Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 This is Cool » Hemophilia » Yesterday 22:27:23
- Jai Ganesh
- Replies: 0
Hemophilia
Gist
Hemophilia is a mostly inherited genetic disorder that affects the blood's ability to clot properly, leading to prolonged bleeding after injuries or even spontaneously. It is caused by deficiencies in specific clotting factors, primarily Factor VIII (Hemophilia A) and Factor IX (Hemophilia B). While more common in males, females can also be affected or carry the gene.
Hemophilia is primarily caused by genetic mutations that affect the body's ability to produce clotting factors, proteins necessary for blood to clot properly. These genetic mutations are typically inherited, following an X-linked recessive pattern. While less common, acquired hemophilia can also occur due to the immune system producing antibodies that interfere with clotting factors.
Summary
Haemophilia (British English), or hemophilia (American English) (from Ancient Greek (haîma) 'blood' and φιλία (philía) 'love of'), is a mostly inherited genetic disorder that impairs the body's ability to make blood clots, a process needed to stop bleeding. This results in people bleeding for a longer time after an injury, easy bruising, and an increased risk of bleeding inside joints or the brain. Those with a mild case of the disease may have symptoms only after an accident or during surgery. Bleeding into a joint can result in permanent damage while bleeding in the brain can result in long term headaches, seizures, or an altered level of consciousness.
There are two main types of haemophilia: haemophilia A, which occurs due to low amounts of clotting factor VIII, and haemophilia B, which occurs due to low levels of clotting factor IX. They are typically inherited from one's parents through an X chromosome carrying a nonfunctional gene. Most commonly found in men, haemophilia can affect women too, though very rarely. A woman would need to inherit two affected X chromosomes to be affected, whereas a man would only need one X chromosome affected. It is possible for a new mutation to occur during early development, or haemophilia may develop later in life due to antibodies forming against a clotting factor. Other types include haemophilia C, which occurs due to low levels of factor XI, Von Willebrand disease, which occurs due to low levels of a substance called von Willebrand factor, and parahaemophilia, which occurs due to low levels of factor V. Haemophilia A, B, and C prevent the intrinsic pathway from functioning properly; this clotting pathway is necessary when there is damage to the endothelium of a blood vessel. Acquired haemophilia is associated with cancers, autoimmune disorders, and pregnancy. Diagnosis is by testing the blood for its ability to clot and its levels of clotting factors.
Prevention may occur by removing an egg, fertilising it, and testing the embryo before transferring it to the uterus. Human embryos in research can be regarded as the technical object/process. Missing blood clotting factors are replaced to treat haemophilia. This may be done on a regular basis or during bleeding episodes. Replacement may take place at home or in hospital. The clotting factors are made either from human blood or by recombinant methods. Up to 20% of people develop antibodies to the clotting factors which makes treatment more difficult. The medication desmopressin may be used in those with mild haemophilia A. Gene therapy treatment was in clinical trials as of 2022, with some approaches and products having received conditional approval. Haemophilia A affects about 1 in 5,000–10,000, while haemophilia B affects about 1 in 40,000 males at birth. As haemophilia A and B are both X-linked recessive disorders, females are rarely severely affected. Some females with a nonfunctional gene on one of the X chromosomes may be mildly symptomatic. Haemophilia C occurs equally in both sexes and is mostly found in Ashkenazi Jews. In the 1800s haemophilia B was common within the royal families of Europe.[5] The difference between haemophilia A and B was determined in 1952.
Details
Hemophilia is a rare, inherited blood disorder that causes your blood to clot less, which results in an increased risk of bleeding and bruising.
Hemophilia happens because your body doesn’t make enough clotting factors, proteins that help your blood form clots. Clotting factors work with your platelets to form blood clots that control bleeding. Low clotting factor levels increase your bleeding risk.
There are several types of hemophilia. Hemophilia may be severe, moderate or mild based on the amount of clotting factors in your blood.
Healthcare providers treat this condition by replacing the missing clotting factors. There isn’t a cure for hemophilia, but people who receive treatment generally live about as long as people who don’t have hemophilia. Providers are researching gene therapy and gene replacement therapy as new ways to treat and possibly cure hemophilia.
Can you develop hemophilia after birth?
Yes, but it rarely happens. Acquired hemophilia, or hemophilia that isn’t inherited, develops when autoantibodies start to attack a specific clotting factor in your blood. (Antibodies are protective proteins your immune system makes. Autoantibodies attack antibodies, essentially attacking your body’s own cells, tissues and proteins.)
Is hemophilia a common disease?
No, it’s not. According to the U.S. Centers for Disease Control and Prevention (CDC) in August 2022, about 33,000 people in the U.S. have hemophilia. Hemophilia typically affects men. Rarely, women may have clotting factor levels that are low enough to develop symptoms like heavy periods.
What are hemophilia types?
There are three types of hemophilia:
* Hemophilia A: This is the most common type of hemophilia. It happens when you don’t have enough clotting factor 8 (factor VIII). The CDC estimates about 10 in 100,000 people have hemophilia A.
* Hemophilia B: Hemophilia B happens when you don’t have enough clotting factor 9 (factor IX.) The CDC estimates about 3 in 100,000 people in the U.S. have hemophilia B.
* Hemophilia C: Hemophilia C is also known as factor 11 (factor XI) deficiency. This hemophilia type is very rare, affecting 1 in 100,000 people.
Is hemophilia a serious illness?
It can be. People with severe hemophilia may develop life-threatening bleeding (hemorrhage), but they’re more likely to develop bleeding in their muscles and joints.
Symptoms and Causes:
What are hemophilia symptoms?
The most significant symptom is unusual or excessive bleeding or bruising.
* People with hemophilia may develop large bruises after minor injuries. This is a sign of bleeding under their skin.
* They may bleed for an unusually long time, whether after surgery, after dental treatment or simply from a cut finger.
* They may start bleeding for no apparent reason, such as sudden nosebleeds.
How much bruising or bleeding you have depends on whether you have severe, moderate or mild hemophilia.
* People with severe hemophilia often have spontaneous bleeding or bleeding for no apparent reason.
* People with moderate hemophilia who have serious injuries may bleed for an unusually long time.
* People with mild hemophilia may have unusual bleeding, but only after a major surgery or injury.
Other symptoms may include:
* Joint pain from internal bleeding. Joints in your ankles, knees, hips and shoulders may ache, swell or feel hot to the touch.
* Bleeding into your brain. People with severe hemophilia very rarely develop life-threatening bleeding into their brains. Brain bleeds may cause persistent headaches or double vision or make you feel very sleepy. If you have hemophilia and have these symptoms, get help right away.
What are hemophilia symptoms in babies and children?
Sometimes, male babies are diagnosed with hemophilia because they bleed more than usual after circumcision. Other times, children develop symptoms a few months after they’re born. Common symptoms include:
* Bleeding: Babies and toddlers may bleed from their mouths after minor injuries, like bumping their mouths on a toy.
* Swollen lumps on their heads: Babies and toddlers who bump their heads often develop goose eggs — large round lumps on their heads.
* Fussiness, irritability or refusal to crawl or walk: These symptoms may happen if babies and toddlers have internal bleeding into a muscle or joint. They may have areas on their bodies that look bruised and swollen, feel warm to your touch or make your child hurt when you gently touch the area.
* Hematomas: A hematoma is a mass of congealed blood that gathers under babies’ or toddlers’ skin. Babies and toddlers may develop hematomas after receiving an injection.
What causes hemophilia?
Certain genes create clotting factors. In inherited hemophilia, the genes carrying instructions for making normal clotting factors mutate or change. The mutated genes may give instructions that end up making abnormal clotting factors or not enough clotting factors. That said, about 20% of all hemophilia cases are spontaneous, meaning you have the disease even though there’s no family history of abnormal bleeding.
Additional Information
Hemophilia is a rare bleeding disorder in which the blood does not clot properly. This can lead to problems with bleeding too much after an injury or surgery. You can also have sudden bleeding inside your body, such as in your joints, muscles, and organs.
Your blood contains many proteins called clotting factors that can help form clots to stop bleeding. People with hemophilia have low levels of one of these factors, usually either factor VIII (8) or factor IX (9). How severe the hemophilia is depends on the amount of factor in the blood. The lower the amount of the factor, the more likely it is that bleeding could happen and might lead to serious health problems.

#2 Re: This is Cool » Miscellany » Yesterday 20:12:28
2335) Valley
Gist
A valley is a low-lying area of land between hills or mountains, often with a river or stream running through it. Valleys are typically formed by erosion from rivers, glaciers, or other geological processes. They can vary in size and shape, from narrow canyons to wide, flat plains.
Summary
A valley is an elongated low area often running between hills or mountains and typically containing a river or stream running from one end to the other. Most valleys are formed by erosion of the land surface by rivers or streams over a very long period. Some valleys are formed through erosion by glacial ice. These glaciers may remain present in valleys in high mountains or polar areas.
At lower latitudes and altitudes, these glacially formed valleys may have been created or enlarged during ice ages but now are ice-free and occupied by streams or rivers. In desert areas, valleys may be entirely dry or carry a watercourse only rarely. In areas of limestone bedrock, dry valleys may also result from drainage now taking place underground rather than at the surface. Rift valleys arise principally from earth movements, rather than erosion. Many different types of valleys are described by geographers, using terms that may be global in use or else applied only locally.
Details
Valleys are depressed areas of land–scoured and washed out by the conspiring forces of gravity, water, and ice. Some hang; others are hollow. They all take the form of a "U" or "V."
Rivers and streams make most primary valley cuts, carving steep-walled sides and a narrow floor that from afar looks like the letter "V." The gradient of the river—how quickly it drops—helps define the steepness of the sides and the width of the floor. Mountain valleys, for example, tend to have near-vertical walls and a narrow channel, but out on the plains, the slopes are shallow and the channel is wide.
As waters wind toward the sea, they add to natural twists in the land by stripping sediment from the outsides of bends and dumping it on the insides. The bulk of the rock and dirt is dredged from the bottom of the channel, a process called down cutting that can ultimately lead to deep, slender chasms like Black Canyon in Colorado's Gunnison National Park.
Types of Valleys
Some river and stream valleys, especially those in the mountains or located near the North and South Poles, are transformed by glaciers.
The massive blocks of snow and ice slowly creep downhill where they will meet the least resistance: valleys already cut by rivers and streams. As the glaciers ooze, they pick up rocks and grind away at the valley floor and sides, pressing the "V" into a "U." When the glacier melts, a U-shaped valley marks the spot where the snow and ice once flowed.
Side valleys are formed by tributaries to streams and rivers and feed the main stem. Where the main channel is carved deeper than the tributary, as commonly occurs during glaciations, the side valleys are left hanging. Waterfalls often cascade from the outlet of the upper valley into the drainage below.
Hollows, like those in Appalachia, are small valleys nestled between mountains or hills.
Giant valleys, called rifts, are found where two pieces of Earth's crust are separated or split apart. One such example is the Great Rift Valley, a rift system stretching from the Middle East to southern Africa.
Additional Information
A valley is elongate depression of the Earth’s surface. Valleys are most commonly drained by rivers and may occur in a relatively flat plain or between ranges of hills or mountains. Those valleys produced by tectonic action are called rift valleys. Very narrow, deep valleys of similar appearance are called gorges. Both of these latter types are commonly cut in flat-lying strata but may occur in other geological situations.
Wherever sufficient rainfall occurs, opportunity exists for the land surface to evolve to the familiar patterns of hills and valleys. There are, of course, hyperarid environments where fluvial activity is minimal. There also are geomorphological settings where the permeability of rocks or sediments induce so much infiltration that water is unable to concentrate on the land surface. Moreover, some landscapes may be so young that insufficient time has elapsed for modification by fluvial action. The role of fluvial action on landscape, including long-term evolutionary processes, is considered here in detail. For additional information on fluvial and hillslope processes relating to valley formation, see river.
Probably the world’s deepest subaerial valley is that of the Kāli Gandaki River in Nepal. Lying between two 8,000-metre (26,000-foot) Himalayan peaks, Dhaulāgiri and Annapūrna, the valley has a total relief of six kilometres (four miles). Because the Himalayas are one of the Earth’s most active areas of tectonic uplift, this valley well illustrates the principle that the most rapid downcutting occurs in areas of the most rapid uplift. The reason for this seeming paradox lies in the energetics of the processes of degradation that characterize valley formation. As will be discussed below, the steeper the gradient or slope of a stream, the greater its expenditure of power on the streambed. Thus, as uplift creates higher relief and steeper slopes, rivers achieve greater power for erosion. As a consequence, the most rapid processes of relief reduction can occur in areas of most rapid relief production.
Perhaps the most famous example of a canyon is the Grand Canyon of the Colorado River in northern Arizona. The Grand Canyon is about 1.6 km (1 mile) deep and 180 metres (590 feet) to 30 km (19 miles) wide and occurs along a 443-km- (275-mile-) long reach where the Colorado River incised into a broad upwarp of sedimentary rocks.

#3 Dark Discussions at Cafe Infinity » Clean Quotes - III » Yesterday 17:19:31
- Jai Ganesh
- Replies: 0
Clean Quotes - III
Indeed, we're strongest when the face of America isn't only a soldier carrying a gun but also a diplomat negotiating peace, a Peace Corps volunteer bringing clean water to a village, or a relief worker stepping off a cargo plane as floodwaters rise. - Colin Powell
2. If we can clean up our world, I'll bet you we can achieve warp drive. - William Shatner
3. The clear and present danger of climate change means we cannot burn our way to prosperity. We already rely too heavily on fossil fuels. We need to find a new, sustainable path to the future we want. We need a clean industrial revolution. - Ban Ki-moon
4. If you don't want to play clean football then go up into the stands. - Diego Maradona
5. I believe climate change is real and that we can save our planet while creating millions of good-paying clean energy jobs. - Hillary Clinton
6. I believe you never get tired by doing work. You get tired when you don't work. When you clean your house, you don't get tired; it gives you satisfaction. - Narendra Modi
7. More than a billion people lack adequate access to clean water. - David Suzuki
8. I like porterhouse steak, rib-eyes and New York strip. This works for me because I have very low cholesterol and low blood pressure. It's not good for everyone; you have to talk to your doctor about that. I also eat fish and cheese. I like clean food prepared as simply as possible. - Sharon Stone.
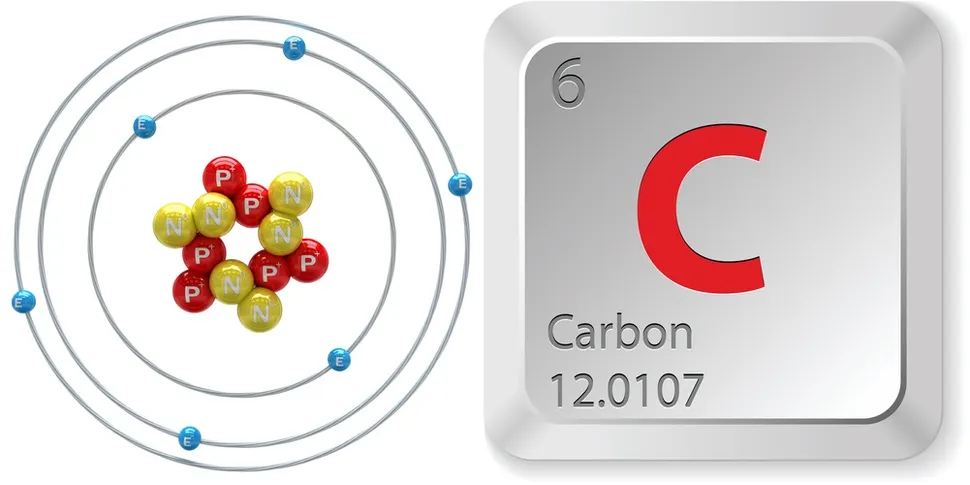
#4 Science HQ » Carbon » Yesterday 16:45:52
- Jai Ganesh
- Replies: 0
Carbon
Gist
Carbon, with the symbol C and atomic number 6, is a nonmetallic chemical element crucial for all known life. It's tetravalent, meaning its atoms can form up to four covalent bonds, and is a key component of organic molecules. Carbon is abundant in the Earth's crust and plays a vital role in the carbon cycle and climate regulation.
Carbon, an essential element for life, is primarily formed in stars through a process called the triple-alpha process. This process involves the fusion of three helium nuclei (alpha particles) to create a carbon nucleus.
Carbon is a fundamental element in life science and biology, serving as the backbone of all living organisms. It is the building block of organic molecules, including DNA, proteins, carbohydrates, and lipids, which are essential for life processes. Carbon's unique ability to form stable bonds with other atoms allows for the creation of diverse and complex molecules necessary for life.
Summary
Carbon (from Latin carbo 'coal') is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the few elements known since antiquity.
Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.
The atoms of carbon can bond together in diverse ways, resulting in various allotropes of carbon. Well-known allotropes include graphite, diamond, amorphous carbon, and fullerenes. The physical properties of carbon vary widely with the allotropic form. For example, graphite is opaque and black, while diamond is highly transparent. Graphite is soft enough to form a streak on paper (hence its name, from the Greek verb which means "to write"), while diamond is the hardest naturally occurring material known. Graphite is a good electrical conductor while diamond has a low electrical conductivity. Under normal conditions, diamond, carbon nanotubes, and graphene have the highest thermal conductivities of all known materials. All carbon allotropes are solids under normal conditions, with graphite being the most thermodynamically stable form at standard temperature and pressure. They are chemically resistant and require high temperature to react even with oxygen.
The most common oxidation state of carbon in inorganic compounds is +4, while +2 is found in carbon monoxide and transition metal carbonyl complexes. The largest sources of inorganic carbon are limestones, dolomites and carbon dioxide, but significant quantities occur in organic deposits of coal, peat, oil, and methane clathrates. Carbon forms a vast number of compounds, with about two hundred million having been described and indexed; and yet that number is but a fraction of the number of theoretically possible compounds under standard conditions.
Details
Carbon (C), nonmetallic chemical element in Group 14 (IVa) of the periodic table. Although widely distributed in nature, carbon is not particularly plentiful—it makes up only about 0.025 percent of Earth’s crust—yet it forms more compounds than all the other elements combined. In 1961 the isotope carbon-12 was selected to replace oxygen as the standard relative to which the atomic weights of all the other elements are measured. Carbon-14, which is radioactive, is the isotope used in radiocarbon dating and radiolabeling.
Element Properties
atomic number : 6
atomic weight : 12.0096 to 12.0116
melting point : 3,550 °C (6,420 °F)
boiling point : 4,827 °C (8,721 °F)
Density
diamond : 3.52 g/{cm}^3
graphite : 2.25 g/{cm}^3
amorphous : 1.9 g/cm3
oxidation states : +2, +3, +4
Properties and uses
On a weight basis, carbon is 19th in order of elemental abundance in Earth’s crust, and there are estimated to be 3.5 times as many carbon atoms as silicon atoms in the universe. Only hydrogen, helium, oxygen, neon, and nitrogen are atomically more abundant in the cosmos than carbon. Carbon is the cosmic product of the “burning” of helium, in which three helium nuclei, atomic weight 4, fuse to produce a carbon nucleus, atomic weight 12.
In the crust of Earth, elemental carbon is a minor component. However, carbon compounds (i.e., carbonates of magnesium and calcium) form common minerals (e.g., magnesite, dolomite, marble, or limestone). Coral and the shells of oysters and clams are primarily calcium carbonate. Carbon is widely distributed as coal and in the organic compounds that constitute petroleum, natural gas, and all plant and animal tissue. A natural sequence of chemical reactions called the carbon cycle—involving conversion of atmospheric carbon dioxide to carbohydrates by photosynthesis in plants, the consumption of these carbohydrates by animals and oxidation of them through metabolism to produce carbon dioxide and other products, and the return of carbon dioxide to the atmosphere—is one of the most important of all biological processes.
Carbon as an element was discovered by the first person to handle charcoal from fire. Thus, together with sulfur, iron, tin, lead, copper, mercury, silver, and gold, carbon was one of the small group of elements well known in the ancient world. Modern carbon chemistry dates from the development of coals, petroleum, and natural gas as fuels and from the elucidation of synthetic organic chemistry, both substantially developed since the 1800s.
Elemental carbon exists in several forms, each of which has its own physical characteristics. Two of its well-defined forms, diamond and graphite, are crystalline in structure, but they differ in physical properties because the arrangements of the atoms in their structures are dissimilar. A third form, called fullerene, consists of a variety of molecules composed entirely of carbon. Spheroidal, closed-cage fullerenes are called buckerminsterfullerenes, or “buckyballs,” and cylindrical fullerenes are called nanotubes. A fourth form, called Q-carbon, is crystalline and magnetic. Yet another form, called amorphous carbon, has no crystalline structure. Other forms—such as carbon black, charcoal, lampblack, coal, and coke—are sometimes called amorphous, but X-ray examination has revealed that these substances do possess a low degree of crystallinity. Diamond and graphite occur naturally on Earth, and they also can be produced synthetically; they are chemically inert but do combine with oxygen at high temperatures, just as amorphous carbon does. Fullerene was serendipitously discovered in 1985 as a synthetic product in the course of laboratory experiments to simulate the chemistry in the atmosphere of giant stars. It was later found to occur naturally in tiny amounts on Earth and in meteorites. Q-carbon is also synthetic, but scientists have speculated that it could form within the hot environments of some planetary cores.
The word carbon probably derives from the Latin carbo, meaning variously “coal,” “charcoal,” “ember.” The term diamond, a corruption of the Greek word adamas, “the invincible,” aptly describes the permanence of this crystallized form of carbon, just as graphite, the name for the other crystal form of carbon, derived from the Greek verb graphein, “to write,” reflects its property of leaving a dark mark when rubbed on a surface. Before the discovery in 1779 that graphite when burned in air forms carbon dioxide, graphite was confused with both the metal lead and a superficially similar substance, the mineral molybdenite.
Pure diamond is the hardest naturally occurring substance known and is a poor conductor of electricity. Graphite, on the other hand, is a soft slippery solid that is a good conductor of both heat and electricity. Carbon as diamond is the most expensive and brilliant of all the natural gemstones and the hardest of the naturally occurring abrasives. Graphite is used as a lubricant. In microcrystalline and nearly amorphous form, it is used as a black pigment, as an adsorbent, as a fuel, as a filler for rubber, and, mixed with clay, as the “lead” of pencils. Because it conducts electricity but does not melt, graphite is also used for electrodes in electric furnaces and dry cells as well as for making crucibles in which metals are melted. Molecules of fullerene show promise in a range of applications, including high-tensile-strength materials, unique electronic and energy-storage devices, and safe encapsulation of flammable gases, such as hydrogen. Q-carbon, which is created by rapidly cooling a sample of elemental carbon whose temperature has been raised to 4,000 K (3,727 °C [6,740 °F]), is harder than diamond, and it can be used to manufacture diamond structures (such as diamond films and microneedles) within its matrix. Elemental carbon is nontoxic.
Each of the “amorphous” forms of carbon has its own specific character, and, hence, each has its own particular applications. All are products of oxidation and other forms of decomposition of organic compounds. Coal and coke, for example, are used extensively as fuels. Charcoal is used as an absorptive and filtering agent and as a fuel and was once widely used as an ingredient in gunpowder. (Coals are elemental carbon mixed with varying amounts of carbon compounds. Coke and charcoal are nearly pure carbon.) In addition to its uses in making inks and paints, carbon black is added to the rubber used in tires to improve its wearing qualities. Bone black, or animal charcoal, can adsorb gases and colouring matter from many other materials.
Carbon, either elemental or combined, is usually determined quantitatively by conversion to carbon dioxide gas, which can then be absorbed by other chemicals to give either a weighable product or a solution with acidic properties that can be titrated.
Additional Information:
Appearance
There are a number of pure forms of this element including graphite, diamond, fullerenes and graphene.
Diamond is a colourless, transparent, crystalline solid and the hardest known material. Graphite is black and shiny but soft. The nano-forms, fullerenes and graphene, appear as black or dark brown, soot-like powders.
Uses
Carbon is unique among the elements in its ability to form strongly bonded chains, sealed off by hydrogen atoms. These hydrocarbons, extracted naturally as fossil fuels (coal, oil and natural gas), are mostly used as fuels. A small but important fraction is used as a feedstock for the petrochemical industries producing polymers, fibres, paints, solvents and plastics etc.
Impure carbon in the form of charcoal (from wood) and coke (from coal) is used in metal smelting. It is particularly important in the iron and steel industries.
Graphite is used in pencils, to make brushes in electric motors and in furnace linings. Activated charcoal is used for purification and filtration. It is found in respirators and kitchen extractor hoods.
Carbon fibre is finding many uses as a very strong, yet lightweight, material. It is currently used in tennis rackets, skis, fishing rods, rockets and aeroplanes.
Industrial diamonds are used for cutting rocks and drilling. Diamond films are used to protect surfaces such as razor blades.
The more recent discovery of carbon nanotubes, other fullerenes and atom-thin sheets of graphene has revolutionised hardware developments in the electronics industry and in nanotechnology generally.
150 years ago the natural concentration of carbon dioxide in the Earth’s atmosphere was 280 ppm. In 2013, as a result of combusting fossil fuels with oxygen, there was 390 ppm. Atmospheric carbon dioxide allows visible light in but prevents some infrared escaping (the natural greenhouse effect). This keeps the Earth warm enough to sustain life. However, an enhanced greenhouse effect is underway, due to a human-induced rise in atmospheric carbon dioxide. This is affecting living things as our climate changes.
Biological role
Carbon is essential to life. This is because it is able to form a huge variety of chains of different lengths. It was once thought that the carbon-based molecules of life could only be obtained from living things. They were thought to contain a ‘spark of life’. However, in 1828, urea was synthesised from inorganic reagents and the branches of organic and inorganic chemistry were united.
Living things get almost all their carbon from carbon dioxide, either from the atmosphere or dissolved in water. Photosynthesis by green plants and photosynthetic plankton uses energy from the sun to split water into oxygen and hydrogen. The oxygen is released to the atmosphere, fresh water and seas, and the hydrogen joins with carbon dioxide to produce carbohydrates.
Some of the carbohydrates are used, along with nitrogen, phosphorus and other elements, to form the other monomer molecules of life. These include bases and sugars for RNA and DNA, and amino acids for proteins.
Living things that do not photosynthesise have to rely on consuming other living things for their source of carbon molecules. Their digestive systems break carbohydrates into monomers that they can use to build their own cellular structures. Respiration provides the energy needed for these reactions. In respiration oxygen rejoins carbohydrates, to form carbon dioxide and water again. The energy released in this reaction is made available for the cells.
Natural abundance
Carbon is found in the sun and other stars, formed from the debris of a previous supernova. It is built up by nuclear fusion in bigger stars.
It is present in the atmospheres of many planets, usually as carbon dioxide. On Earth, the concentration of carbon dioxide in the atmosphere is currently 390 ppm and rising.
Graphite is found naturally in many locations. Diamond is found in the form of microscopic crystals in some meteorites. Natural diamonds are found in the mineral kimberlite, sources of which are in Russia, Botswana, DR Congo, Canada and South Africa.
In combination, carbon is found in all living things. It is also found in fossilised remains in the form of hydrocarbons (natural gas, crude oil, oil shales, coal etc) and carbonates (chalk, limestone, dolomite etc).
#5 Re: Jai Ganesh's Puzzles » General Quiz » Yesterday 14:39:34
Hi,
#10455. What does the term in Biology Barr body mean?
#10456. What does the term in Biology Basal body mean?
#6 Re: Jai Ganesh's Puzzles » English language puzzles » Yesterday 14:22:03
Hi,
#5645. What does the noun pheasant mean?
#5646. What does the adjective philanthropic mean?
#7 Re: Jai Ganesh's Puzzles » Doc, Doc! » Yesterday 14:00:01
Hi,
#2414. What does the medical term Neonatal teeth mean?
#8 Re: Jai Ganesh's Puzzles » 10 second questions » Yesterday 13:47:41
Hi,
#9680.
#9 Re: Jai Ganesh's Puzzles » Oral puzzles » Yesterday 13:41:13
Hi,
#6185.
#10 Re: Exercises » Compute the solution: » Yesterday 13:19:12
Hi,
2426.
#11 Jokes » Dentist Jokes - IV » Yesterday 12:51:38
- Jai Ganesh
- Replies: 0
Q: Who has the most dangerous job in Transylvania?
A: Dracula's dentist.
* * *
Q: Why does a dentist seem moody?
A: Because he always looks down in the mouth.
* * *
Q: Why did the cheerleader go to the dentist?
A: She needed a root canal.
* * *
Q: Why did the Tooth Fairy go to a psychiatrist?
A: She no longer believed in herself.
* * *
Q: What did the werewolf eat after he'd had his teeth taken out?
A: The dentist.
* * *
#12 Re: This is Cool » Miscellany » 2025-07-16 20:20:01
2334) Sea
Gist
A sea is a large body of salt water. There are particular seas and the sea. The sea commonly refers to the ocean, the interconnected body of seawaters that spans most of Earth.
Summary
A sea is a large body of salt water. It may be an ocean, or may be a large saltwater lake which like the Caspian Sea, lacks a natural outlet.
Characteristics:
Seawater
Seawater is salty. The open ocean has about 35 grams (1.2 oz) solids per litre, a salinity of 35 part per thousand. The Mediterranean Sea is a little higher at 37part per thousand and the Dead Sea has as much as 300 grams (11 oz) dissolved solids per litre. Sodium chloride is the main salt present, making up about 85% of the solids in solution. There are also 5 grams (0.18 oz) per litre of the chlorides of other metals such as potassium and magnesium and 3 grams (0.11 oz) of sulphates, carbonates, bromides and other salts. A kilogram (2.2 lb) of salt can be found in 28 litres or one cubic foot of typical ocean water. Despite differences in the levels of salinity in different seas, the relative composition of the dissolved salts is very stable throughout the world's oceans.
Temperature
The temperature of the sea depends on the amount of sunlight falling on the surface. In the tropics, with the sun nearly overhead, the temperature of the surface layers can rise to over 30 °C (86 °F). Near the poles the temperature in balance with the sea ice is about −2 °C (28 °F). Cold water is denser than warm water and tends to sink. There is a continuous circulation of water in the oceans. Warm surface currents cool as they move away from the tropics, the water becomes denser and sinks. The cold water moves back towards the equator as a deep sea current, driven by changes in the temperature and density of the water, eventually welling up again towards the surface. Deep sea water has a temperature between −2 °C (28 °F) and 5 °C (41 °F) in all parts of the globe.
Oxygen
The amount of oxygen found in seawater depends mostly on the plants growing in it. These are mainly algae, including phytoplankton, but also include some vascular plants such as seagrasses. In daylight the photosynthetic activity of these plants produces oxygen which dissolves in the seawater where it is used by marine animals. At night, photosynthesis stops, and the amount of dissolved oxygen declines. In the deep sea where not enough light penetrates for plants to grow, there is very little dissolved oxygen.
Seawater is a little alkaline and during historic times has had a pH of about 8.2. The pH is expected to reach 7.7 by the year 2100, an increase of 320% in acidity in a century. One important element for the formation of skeletal material in marine animals is calcium but it is easily precipitated out in the form of calcium carbonate as the sea becomes more acid. This is likely to have profound effects on certain planktonic marine organisms because their ability to form shells will be reduced. These include single-celled algae called coccolithophorids and foraminifera. These are important parts of the food chain. Reducing their numbers will have significant results. In tropical areas, corals will be affected by a lack of calcium, with knock-on effects for other reef residents.
Waves
Wind blowing over the surface of a body of water forms waves. The friction between air and water caused by a gentle breeze on a pond causes ripples to form. A strong blow over the ocean causes larger waves as the moving air pushes against the raised ridges of water. The waves reach their greatest height when the rate at which they travel nearly matches the speed of the wind. The waves form at right angles to the direction from which the wind blows. In open water, if the wind continues to blow, as happens in the Roaring Forties in the southern hemisphere, long, organized masses of water called swell roll across the ocean.
Details
The phrase “the Seven Seas” has been around for centuries, but that term really refers to different parts of the ocean and several other large bodies of water. There are actually more than seven seas in the world. But what makes a sea different from other bodies of water?
That is not an easy question to answer, because the definition of a sea leaves some room for interpretation. In general, a sea is defined as a portion of the ocean that is partly surrounded by land. Given that definition, there are about 50 seas around the world. But that number includes water bodies not always thought of as seas, such as the Gulf of Mexico and the Hudson Bay.
Moreover, in some cases, a sea is completely landlocked. The Caspian Sea is the most famous example, though this sea, which lies between Russia and Iran, is also referred to as the world’s largest lake. Other seas surrounded by land include the Aral Sea and the Dead Sea. They contain saltwater and have been called seas for many years, but many oceanographers and geographers are more inclined to call them lakes.
Still, that leaves dozens of water bodies that fit the traditional definition of a sea, even though they can be quite different from one another. A sea can be more than 2.6 million square kilometers (1 million square miles) in area, such as the Caribbean Sea. Or, it can be as tiny as the Sea of Marmara, which is less than 12,950 square kilometers (5,000 square miles) in area. This tiny Turkish sea connects the Aegean Sea and the Black Sea.
A sea can also be very warm for most of the year. The Red Sea, for instance, has an average temperature of around 30 degrees Celsius (86 degrees Fahrenheit). It is also the saltiest sea, containing 41 parts of salt per 1,000 parts of seawater. Seas can be quite cold, too. The Greenland Sea, for instance, has surface water that hovers near the freezing mark most of the year.
The variety of the sizes, temperatures, and locations of the Earth’s seas also means that the marine ecosystems within each sea can vary greatly from one to the other. The Baltic Sea in Scandinavia is the world’s youngest sea having formed between 10 thousand and 15 thousand years ago from glacial erosion. It contains a unique mixture of saltwater and freshwater, making it the largest brackish water body on the planet. As a result, the Baltic Sea contains a small, but rare, variety of freshwater and saltwater plants and animals that have been able to adapt to their brackish environment.
Not surprisingly, the diversity of the world’s seas also draws National Geographic explorers, such as oceanographer Katy Croff Bell. She was part of the crew aboard the exploration vessel Nautilus, a ship that shared its scientific discoveries in the Mediterranean Sea, the Black Sea, and elsewhere with students around the world in online lessons and chats. She says the seas—big and small, cold and warm—can teach scientists about the rest of the world. “We’re going to places that have never been explored to see what’s there,” Bell told MIT Technology Review in 2015. “There are things we can’t even conceive of out there, and it will take a long, long time to fully understand our own planet.”
Additional Information
A sea is a large body of salt water. There are particular seas and the sea. The sea commonly refers to the ocean, the interconnected body of seawaters that spans most of Earth. Particular seas are either marginal seas, second-order sections of the oceanic sea (e.g. the Mediterranean Sea), or certain large, nearly landlocked bodies of water.
The salinity of water bodies varies widely, being lower near the surface and the mouths of large rivers and higher in the depths of the ocean; however, the relative proportions of dissolved salts vary little across the oceans. The most abundant solid dissolved in seawater is sodium chloride. The water also contains salts of magnesium, calcium, potassium, and mercury, among other elements, some in minute concentrations. A wide variety of organisms, including bacteria, protists, algae, plants, fungi, and animals live in various marine habitats and ecosystems throughout the seas. These range vertically from the sunlit surface and shoreline to the great depths and pressures of the cold, dark abyssal zone, and in latitude from the cold waters under polar ice caps to the warm waters of coral reefs in tropical regions. Many of the major groups of organisms evolved in the sea and life may have started there.
The ocean moderates Earth's climate and has important roles in the water, carbon, and nitrogen cycles. The surface of water interacts with the atmosphere, exchanging properties such as particles and temperature, as well as currents. Surface currents are the water currents that are produced by the atmosphere's currents and its winds blowing over the surface of the water, producing wind waves, setting up through drag slow but stable circulations of water, as in the case of the ocean sustaining deep-sea ocean currents. Deep-sea currents, known together as the global conveyor belt, carry cold water from near the poles to every ocean and significantly influence Earth's climate. Tides, the generally twice-daily rise and fall of sea levels, are caused by Earth's rotation and the gravitational effects of the Moon and, to a lesser extent, of the Sun. Tides may have a very high range in bays or estuaries. Submarine earthquakes arising from tectonic plate movements under the oceans can lead to destructive tsunamis, as can volcanoes, huge landslides, or the impact of large meteorites.
The seas have been an integral element for humans throughout history and culture. Humans harnessing and studying the seas have been recorded since ancient times and evidenced well into prehistory, while its modern scientific study is called oceanography and maritime space is governed by the law of the sea, with admiralty law regulating human interactions at sea. The seas provide substantial supplies of food for humans, mainly fish, but also shellfish, mammals and seaweed, whether caught by fishermen or farmed underwater. Other human uses of the seas include trade, travel, mineral extraction, power generation, warfare, and leisure activities such as swimming, sailing, and scuba diving. Many of these activities create marine pollution.

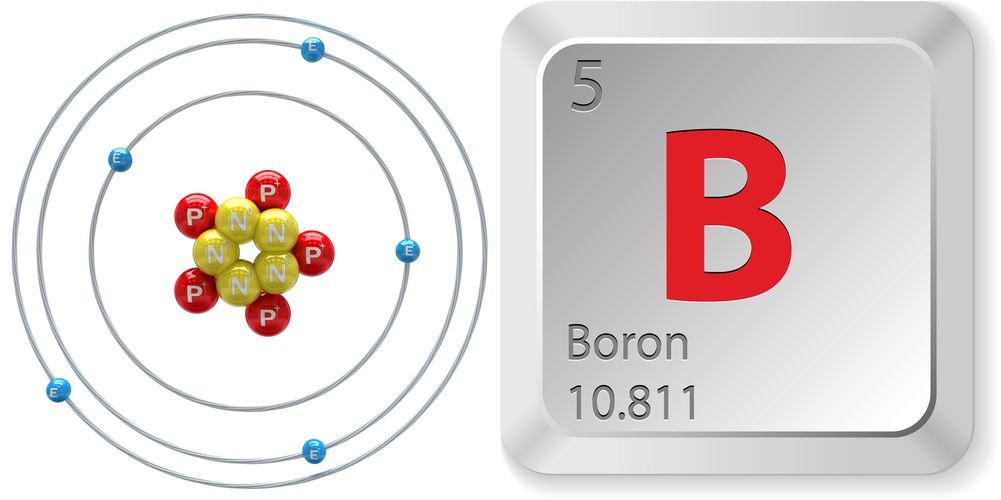
#13 Science HQ » Boron » 2025-07-16 16:07:50
- Jai Ganesh
- Replies: 0
Boron
Gist
Boron is an extremely valuable mineral and it is used in many products from cookware and medicine to nuclear waste storage and space exploration. Boron compounds are mainly used in borosilicate glass products, but are also used in agriculture, in fire retardants, and in soaps and detergents.
Summary
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.
Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals.
Elemental boron is found in small amounts in meteoroids, but chemically uncombined boron is not otherwise found naturally on Earth.
Several allotropes exist: amorphous boron is a brown powder; crystalline boron is silvery to black, extremely hard (9.3 on the Mohs scale), and a poor electrical conductor at room temperature electrical conductivity). The primary use of the element itself is as boron filaments with applications similar to carbon fibers in some high-strength materials.
Boron is primarily used in chemical compounds. About half of all production consumed globally is an additive in fiberglass for insulation and structural materials. The next leading use is in polymers and ceramics in high-strength, lightweight structural and heat-resistant materials. Borosilicate glass is desired for its greater strength and thermal shock resistance than ordinary soda lime glass. As sodium perborate, it is used as a bleach. A small amount is used as a dopant in semiconductors, and reagent intermediates in the synthesis of organic fine chemicals. A few boron-containing organic pharmaceuticals are used or are in study. Natural boron is composed of two stable isotopes, one of which (boron-10) has a number of uses as a neutron-capturing agent.
Borates have low toxicity in mammals (similar to table salt) but are more toxic to arthropods and are occasionally used as insecticides. Boron-containing organic antibiotics are known. Although only traces are required, it is an essential plant nutrient.
Details
Boron (B), chemical element, semimetal of main Group 13 (IIIa, or boron group) of the periodic table, essential to plant growth and of wide industrial application.
Element Properties
atomic number : 5
atomic weight : [10.806, 10.821]
melting point : 2,200 °C (4,000 °F)
boiling point : 2,550 °C (4,620 °F)
specific gravity : 2.34 (at 20 °C [68 °F])
oxidation state : +3
Properties, occurrence, and uses
Pure crystalline boron is a black, lustrous semiconductor; i.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. It is hard enough (9.3 on Mohs scale) to scratch some abrasives, such as carborundum, but too brittle for use in tools. It constitutes about 0.001 percent by weight of Earth’s crust. Boron occurs combined as borax, kernite, and tincalconite (hydrated sodium borates), the major commercial boron minerals, especially concentrated in the arid regions of California, and as widely dispersed minerals such as colemanite, ulexite, and tourmaline. Sassolite—natural boric acid—occurs especially in Italy.
Boron was first isolated (1808) by French chemists Joseph-Louis Gay-Lussac and Louis-Jacques Thenard and independently by British chemist Sir Humphry Davy by heating boron oxide (B2O3) with potassium metal. The impure amorphous product, a brownish black powder, was the only form of boron known for more than a century. Pure crystalline boron may be prepared with difficulty by reduction of its bromide or chloride (BBr3, BCl3) with hydrogen on an electrically heated tantalum filament.
Limited quantities of elemental boron are widely used to increase hardness in steel. Added as the iron alloy ferroboron, it is present in many steels, usually in the range 0.001 to 0.005 percent. Boron is also used in the nonferrous-metals industry, generally as a deoxidizer, in copper-base alloys and high-conductance copper as a degasifier, and in aluminum castings to refine the grain. In the semiconductor industry, small, carefully controlled amounts of boron are added as a doping agent to silicon and germanium to modify electrical conductivity.
In the form of boric acid or borates, traces of boron are necessary for growth of many land plants and thus are indirectly essential for animal life. Typical effects of long-term boron deficiency are stunted, misshapen growth; vegetable “brown heart” and sugar beet “dry rot” are among the disorders due to boron deficiency. Boron deficiency can be alleviated by the application of soluble borates to the soil. In excess quantities, however, borates act as unselective herbicides. Gigantism of several species of plants growing in soil naturally abundant in boron has been reported. It is not yet clear what the precise role of boron in plant life is, but most researchers agree that the element is in some way essential for the normal growth and functioning of apical meristems, the growing tips of plant shoots.
Pure boron exists in at least four crystalline modifications or allotropes. Closed cages containing 12 boron atoms arranged in the form of an icosahedron occur in the various crystalline forms of elemental boron.
Crystalline boron is almost inert chemically at ordinary temperatures. Boiling hydrochloric acid does not affect it, and hot concentrated nitric acid only slowly converts finely powdered boron to boric acid (H3BO3). Boron in its chemical behaviour is nonmetallic.
In nature, boron consists of a mixture of two stable isotopes—boron-10 (19.9 percent) and boron-11 (80.1 percent); slight variations in this proportion produce a range of ±0.003 in the atomic weight. Both nuclei possess nuclear spin (rotation of the atomic nuclei); that of boron-10 has a value of 3 and that of boron-11, 3/2, the values being dictated by quantum factors. These isotopes are therefore of use in nuclear magnetic resonance spectroscopy, and spectrometers specially adapted to detecting the boron-11 nucleus are available commercially. The boron-10 and boron-11 nuclei also cause splitting in the resonances (that is, the appearance of new bands in the resonance spectra) of other nuclei (e.g., those of hydrogen atoms bonded to boron).
Additional Information:
Appearance
Pure boron is a dark amorphous powder.
Uses
Amorphous boron is used as a rocket fuel igniter and in pyrotechnic flares. It gives the flares a distinctive green colour.
The most important compounds of boron are boric (or boracic) acid, borax (sodium borate) and boric oxide. These can be found in eye drops, mild antiseptics, washing powders and tile glazes. Borax used to be used to make bleach and as a food preservative.
Boric oxide is also commonly used in the manufacture of borosilicate glass (Pyrex). It makes the glass tough and heat resistant. Fibreglass textiles and insulation are made from borosilcate glass.
Sodium octaborate is a flame retardant.
The isotope boron-10 is good at absorbing neutrons. This means it can be used to regulate nuclear reactors. It also has a role in instruments used to detect neutrons.
Biological role
Boron is essential for the cell walls of plants. It is not considered poisonous to animals, but in higher doses it can upset the body’s metabolism. We take in about 2 milligrams of boron each day from our food, and about 60 grams in a lifetime. Some boron compounds are being studied as a possible treatment for brain tumours.
Natural abundance
Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. Extensive borax deposits are found in Turkey. However, by far the most important source of boron is rasorite. This is found in the Mojave Desert in California, USA.
High-purity boron is prepared by reducing boron trichloride or tribromide with hydrogen, on electrically heated filaments. Impure, or amorphous, boron can be prepared by heating the trioxide with magnesium powder.
#14 Dark Discussions at Cafe Infinity » Clean Quotes - II » 2025-07-16 15:31:24
- Jai Ganesh
- Replies: 0
Clean Quotes - II
1. If they had told me I was the janitor and would have to mop up and clean the toilets after the show in order to play, I probably would have done it. - Bruce Springsteen
2. Forgiveness is the answer to the child's dream of a miracle by which what is broken is made whole again, what is soiled is made clean again. - Dag Hammarskjold
3. Whoso will pray, he must fast and be clean, And fat his soul, and make his body lean. - Geoffrey Chaucer
4. There's a very fundamental basic value system that I think America was built upon, and that's mutual respect, honor, integrity and concern for our environment and the right to clean water. And we have moved away from it. - Erin Brockovich
5. I will certainly not join politics. I would like to be remembered as a clean businessman who has not partaken in any twists and turns beneath the surface, and one who has been reasonably successful. - Ratan Tata
6. I want American Dream growth - lots of new businesses, well-paying jobs, and American leadership in new industries, like clean energy and biotechnology. - William J. Clinton
7. Information, education, skills, healthcare, livelihood, financial inclusion, small and village enterprises, opportunities for women, conservation of natural resources, distributed clean energy - entirely new possibilities have emerged to change the development model. - Narendra Modi
8. If I see something dirty or untidy, I have to clean it up. - Indira Gandhi.
#15 Re: Jai Ganesh's Puzzles » General Quiz » 2025-07-16 14:27:41
Hi,
#10453. What does the term in Biology Bacteria mean?
#10454. What does the term in Biology Bacteriophage mean?
#16 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-07-16 14:12:49
Hi,
#5643. What does the verb (used with object) braise mean?
#5644. What does the verb (used with object) browse mean?
#17 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-07-16 13:59:45
Hi,
#2413. What does the medical term Nasal bone mean?
#18 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-07-16 13:48:03
Hi,
#9679.
#19 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-07-16 13:41:38
Hi,
#6184.
#20 Re: Exercises » Compute the solution: » 2025-07-16 13:18:46
Hi,
2425.
#21 Jokes » Dentist Jokes - III » 2025-07-16 13:08:25
- Jai Ganesh
- Replies: 0
Q: What did the dentist see at the North Pole?
A: A molar bear.
* * *
Q: What did the dentist say to the golfer?
A: "You have a hole in one. "
* * *
Q: Why did the king go to the dentist?
A: To get a new crown!
* * *
Q: Why did the deer need braces?
A: He had buck teeth.
* * *
Q: What was the dentist doing in Panama?
A: Looking for the Root Canal!
* * *
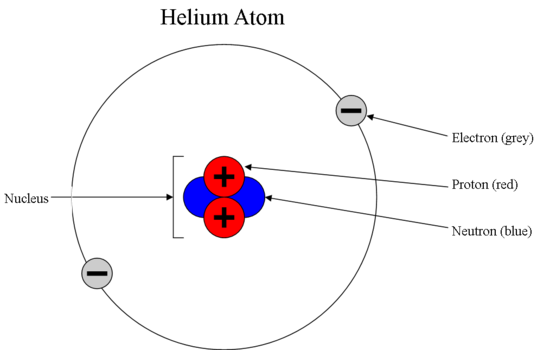
#22 Re: This is Cool » Subatomic Particle » 2025-07-16 00:57:00
Explanation given supra. The source is available in #2 and #3.
#23 Science HQ » Beryllium » 2025-07-15 22:04:32
- Jai Ganesh
- Replies: 0
Beryllium
Gist
Beryllium is a chemical element with the symbol Be and atomic number 4. It's a relatively rare, hard, and lightweight alkaline earth metal known for its high strength-to-weight ratio and stiffness. Beryllium is used in various applications, including aerospace, nuclear reactors, and precision instruments, due to its unique properties.
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a hard, strong, lightweight, and brittle alkaline earth metal, typically a steel-gray color. It's known for its high melting point, good electrical and thermal conductivity, and transparency to X-rays. Beryllium is used in a variety of high-tech applications, including aerospace components, nuclear reactors, and electronics.
Summary
Beryllium is a chemical element; it has symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form minerals. Gemstones high in beryllium include beryl (aquamarine, emerald, red beryl) and chrysoberyl. It is a relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into heavier elements. Beryllium constitutes about 0.0004 percent by mass of Earth's crust. The world's annual beryllium production of 220 tons is usually manufactured by extraction from the mineral beryl, a difficult process because beryllium bonds strongly to oxygen.
In structural applications, the combination of high flexural rigidity, thermal stability, thermal conductivity and low density (1.85 times that of water) make beryllium a desirable aerospace material for aircraft components, missiles, spacecraft, and satellites. Because of its low density and atomic mass, beryllium is relatively transparent to X-rays and other forms of ionizing radiation; therefore, it is the most common window material for X-ray equipment and components of particle detectors. When added as an alloying element to aluminium, copper (notably the alloy beryllium copper), iron, or nickel, beryllium improves many physical properties. For example, tools and components made of beryllium copper alloys are strong and hard and do not create sparks when they strike a steel surface. In air, the surface of beryllium oxidizes readily at room temperature to form a passivation layer 1–10 nm thick that protects it from further oxidation and corrosion. The metal oxidizes in bulk (beyond the passivation layer) when heated above 500 °C (932 °F), and burns brilliantly when heated to about 2,500 °C (4,530 °F).
The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times because of the toxicity of inhaled beryllium-containing dusts that can cause a chronic life-threatening allergic disease, berylliosis, in some people. Berylliosis is typically manifested by chronic pulmonary fibrosis and, in severe cases, right sided heart failure and death.
Details
Beryllium (Be), chemical element, the lightest member of the alkaline-earth metals of Group 2 (IIa) of the periodic table, used in metallurgy as a hardening agent and in many outer space and nuclear applications.
Element Properties
atomic number : 4
atomic weight : 9.0121831
melting point : 1,287 °C (2,349 °F)
boiling point : 2,471 °C (4,480 °F)
specific gravity : 1.85 at 20 °C (68 °F)
oxidation state : +2
Occurrence, properties, and uses
Beryllium is a steel-gray metal that is quite brittle at room temperature, and its chemical properties somewhat resemble those of aluminum. It does not occur free in nature. Beryllium is found in beryl and emerald, minerals that were known to the ancient Egyptians. Although it had long been suspected that the two minerals were similar, chemical confirmation of this did not occur until the late 18th century. Emerald is now known to be a green variety of beryl. Beryllium was discovered (1798) as the oxide by French chemist Nicolas-Louis Vauquelin in beryl and in emeralds and was isolated (1828) as the metal independently by German chemist Friedrich Wöhler and French chemist Antoine A.B. Bussy by the reduction of its chloride with potassium. Beryllium is widely distributed in Earth’s crust and is estimated to occur in Earth’s igneous rocks to the extent of 0.0002 percent. Its cosmic abundance is 20 on the scale in which silicon, the standard, is 1,000,000. The United States has about 60 percent of the world’s beryllium and is by far the largest producer of beryllium; other major producing countries include China, Mozambique, and Brazil.
There are about 30 recognized minerals containing beryllium, including beryl (Al2Be3Si6O18, a beryllium aluminum silicate), bertrandite (Be4Si2O7(OH)2, a beryllium silicate), phenakite (Be2SiO4), and chrysoberyl (BeAl2O4). (The precious forms of beryl, emerald and aquamarine, have a composition closely approaching that given above, but industrial ores contain less beryllium; most beryl is obtained as a by-product of other mining operations, with the larger crystals being picked out by hand.) Beryl and bertrandite have been found in sufficient quantities to constitute commercial ores from which beryllium hydroxide or beryllium oxide is industrially produced. The extraction of beryllium is complicated by the fact that beryllium is a minor constituent in most ores (5 percent by mass even in pure beryl, less than 1 percent by mass in bertrandite) and is tightly bound to oxygen. Treatment with acids, roasting with complex fluorides, and liquid-liquid extraction have all been employed to concentrate beryllium in the form of its hydroxide. The hydroxide is converted to fluoride via ammonium beryllium fluoride and then heated with magnesium to form elemental beryllium. Alternatively, the hydroxide can be heated to form the oxide, which in turn can be treated with carbon and chlorine to form beryllium chloride; electrolysis of the molten chloride is then used to produce the metal. The element is purified by vacuum melting.
Beryllium is the only stable light metal with a relatively high melting point. Although it is readily attacked by alkalies and nonoxidizing acids, beryllium rapidly forms an adherent oxide surface film that protects the metal from further air oxidation under normal conditions. These chemical properties, coupled with its excellent electrical conductivity, high heat capacity and conductivity, good mechanical properties at elevated temperatures, and very high modulus of elasticity (one-third greater than that of steel), make it valuable for structural and thermal applications. Beryllium’s dimensional stability and its ability to take a high polish have made it useful for mirrors and camera shutters in space, military, and medical applications and in semiconductor manufacturing. Because of its low atomic weight, beryllium transmits X-rays 17 times as well as aluminum and has been extensively used in making windows for X-ray tubes. Beryllium is fabricated into gyroscopes, accelerometers, and computer parts for inertial guidance instruments and other devices for missiles, aircraft, and space vehicles, and it is used for heavy-duty brake drums and similar applications in which a good heat sink is important. Its ability to slow down fast neutrons has found considerable application in nuclear reactors.
Much beryllium is used as a low-percentage component of hard alloys, especially with copper as the main constituent but also with nickel- and iron-based alloys, for products such as springs. Beryllium-copper (2 percent beryllium) is made into tools for use when sparking might be dangerous, as in powder factories. Beryllium itself does not reduce sparking, but it strengthens the copper (by a factor of 6), which does not form sparks upon impact. Small amounts of beryllium added to oxidizable metals generate protecting surface films, reducing inflammability in magnesium and tarnishing in silver alloys.
Neutrons were discovered (1932) by British physicist Sir James Chadwick as particles ejected from beryllium bombarded by alpha particles from a radium source. Since then beryllium mixed with an alpha emitter such as radium, plutonium, or americium has been used as a neutron source. The alpha particles released by radioactive decay of radium atoms react with atoms of beryllium to give, among the products, neutrons with a wide range of energies—up to about 5 × 106 electron volts (eV). If radium is encapsulated, however, so that none of the alpha particles reach beryllium, neutrons of energy less than 600,000 eV are produced by the more-penetrating gamma radiation from the decay products of radium. Historically important examples of the use of beryllium/radium neutron sources include the bombardment of uranium by German chemists Otto Hahn and Fritz Strassmann and Austrian-born physicist Lise Meitner, which led to the discovery of nuclear fission (1939), and the triggering in uranium of the first controlled-fission chain reaction by Italian-born physicist Enrico Fermi (1942).
The only naturally occurring isotope is the stable beryllium-9, although 11 other synthetic isotopes are known. Their half-lives range from 1.5 million years (for beryllium-10, which undergoes beta decay) to {6.7} × {10}^{-17} second for beryllium-8 (which decays by two-proton emission). The decay of beryllium-7 (53.2-day half-life) in the Sun is the source of observed solar neutrinos.
Additional Information:
Appearance
Beryllium is a silvery-white metal. It is relatively soft and has a low density.
Uses
Beryllium is used in alloys with copper or nickel to make gyroscopes, springs, electrical contacts, spot-welding electrodes and non-sparking tools. Mixing beryllium with these metals increases their electrical and thermal conductivity.
Other beryllium alloys are used as structural materials for high-speed aircraft, missiles, spacecraft and communication satellites.
Beryllium is relatively transparent to X-rays so ultra-thin beryllium foil is finding use in X-ray lithography. Beryllium is also used in nuclear reactors as a reflector or moderator of neutrons.
The oxide has a very high melting point making it useful in nuclear work as well as having ceramic applications.
Biological role
Beryllium and its compounds are toxic and carcinogenic. If beryllium dust or fumes are inhaled, it can lead to an incurable inflammation of the lungs called berylliosis.
Natural abundance
Beryllium is found in about 30 different mineral species. The most important are beryl (beryllium aluminium silicate) and bertrandite (beryllium silicate). Emerald and aquamarine are precious forms of beryl.
The metal is usually prepared by reducing beryllium fluoride with magnesium metal.

#24 Dark Discussions at Cafe Infinity » Clean Quotes - I » 2025-07-15 17:02:37
- Jai Ganesh
- Replies: 0
Clean Quotes - I
1. Let everyone sweep in front of his own door, and the whole world will be clean. - Johann Wolfgang von Goethe
2. Water is life, and clean water means health. - Audrey Hepburn
3. That's been one of my mantras - focus and simplicity. Simple can be harder than complex: You have to work hard to get your thinking clean to make it simple. But it's worth it in the end because once you get there, you can move mountains. - Steve Jobs
4. Keep close to Nature's heart... and break clear away, once in awhile, and climb a mountain or spend a week in the woods. Wash your spirit clean. - John Muir
5. We learned about gratitude and humility - that so many people had a hand in our success, from the teachers who inspired us to the janitors who kept our school clean... and we were taught to value everyone's contribution and treat everyone with respect. - Michelle Obama
6. Better keep yourself clean and bright; you are the window through which you must see the world. - George Bernard Shaw
7. I aways rewrite each day up to the point where I stopped. When it is all finished, naturally you go over it. You get another chance to correct and rewrite when someone else types it, and you see it clean in type. The last chance is in the proofs. You're grateful for these different chances. - Ernest Hemingway
8. Lemons clean everything. It's the greatest disinfectant. - Sandra Bullock.
#25 This is Cool » Subatomic Particle » 2025-07-15 16:33:56
- Jai Ganesh
- Replies: 2
Subatomic particle
Gist
Subatomic particles are particles smaller than an atom. They include protons, neutrons, and electrons, which are the fundamental building blocks of atoms. Additionally, there are other subatomic particles like quarks, leptons (including electrons, muons, and neutrinos), and bosons, some of which are fundamental and others are composite particles.
Summary
A subatomic particle is any of various self-contained units of matter or energy that are the fundamental constituents of all matter. Subatomic particles include electrons, the negatively charged, almost massless particles that nevertheless account for most of the size of the atom, and they include the heavier building blocks of the small but very dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. But these basic atomic components are by no means the only known subatomic particles. Protons and neutrons, for instance, are themselves made up of elementary particles called quarks, and the electron is only one member of a class of elementary particles that also includes the muon and the neutrino. More-unusual subatomic particles—such as the positron, the antimatter counterpart of the electron—have been detected and characterized in cosmic ray interactions in Earth’s atmosphere. The field of subatomic particles has expanded dramatically with the construction of powerful particle accelerators to study high-energy collisions of electrons, protons, and other particles with matter. As particles collide at high energy, the collision energy becomes available for the creation of subatomic particles such as mesons and hyperons. Finally, completing the revolution that began in the early 20th century with theories of the equivalence of matter and energy, the study of subatomic particles has been transformed by the discovery that the actions of forces are due to the exchange of “force” particles such as photons and gluons. More than 200 subatomic particles have been detected—most of them highly unstable, existing for less than a millionth of a second—as a result of collisions produced in cosmic ray reactions or particle accelerator experiments. Theoretical and experimental research in particle physics, the study of subatomic particles and their properties, has given scientists a clearer understanding of the nature of matter and energy and of the origin of the universe.
The current understanding of the state of particle physics is integrated within a conceptual framework known as the Standard Model. The Standard Model provides a classification scheme for all the known subatomic particles based on theoretical descriptions of the basic forces of matter.
Details
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, like a proton or a neutron, composed of three quarks; or a meson, composed of two quarks), or an elementary particle, which is not composed of other particles (for example, quarks; or electrons, muons, and tau particles, which are called leptons). Particle physics and nuclear physics study these particles and how they interact. Most force-carrying particles like photons or gluons are called bosons and, although they have quanta of energy, do not have rest mass or discrete diameters (other than pure energy wavelength) and are unlike the former particles that have rest mass and cannot overlap or combine which are called fermions. The W and Z bosons, however, are an exception to this rule and have relatively large rest masses at approximately 80 GeV/c^2 and 90 GeV/c^2 respectively.
Experiments show that light could behave like a stream of particles (called photons) as well as exhibiting wave-like properties. This led to the concept of wave–particle duality to reflect that quantum-scale particles behave both like particles and like waves; they are occasionally called wavicles to reflect this.
Another concept, the uncertainty principle, states that some of their properties taken together, such as their simultaneous position and momentum, cannot be measured exactly. Interactions of particles in the framework of quantum field theory are understood as creation and annihilation of quanta of corresponding fundamental interactions. This blends particle physics with field theory.
Even among particle physicists, the exact definition of a particle has diverse descriptions. These professional attempts at the definition of a particle include:
* A particle is a collapsed wave function
* A particle is an excitation of a quantum field
* A particle is an irreducible representation of the Poincaré group
* A particle is an observed thing.
Additional Information
Subatomic Particles are the particles inside an atom. They are self-contained units of matter or energy and are the fundamental constituents of all matter. Initially, the atom was considered to be the fundamental particle which constitutes all the matter. However, with later experiments and discoveries, it was revealed that the atom is itself constituted of several particles such as Electrons, Protons and Neutrons. Further research in particle physics revealed that even protons and neutrons are composite particles, made up of some other subatomic elementary particles.
Types of Subatomic Particles
Atoms are considered the basic building blocks of matter. It was John Dalton who in 1803 postulated that an atom is indestructible and is the fundamental unit of matter. This was proved wrong by J.J. Thomson in 1897.
Electrons
Electrons were discovered by J.J Thomson after many experiments involving cathode rays. He demonstrated the ratio of mass to electric charge of cathode rays. He confirmed that cathode rays are fundamental particles that are negatively charged; these cathode rays became known as electrons.... Read more at: https://vajiramandravi.com/upsc-exam/subatomic-particles/
Protons
Eugene Goldstein in 1886 showed the existence of a positively charged particle in an atom. However, the actual discovery of protons is credited to Ernest Rutherford during his experiment on the scattering of α-particles.
Neutrons
It was inevitable from Rutherford’s experiment that there must be a neutral, sub-nuclear particle with a mass closely equal to protons. James Chadwick in 1932 discovered the neutrons.
Fundamental Particles
Subatomic particles can be further divided into elementary (fundamental) particles and composite particles (made up of elementary particles).