimir Putin
5. Even in Hollywood, filmmakers are re-creating classics at regular intervals. - Mohanlal
6. I like all kinds of music, be it old Hindi movie songs or English classics. - Dimple Kapadia
7. Like every actor I too have some 'classics' in the closet, but I am not keen on adding to that collection. - Mahima Chaudhry.
#113 Jokes » Dancer Jokes - III » 2025-07-05 21:41:49
- Jai Ganesh
- Replies: 0
Q: What dance do women do when summer is over?
A: Tango (tan-go).
* * *
Q: What do you call dancing candy?
A: Sour cabbage patch kids.
* * *
Q: Which dance will a chicken not do?
A: The foxtrot!
* * *
Q: Where do fortune tellers dance?
A: At the crystal ball.
* * *
Q: Why did the dancer say in math class?
A: 2, 4, 6, 8.
* * *
#114 Science HQ » Copper » 2025-07-05 21:26:30
- Jai Ganesh
- Replies: 0
Copper
Gist
Copper is a reddish-brown metallic chemical element, known for its excellent conductivity of heat and electricity. It has the symbol Cu and atomic number 29. Copper is a soft, malleable, and ductile metal used in various applications, including electrical wiring, plumbing, and as a component in many alloys.
Copper is a reddish-brown metal known for its excellent electrical and thermal conductivity, malleability, and ductility. It's also highly corrosion-resistant, especially when it develops a protective patina. These characteristics make it a versatile material used in various applications, including electrical wiring, plumbing, and heat exchangers.
Summary
Copper is a chemical element; it has symbol Cu (from Latin cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement.
Copper is one of the few metals that can occur in nature in a directly usable, unalloyed metallic form. This means that copper is a native metal. This led to very early human use in several regions, from c. 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, c. 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create bronze, c. 3500 BC.
Commonly encountered compounds are copper(II) salts, which often impart blue or green colors to such minerals as azurite, malachite, and turquoise, and have been used widely and historically as pigments.
Copper used in buildings, usually for roofing, oxidizes to form a green patina of compounds called verdigris. Copper is sometimes used in decorative art, both in its elemental metal form and in compounds as pigments. Copper compounds are used as bacteriostatic agents, fungicides, and wood preservatives.
Copper is essential to all living organisms as a trace dietary mineral because it is a key constituent of the respiratory enzyme complex cytochrome c oxidase. In molluscs and crustaceans, copper is a constituent of the blood pigment hemocyanin, replaced by the iron-complexed hemoglobin in fish and other vertebrates. In humans, copper is found mainly in the liver, muscle, and bone. The adult body contains between 1.4 and 2.1 mg of copper per kilogram of body weight.
Details
Copper (Cu), chemical element, a reddish, extremely ductile metal of Group 11 (Ib) of the periodic table that is an unusually good conductor of electricity and heat. Copper is found in the free metallic state in nature. This native copper was first used (c. 8000 bce) as a substitute for stone by Neolithic (New Stone Age) humans. Metallurgy dawned in Mesopotamia as copper was cast to shape in molds (c. 4000 bce), was reduced to metal from ores with fire and charcoal, and was intentionally alloyed with tin as bronze (c. 3500 bce). The Roman supply of copper came almost entirely from Cyprus. It was known as aes Cyprium, “metal of Cyprus,” shortened to cyprium and later corrupted to cuprum.
Element Properties
atomic number : 29
atomic weight : 63.546
melting point : 1,083 °C (1,981 °F)
boiling point : 2,567 °C (4,653 °F)
density : 8.96 at 20 °C (68 °F)
valence : 1, 2
Occurrence, uses, and properties
Native copper is found at many locations as a primary mineral in basaltic lavas and also as reduced from copper compounds, such as sulfides, math, chlorides, and carbonates. (For mineralogical properties of copper, see the table of native elements.) Copper occurs combined in many minerals, such as chalcocite, chalcopyrite, bornite, cuprite, malachite, and azurite. It is present in the ashes of seaweeds, in many sea corals, in the human liver, and in many mollusks and arthropods. Copper plays the same role of oxygen transport in the hemocyanin of blue-blooded mollusks and crustaceans as iron does in the hemoglobin of red-blooded animals. The copper present in humans as a trace element helps catalyze hemoglobin formation. A porphyry copper deposit in the Andes Mountains of Chile is the greatest known deposit of the mineral. By the early 21st century Chile had become the world’s leading producer of copper. Other major producers include Peru, China, and the United States.
Copper is commercially produced mainly by smelting or leaching, usually followed by electrodeposition from sulfate solutions. The major portion of copper produced in the world is used by the electrical industries; most of the remainder is combined with other metals to form alloys. (It is also technologically important as an electroplated coating.) Important series of alloys in which copper is the chief constituent are brasses (copper and zinc), bronzes (copper and tin), and nickel silvers (copper, zinc, and nickel, no silver). There are many useful alloys of copper and nickel, including Monel; the two metals are completely miscible. Copper also forms an important series of alloys with aluminum, called aluminum bronzes. Beryllium copper (2 percent Be) is an unusual copper alloy in that it can be hardened by heat treatment. Copper is a part of many coinage metals. Long after the Bronze Age passed into the Iron Age, copper remained the metal second in use and importance to iron. By the 1960s, however, cheaper and much more plentiful aluminum had moved into second place in world production.
Copper is one of the most ductile metals, not especially strong or hard. Strength and hardness are appreciably increased by cold-working because of the formation of elongated crystals of the same face-centred cubic structure that is present in the softer annealed copper. Common gases, such as oxygen, nitrogen, carbon dioxide, and sulfur dioxide are soluble in molten copper and greatly affect the mechanical and electrical properties of the solidified metal. The pure metal is second only to silver in thermal and electrical conductivity. Natural copper is a mixture of two stable isotopes: copper-63 (69.15 percent) and copper-65 (30.85 percent).
Because copper lies below hydrogen in the electromotive series, it is not soluble in acids with the evolution of hydrogen, though it will react with oxidizing acids, such as nitric and hot, concentrated sulfuric acid. Copper resists the action of the atmosphere and seawater. Exposure for long periods to air, however, results in the formation of a thin green protective coating (patina) that is a mixture of hydroxocarbonate, hydroxosulfate, and small amounts of other compounds. Copper is a moderately noble metal, being unaffected by nonoxidizing or noncomplexing dilute acids in the absence of air. It will, however, dissolve readily in nitric acid and in sulfuric acid in the presence of oxygen. It is also soluble in aqueous ammonia or potassium cyanide in the presence of oxygen because of the formation of very stable cyano complexes upon dissolution. The metal will react at red heat with oxygen to give cupric oxide, CuO, and, at higher temperatures, cuprous oxide, Cu2O. It reacts on heating with sulfur to give cuprous sulfide, Cu2S.
Principal compounds
Copper forms compounds in the oxidation states +1 and +2 in its normal chemistry, although under special circumstances some compounds of trivalent copper can be prepared. It has been shown that trivalent copper survives no more than a few seconds in an aqueous solution.
cuprite
cupriteCuprite from Namibia.
Copper(I) (cuprous) compounds are all diamagnetic and, with few exceptions, colourless. Among the important industrial compounds of copper(I) are cuprous oxide (Cu2O), cuprous chloride (Cu2Cl2), and cuprous sulfide (Cu2S). Cuprous oxide is a red or reddish brown crystal or powder that occurs in nature as the mineral cuprite. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. The pure compound is insoluble in water but soluble in hydrochloric acid or ammonia. Cuprous oxide is used principally as a red pigment for antifouling paints, glasses, porcelain glazes, and ceramics and as a seed or crop fungicide.
Cuprous chloride is a whitish to grayish solid that occurs as the mineral nantokite. It is usually prepared by reduction of copper(II) chloride with metallic copper. The pure compound is stable in dry air. Moist air converts it to a greenish oxygenated compound, and upon exposure to light it is transformed into copper(II) chloride. It is insoluble in water but dissolves in concentrated hydrochloric acid or in ammonia because of the formation of complex ions. Cuprous chloride is used as a catalyst in a number of organic reactions, notably the synthesis of acrylonitrile from acetylene and hydrogen cyanide; as a decolourizing and desulfurizing agent for petroleum products; as a denitrating agent for cellulose; and as a condensing agent for soaps, fats, and oils.
Cuprous sulfide occurs in the form of black powder or lumps and is found as the mineral chalcocite. Large quantities of the compound are obtained by heating cupric sulfide (CuS) in a stream of hydrogen. Cuprous sulfide is insoluble in water but soluble in ammonium hydroxide and nitric acid. Its applications include use in solar cells, luminous paints, electrodes, and certain varieties of solid lubricants.
Copper(II) compounds of commercial value include cupric oxide (CuO), cupric chloride (CuCl2), and cupric sulfate (CuSO4). Cupric oxide is a black powder that occurs as the minerals tenorite and paramelaconite. Large amounts are produced by roasting mixed copper oxide ores in a furnace at a temperature below 1,030 °C (1,900 °F). The pure compound can be dissolved in acids and alkali cyanides. Cupric oxide is employed as a pigment (blue to green) for glasses, porcelain glazes, and artificial gems. It is also used as a desulfurizing agent for petroleum gases and as an oxidation catalyst.
Cupric chloride is a yellowish to brown powder that readily absorbs moisture from the air and turns into the greenish blue hydrate, CuCl2∙2H2O. The hydrate is commonly prepared by passing chlorine and water in a contacting tower packed with metallic copper. The anhydrous salt is obtained by heating the hydrate to 100 °C (212 °F). Like cuprous chloride, cupric chloride is used as a catalyst in a number of organic reactions—e.g., in chlorination of hydrocarbons. In addition, it serves as a wood preservative, mordant (fixative) in the dyeing and printing of fabrics, disinfectant, feed additive, and pigment for glass and ceramics.
Cupric sulfate is a salt formed by treating cupric oxide with sulfuric acid. It forms as large, bright blue crystals containing five molecules of water (CuSO4∙5H2O) and is known in commerce as blue vitriol. The anhydrous salt is produced by heating the hydrate to 150 °C (300 °F). Cupric sulfate is utilized chiefly for agricultural purposes, as a pesticide, germicide, feed additive, and soil additive. Among its minor uses are as a raw material in the preparation of other copper compounds, as a reagent in analytic chemistry, as an electrolyte for batteries and electroplating baths, and in medicine as a locally applied fungicide, bactericide, and astringent.
Other important copper(II) compounds include cupric carbonate, Cu2(OH)2CO3, which is prepared by adding sodium carbonate to a solution of copper sulfate and then filtering and drying the product. It is used as a colouring agent.
Additional Information:
Appearance
A reddish-gold metal that is easily worked and drawn into wires.
Uses
Historically, copper was the first metal to be worked by people. The discovery that it could be hardened with a little tin to form the alloy bronze gave the name to the Bronze Age.
Traditionally it has been one of the metals used to make coins, along with silver and gold. However, it is the most common of the three and therefore the least valued. All US coins are now copper alloys, and gun metals also contain copper.
Most copper is used in electrical equipment such as wiring and motors. This is because it conducts both heat and electricity very well, and can be drawn into wires. It also has uses in construction (for example roofing and plumbing), and industrial machinery (such as heat exchangers).
Copper sulfate is used widely as an agricultural poison and as an algicide in water purification.
Copper compounds, such as Fehling’s solution, are used in chemical tests for sugar detection.
Biological role
Copper is an essential element. An adult human needs around 1.2 milligrams of copper a day, to help enzymes transfer energy in cells. Excess copper is toxic.
Genetic diseases, such as Wilson’s disease and Menkes’ disease, can affect the body’s ability to use copper properly.
Unlike mammals, which use iron (in haemoglobin) to transport oxygen around their bodies, some crustaceans use copper complexes.
Natural abundance
Copper metal does occur naturally, but by far the greatest source is in minerals such as chalcopyrite and bornite. Copper is obtained from these ores and minerals by smelting, leaching and electrolysis. The major copper-producing countries are Chile, Peru and China.

#115 Re: Jai Ganesh's Puzzles » General Quiz » 2025-07-05 17:27:14
Hi,
#10435. What does the term in Biology Antibiotic mean?
#10436. What does the term in Biology Archaea mean?
#116 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-07-05 17:04:54
Hi,
#5625. What does the verb (used with or without object) masticate mean?
#5626. What does the noun mastiff mean?
#117 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-07-05 16:34:12
Hi,
#2403. What does the medical term Olfaction mean?
#118 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-07-05 16:16:59
Hi,
#9668.
#119 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-07-05 16:03:43
Hi,
#6173.
#120 Re: Introductions » Hi » 2025-07-05 15:44:32
Hi,
Welcome to the forum!
#121 Re: Exercises » Compute the solution: » 2025-07-05 15:32:00
Hi,
2414.
#122 Dark Discussions at Cafe Infinity » Classical Quotes - V » 2025-07-04 21:27:16
- Jai Ganesh
- Replies: 0
Classical Quotes - V
1.Suddenly I've got an overwhelming desire to surround myself with the aura of classical and Romantic art. - Sylvester Stallone
2. First of all, we have to go back to the classical time control. - Anatoly Karpov
3. Growing up with country, R&B, gospel, and classical music from my grandmother and pop, Tuskegee was the perfect melting pot for my influences as a writer. - Lionel Richie
4. I used to dance for seventeen years -classical ballet, which was very disciplined. I like yoga and Pilates, but I don't have the discipline to go to the gym. - Penelope Cruz
5. I loved 'Fantasia' as a kid because it filled me with wonder, enchantment and awe. It was my first real introduction into classical music. It was totally inspiring to me. - Nicolas Cage
6. I love the Russian classics very much, the Russian classical literature. But I also read modern literature. As far as Russian literature is concerned, I am very fond of Tolstoy and Chekhov, and I also enjoy reading Gogol very much. - Vladimir Putin
7. If you ever ask me what my all-time dream character is, my answer will be Mia Tansen, the great composer-musician in Hindustani classical music. And ideally, the film should be directed by a person like Bhansali who is a great director and has a marvelous sense of classical music. - Jackie Shroff
8. I was pretty much a goody-two shoes at school - a bit boring, didn't get in trouble with teachers - it was classical Yorkshire: a lot of respect to your elders. Once I started playing cricket that sort of slipped away. - Joe Root.
#123 Jokes » Dancer Jokes - II » 2025-07-04 20:22:07
- Jai Ganesh
- Replies: 0
Life isn't about waiting for the storm to pass, it's about learning to dance in the rain.
* * *
Q: Where can you dance in California?
A: San Fran-disco.
* * *
Q: What did the groovy bank robber say?
A: Everybody get down!
* * *
Q: What do you get if you cross an insect and a dance?
A: A cricket ball!
* * *
Q: Why do ants dance on jam jars?
A: Because the jar says 'twist to open'.
* * *
#124 Re: Jai Ganesh's Puzzles » General Quiz » 2025-07-04 16:32:21
Hi,
#10433. What does the term in Biology Aerobic organism mean?
#10434. What does the term in Biology amino acid (Biochemistry) mean?
#125 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-07-04 15:34:59
Hi,
#5623. What does the noun ignoramus mean?
#5624. What does the noun ignominy mean?