Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2676 2026-01-20 19:31:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2476) Fractional Distillation
Gist
Fractional distillation is the most common form of separation technology used in petroleum refineries, petrochemical and chemical plants, natural gas processing and cryogenic air separation plants. In most cases, the distillation is operated at a continuous steady state.
Fractional distillation is a method to separate a liquid mixture into its parts (fractions) by heating it, relying on the components' different boiling points, especially when those boiling points are close (less than 25°C apart). It uses a fractionating column with obstacles to create multiple cycles of vaporization and condensation, allowing for a purer separation of components than simple distillation.
Summary
The various components of crude oil have different sizes, weights and boiling temperatures; so, the first step is to separate these components. Because they have different boiling temperatures, they can be separated easily by a process called fractional distillation. The steps of fractional distillation are as follows:
* You heat the mixture of two or more substances (liquids) with different boiling points to a high temperature. Heating is usually done with high pressure steam to temperatures of about 1112 degrees Fahrenheit / 600 degrees Celsius.
* The mixture boils, forming vapor (gases); most substances go into the vapor phase.
* The vapor enters the bottom of a long column (fractional distillation column) that is filled with trays or plates. The trays have many holes or bubble caps (like a loosened cap on a soda bottle) in them to allow the vapor to pass through. They increase the contact time between the vapor and the liquids in the column and help to collect liquids that form at various heights in the column. There is a temperature difference across the column (hot at the bottom, cool at the top).
* The vapor rises in the column.
* As the vapor rises through the trays in the column, it cools.
* When a substance in the vapor reaches a height where the temperature of the column is equal to that substance's boiling point, it will condense to form a liquid. (The substance with the lowest boiling point will condense at the highest point in the column; substances with higher boiling points will condense lower in the column.).
* The trays collect the various liquid fractions.
* The collected liquid fractions may pass to condensers, which cool them further, and then go to storage tanks, or they may go to other areas for further chemical processing.
Fractional distillation is useful for separating a mixture of substances with narrow differences in boiling points, and is the most important step in the refining process.
Very few of the components come out of the fractional distillation column ready for market. Many of them must be chemically processed to make other fractions. For example, only 40% of distilled crude oil is gasoline; however, gasoline is one of the major products made by oil companies. Rather than continually distilling large quantities of crude oil, oil companies chemically process some other fractions from the distillation column to make gasoline; this processing increases the yield of gasoline from each barrel of crude oil.
Details
Fractional distillation is the most common form of separation technology used in petroleum refineries, petrochemical and chemical plants, natural gas processing and cryogenic air separation plants. In most cases, the distillation is operated at a continuous steady state. New feed is always being added to the distillation column and products are always being removed. Unless the process is disturbed due to changes in feed, heat, ambient temperature, or condensing, the amount of feed being added and the amount of product being removed are normally equal. This is known as continuous, steady-state fractional distillation.
Industrial distillation is typically performed in large, vertical cylindrical columns known as "distillation or fractionation towers" or "distillation columns" with diameters ranging from about 0.65 to 6 meters (2 to 20 ft) and heights ranging from about 6 to 60 meters (20 to 197 ft) or more. The distillation towers have liquid outlets at intervals up the column which allow for the withdrawal of different fractions or products having different boiling points or boiling ranges. By increasing the temperature of the product inside the columns, the different products are separated. The "lightest" products (those with the lowest boiling point) exit from the top of the columns and the "heaviest" products (those with the highest boiling point) exit from the bottom of the column.
For example, fractional distillation is used in oil refineries to separate crude oil into useful substances (or fractions) having different hydrocarbons of different boiling points. The crude oil fractions with higher boiling points:
* have more carbon atoms
* have higher molecular weights
* are less branched-chain alkanes
* are darker in color
* are more viscous
* are more difficult to ignite and to burn
Large-scale industrial towers use reflux to achieve a more complete separation of products. Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Inside the tower, the reflux liquid flowing downwards provides the cooling needed to condense the vapors flowing upwards, thereby increasing the effectiveness of the distillation tower. The more reflux is provided for a given number of theoretical plates, the better the tower's separation of lower boiling materials from higher boiling materials. Alternatively, the more reflux provided for a given desired separation, the fewer theoretical plates are required.
Crude oil is separated into fractions by fractional distillation. The fractions at the top of the fractionating column have lower boiling points than the fractions at the bottom. All of the fractions are processed further in other refining units.
Fractional distillation is also used in air separation, producing liquid oxygen, liquid nitrogen, and highly concentrated argon. Distillation of chlorosilanes also enable the production of high-purity silicon for use as a semiconductor.
In industrial uses, sometimes a packing material is used in the column instead of trays, especially when low-pressure drops across the column are required, as when operating under vacuum. This packing material can either be random dumped packing (1–3 in (25–76 mm) wide) such as Raschig rings or structured sheet metal. Typical manufacturers are Koch, Sulzer, and other companies. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfer takes place. Unlike conventional tray distillation in which every tray represents a separate point of vapor liquid equilibrium the vapor-liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns it is useful to compute several "theoretical plates" to denote the separation efficiency of the packed column concerning more traditional trays. Differently shaped packings have different surface areas and porosity. Both of these factors affect packing performance.
Design and operation of a distillation column depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the McCabe–Thiele method or the Fenske equation can be used. For a multi-component feed, simulation models are used both for design and operation.
Moreover, the efficiencies of the vapor-liquid contact devices (referred to as plates or trays) used in distillation columns are typically lower than that of a theoretical 100% efficient equilibrium stage. Hence, a distillation column needs more plates than the number of theoretical vapor-liquid equilibrium stages.
Reflux refers to the portion of the condensed overhead product that is returned to the tower. The reflux flowing downwards provides the cooling required for condensing the vapors flowing upwards. The reflux ratio, which is the ratio of the (internal) reflux to the overhead product, is conversely related to the theoretical number of stages required for efficient separation of the distillation products. Fractional distillation towers or columns are designed to achieve the required separation efficiently. The design of fractionation columns is normally made in two steps; a process design, followed by a mechanical design. The purpose of the process design is to calculate the number of required theoretical stages and stream flows including the reflux ratio, heat reflux, and other heat duties. The purpose of the mechanical design, on the other hand, is to select the tower internals, column diameter, and height. In most cases, the mechanical design of fractionation towers is not straightforward. For the efficient selection of tower internals and the accurate calculation of column height and diameter, many factors must be taken into account. Some of the factors involved in design calculations include feed load size and properties and the type of distillation column used.
The two major types of distillation columns used are tray and packing columns. Packing columns are normally used for smaller towers and loads that are corrosive or temperature-sensitive or for vacuum service where pressure drop is important. Tray columns, on the other hand, are used for larger columns with high liquid loads. They first appeared on the scene in the 1820s. In most oil refinery operations, tray columns are mainly used for the separation of petroleum fractions at different stages of oil refining.
In the oil refining industry, the design and operation of fractionation towers is still largely accomplished on an empirical basis. The calculations involved in the design of petroleum fractionation columns require in the usual practice the use of numerable charts, tables, and complex empirical equations. In recent years, however, a considerable amount of work has been done to develop efficient and reliable computer-aided design procedures for fractional distillation.
Additional Information
The inside of a fractional distilling column has sets of perforated trays. Each perforation is fitted with a bubble cap. Very hot, vaporized crude oil is pumped into the bottom of the column and rises up through the perforations. The bubble cap forces the oil vapor to bubble through liquid on the tray. This causes the vapor to cool as it flows upward and to condense into liquids. Excess liquid overflows through a pipe called a downcomer to the tray below. At various levels in the column, liquid is drawn off. The heavier products, such as straight-run heavy gas oil, are taken from the bottom part of the column and the lighter products, such as straight-run gasoline, are taken from the top. Straight-run natural gas comes out the top, and straight-run residuum comes out the bottom.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2677 2026-01-21 16:44:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2476) The Alps
The Alps
Gist
The Alps are Europe's highest and most extensive mountain range, forming a crescent across eight countries (France, Switzerland, Italy, Austria, Germany, Slovenia, Liechtenstein, Monaco), famous for Mont Blanc, Matterhorn, stunning scenery, skiing, biodiversity, and unique climate zones, originating from tectonic plate collision and providing vital water sources for Europe.
Which countries are in the Alps?
They stretch across eight countries: France, Switzerland, Italy, Monaco, Liechtenstein, Austria, Germany and Slovenia. The mountains were formed millions of years ago as two giant tectonic plates collided, creating some of the highest mountains in Europe, like the Matterhorn, Mont Blanc, and the Eiger.
Summary
The Alps are some of the highest and most extensive mountain ranges in Europe, stretching approximately 1,200 km (750 mi) across eight Alpine countries (from west to east): Monaco, France, Switzerland, Italy, Liechtenstein, Germany, Austria, Slovenia, and Hungary.
The Alpine arch extends from Nice on the western Mediterranean to Trieste on the Adriatic and Vienna at the beginning of the Pannonian Basin. The mountains were formed over tens of millions of years as the African and Eurasian tectonic plates collided. Extreme shortening caused by the event resulted in marine sedimentary rocks rising by thrusting and folding into high mountain peaks such as Mont Blanc and the Matterhorn.
Mont Blanc spans the French–Italian border, and at 4,809 m (15,778 ft) is the highest mountain in the Alps. The Alpine region area contains 82 peaks higher than 4,000 m (13,000 ft).
The altitude and size of the range affect the climate in Europe; in the mountains, precipitation levels vary greatly and climatic conditions consist of distinct zones. Wildlife such as ibex live in the higher peaks to elevations of 3,400 m (11,155 ft), and plants such as edelweiss grow in rocky areas in lower elevations as well as in higher elevations.
Evidence of human habitation in the Alps goes back to the Palaeolithic era. A mummified man ("Ötzi"), determined to be 5,000 years old, was discovered on a glacier at the Austrian–Italian border in 1991.
By the 6th century BC, the Celtic La Tène culture was well established. Hannibal notably crossed the Alps with a herd of elephants, and the Romans had settlements in the region. In 1800, Napoleon crossed one of the mountain passes with an army of 40,000. The 18th and 19th centuries saw an influx of naturalists, writers, and artists, in particular, the Romanticists, followed by the Golden Age of Alpinism as mountaineers began to ascend the peaks of the Alps.
The Alpine region has a strong cultural identity. Traditional practices such as farming, cheese making, and woodworking still thrive in Alpine villages. However, the tourist industry began to grow early in the 20th century and expanded significantly after World War II, eventually becoming the dominant industry by the end of the century.
The Winter Olympic Games have been hosted in the Swiss, French, Italian, Austrian and German Alps. As of 2010, the region is home to 14 million people and has 120 million annual visitors.
Details
Alps, a small segment of a discontinuous mountain chain that stretches from the Atlas Mountains of North Africa across southern Europe and Asia to beyond the Himalayas. The Alps extend north from the subtropical Mediterranean coast near Nice, France, to Lake Geneva before trending east-northeast to Vienna (at the Vienna Woods). There they touch the Danube River and meld with the adjacent plain. The Alps form part of France, Italy, Switzerland, Germany, Austria, Slovenia, Croatia, Bosnia and Herzegovina, Montenegro, Serbia, and Albania. Only Switzerland and Austria can be considered true Alpine countries, however. Some 750 miles (1,200 kilometres) long and more than 125 miles wide at their broadest point between Garmisch-Partenkirchen, Germany, and Verona, Italy, the Alps cover more than 80,000 square miles (207,000 square kilometres). They are the most prominent of western Europe’s physiographic regions.
Though they are not as high and extensive as other mountain systems uplifted during the Paleogene and Neogene periods (i.e., about 65 million to 2.6 million years ago)—such as the Himalayas and the Andes and Rocky mountains—they are responsible for major geographic phenomena. The Alpine crests isolate one European region from another and are the source of many of Europe’s major rivers, such as the Rhône, Rhine, Po, and numerous tributaries of the Danube. Thus, waters from the Alps ultimately reach the North, Mediterranean, Adriatic, and Black seas. Because of their arclike shape, the Alps separate the marine west-coast climates of Europe from the Mediterranean areas of France, Italy, and the Balkan region. Moreover, they create their own unique climate based on both the local differences in elevation and relief and the location of the mountains in relation to the frontal systems that cross Europe from west to east. Apart from tropical conditions, most of the other climates found on the Earth may be identified somewhere in the Alps, and contrasts are sharp.
A distinctive Alpine pastoral economy that evolved through the centuries has been modified since the 19th century by industry based on indigenous raw materials, such as the industries in the Mur and Mürz valleys of southern Austria that used iron ore from deposits near Eisenerz. Hydroelectric power development at the end of the 19th and beginning of the 20th centuries, often involving many different watersheds, led to the establishment in the lower valleys of electricity-dependent industries, manufacturing such products as aluminum, chemicals, and specialty steels. Tourism, which began in the 19th century in a modest way, became, after World War II, a mass phenomenon. Thus, the Alps are a summer and winter playground for millions of European urban dwellers and annually attract tourists from around the world. Because of this enormous human impact on a fragile physical and ecological environment, the Alps are likely the most threatened mountain system in the world.
Physical features:
Geology
The Alps emerged during the Alpine orogeny, an event that began about 65 million years ago as the Mesozoic Era was drawing to a close. A broad outline helps to clarify the main episodes of a complicated process. At the end of the Paleozoic Era, about 250 million years ago, eroded Hercynian mountains, similar to the present Massif Central in France and Bohemian Massif embracing parts of Germany, Austria, Poland, and the Czech Republic, stood where the Alps are now located. A large landmass, formed of crystalline rocks and known as Tyrrhenia, occupied what is today the western Mediterranean basin, whereas much of the rest of Europe was inundated by a vast sea. During the Mesozoic (about 250 million to 65 million years ago) Tyrrhenia was slowly leveled by the forces of erosion. The eroded materials were carried southward by river action and deposited at the bottom of a vast ocean known as the Tethys Sea, where they were slowly transformed into horizontal layers of rock composed of limestone, clay, shale, and sandstone.
About 44 million years ago, relentless and powerful pressures from the south first formed the Pyrenees and then the Alps, as the deep layers of rock that had settled into the Tethys Sea were folded around and against the crystalline bedrock and raised with the bedrock to heights approaching the present-day Himalayas. These tectonic movements lasted until 9 million years ago. Tyrrhenia sank at the beginning of the Quaternary Period, about 2.6 million years ago, but remnants of its mass, such as the rugged Estéral region west of Cannes, are still found in the western Mediterranean. Throughout the Quaternary Period, erosive forces gnawed steadily at the enormous block of newly folded and upthrust mountains, forming the general outlines of the present-day landscape.
The landscape was further modeled during the Quaternary by Alpine glaciation and by expanding ice tongues, some reaching depths of nearly 1 mile (1.6 kilometres), that filled in the valleys and overflowed onto the plains. Amphitheatre-like cirques, arête ridges, and majestic peaks such as the Matterhorn and Grossglockner were shaped from the mountaintops; the valleys were widened and deepened into general U-shapes, and immense waterfalls, like the Staubbach and Trümmelbach falls in the Lauterbrunnen Valley of the Bernese Alps, poured forth from hanging valleys hundreds of feet above the main valley floors; elongated lakes of great depth such as Lake Annecy in France, Lake Constance, bordering Switzerland, Germany, and Austria, and the lakes of the Salzkammergut in Austria filled in many of the ice-scoured valleys; and enormous quantities of sands and gravels were deposited by the melting glaciers, and landslides—following the melting of much of the ice—filled in sections of the valley floors. The hills east of Sierre in the Rhône valley are an example of this last phenomenon, and they mark the French–German language divide in this area.
When the ice left the main valleys, there was renewed river downcutting, both in the lateral and transverse valleys. The river valleys have been eroded to relatively low elevations that are well below those of the surrounding mountains. Thus, Aosta, Italy, in the Pennine Alps, and Sierre, Switzerland, look up to peaks that tower a mile and a half above them. In the valley of the Arve River near Mont Blanc, the difference in relief is more than 13,100 feet.
Glaciation therefore modified what otherwise would have been a harsher physical environment: the climate was much milder in the valleys than on the surrounding heights, settlement could be established deeper into the mountains, communication was facilitated, and soils were inherently more fertile because of morainic deposits. Vigorous glacial erosion continues in modern times. Many hundreds of square miles of Alpine glaciers, such as those in the Ortles and Adamello ranges and such deep-valley glaciers as the Aletsch Glacier near Brig, Switzerland, are still found in the Alps. The summer runoff from these ice masses is instrumental in filling the deep reservoirs used to generate hydroelectricity.
Physiography
The Alps present a great variety of elevations and shapes, ranging from the folded sediments forming the low-lying pre-Alps that border the main range everywhere except in northwestern Italy to the crystalline massifs of the inner Alps that include the Belledonne and Mont Blanc in France, the Aare and Gotthard in Switzerland, and the Tauern in Austria. From the Mediterranean to Vienna, the Alps are divided into Western, Central, and Eastern segments, each of which consists of several distinct ranges.
The Western Alps trend north from the coast through southeastern France and northwestern Italy to Lake Geneva and the Rhône valley in Switzerland. Their forms include the low-lying arid limestones of the Maritime Alps near the Mediterranean, the deep cleft of the Verdon Canyon in France, the crystalline peaks of the Mercantour Massif, and the glacier-covered dome of Mont Blanc, which at 15,771 feet (4,807 metres) is the highest peak in the Alps. Rivers from these ranges flow west into the Rhône and east into the Po.
The Central Alps occupy an area from the Great St. Bernard Pass east of Mont Blanc on the Swiss-Italian border to the region of the Splügen Pass north of Lake Como. Within this territory are such distinctive peaks as the Dufourspitze, Weisshorn, Matterhorn, and Finsteraarhorn, all 14,000 feet high. In addition, the great glacial lakes—Como and Maggiore in the south, part of the drainage system of the Po; and Thun, Brienz, and Lucerne (Vierwaldstättersee) in the north—fall within this zone.
The Eastern Alps, consisting in part of the Rätische range in Switzerland, the Dolomite Alps in Italy, the Bavarian Alps of southern Germany and western Austria, the Tauern Mountains in Austria, the Julian Alps in northeastern Italy and northern Slovenia, and the Dinaric Alps along the western edge of the Balkan Peninsula, generally have a northerly and southeasterly drainage pattern. The Inn, Lech, and Isar rivers in Germany and the Salzach and Enns in Austria flow into the Danube north of the Alps, while the Mur and Drau (Austria) and Sava (Balkan region) rivers discharge into the Danube east and southeast of the Alps. Within the Eastern Alps in Italy, Lake Garda drains into the Po, whereas the Adige, Piave, Tagliamento, and Isonzo pour into the Gulf of Venice.
Differences in relief within the Alps are considerable. The highest mountains, composed of autochthonous crystalline rocks, are found in the west in the Mont Blanc massif and also in the massif centring on Finsteraarhorn (14,022 feet) that divides the cantons of Valais and Bern. Other high chains include the crystalline rocks of the Mount Blanche nappe—which includes the Weisshorn (14,780 feet)—and the nappe of Monte Rosa Massif, sections of which mark the frontier between Switzerland and Italy. Farther to the east, Bernina Peak is the last of the giants over 13,120 feet (4,000 metres). In Austria the highest peak, the Grossglockner, reaches only 12,460 feet; Germany’s highest point, the Zugspitze in the Bavarian Alps, only 9,718 feet; and the highest point of Slovenia and the Julian Alps, Triglav, only 9,396 feet. Some of the lowest areas within the Western Alps are found at the delta of the Rhône River where the river enters Lake Geneva, 1,220 feet. In the valleys of the Eastern Alps north of Venice, elevations of only about 300 feet are common.
Climate of the Alps
The location of the Alps, as well as the great variations in their elevations and exposure, give rise to extreme differences in climate, not only among separate ranges but also within a particular range itself. Because of their central location in Europe, the Alps are affected by four main climatic influences: from the west flows the relatively mild, moist air of the Atlantic; cool or cold polar air descends from northern Europe; continental air masses, cold and dry in winter and hot in summer, dominate in the east; and, to the south, warm Mediterranean air flows northward. Daily weather is influenced by the location and passage of cyclonic storms and the direction of the accompanying winds as they pass over the mountains.
Temperature extremes and annual precipitation are related to the physiography of the Alps. The valley bottoms clearly stand out because generally they are warmer and drier than the surrounding heights. In winter nearly all precipitation above 5,000 feet is in the form of snow, and depths from 10 to 33 feet or more are common. Snow cover lasts from approximately mid-November to the end of May at the 6,600-foot level, blocking the high mountain passes; nevertheless, relatively snowless winters can occur. Mean January temperatures on the valley floors range from 23 to 39 °F (−5 to 4 °C) to as high as 46 °F (8 °C) in the mountains bordering the Mediterranean, whereas mean July temperatures range between 59 and 75 °F (15 and 24 °C). Temperature inversions are frequent, especially during autumn and winter, and the valleys often fill with fog and stagnant air for days at a time. At those times the levels above 3,300 feet can be warmer and sunnier than the low-lying valley bottoms. Winds can play a prominent role in daily weather and microclimatic conditions.
A foehn wind can last from two to three days and blows either south–north or north–south, depending on the tracking of cyclonic storms. The air mass of such a wind is cooled adiabatically as it passes upward to the mountain crests, which precipitates either rain or snow and retards the rate of cooling. When this drier air descends on the lee side, it is adiabatically warmed by compression at a constant rate and therefore has a higher temperature at the same altitude than when it began its upward flow. Snow in the affected areas disappears rapidly.
Avalanches, one of the great destructive forces of nature, are an ever-present danger during the period from late November to early June. Most follow well-defined paths, but much of the fear of avalanches is related to the difficulty of predicting where and when they will strike. Avalanches are a greater threat to human life and property in the Alps than in other mountain ranges because of the region’s relatively high population density and the tens of millions of tourists who visit each year. The development of ski resorts also has depleted many forests, which serve as natural barriers against avalanches.
Additional Information:
Geography:
The Alps are the highest and most extensive mountain range system that lie in south-central Europe. The mountain range stretches approximately 750 miles (1,200 kilometers) in a crescent shape across eight Alpine countries: France, Switzerland, Monaco, Italy, Liechtenstein, Austria, Germany, and Slovenia.
Ecology:
The Alps are an interzonal mountain system (Orobiome), or a “transition area” between Central and Mediterranean Europe. The Alps have high habitat diversity, with 200 habitats classified throughout the mountain range. This mountain range is home to a high level of biodiversity.
According to the World Wildlife Fund (WWF), there are over 4,500 species of plants, 200 bird species, 21 amphibian species, 15 reptile species, and 80 mammal species. Many of these species have made adaptations to the harsh cold conditions and high altitudes.
None of the 80 mammal species found in the Alps are “strictly” endemic, meaning they occur in the Alps and nowhere else in the world. Some of the larger carnivore species found in the Alps include the Eurasian lynx, the wolf, and the brown bear, and herbivores include the Alpine ibex, the chamois. These populations have been reduced in size or fragmented into small groups. There are also numerous rodent species found in the Alps, such as voles and marmots.
There are about 200 breeding bird species, as well as an equal number of migratory species, in the Alps. The largest bird species found in the Alps are the golden eagle and the bearded vulture. The alpine chough is the most common bird found in the region.
Of the 21 total species of amphibians, only one is endemic, Salamandra lanzai. There are fifteen reptile species present, including adders and vipers.
According to WWF, the Alps are one of the regions with the richest flora and fauna in Europe, second only to the Mediterranean. There are about 4,500 species of vascular plants, 800 species of mosses, 300 liverworts, 2500 lichens and more than 5000 fungi found in the Alps. About 8 percent of the vascular species are endemic. The variety of habitats in the Alps helps to promote the uniqueness of Alpine flora, along with the harsh environmental conditions that drive species to change and adapt.
Highest peak:
Mont Blanc is the highest peak at 15,776 feet (4,808 m). It is the second-highest and second most prominent mountain in Europe and the eleventh most prominent mountain summit in the world. The Mont Blanc massif spans across France, Italy, and Switzerland, while the summit itself lies on the border between France and Italy. There are about a hundred peaks higher than 13,000 ft (4,000 m) in the Alpine region.
Rivers:
The Alps provide lowland Europe with drinking water, irrigation, and hydroelectric power. Although the area is only about 11% of the surface area of Europe, the Alps provide up to 90% of water to lowland Europe. Major European rivers flow from the Alps, including the Rhine, the Rhône, the Inn, and the Po.
Glaciers:
The major glacierized areas in the Alps are situated along the crest of the mountain chain, with the largest glaciers often found at the highest elevations. There are smaller glaciers scattered throughout the Alps.
The Swiss Alps is known for glaciers, containing around 1,800 glaciers. The region’s glaciers include the longest glacier in the Alps: the Aletsch Glacier.
Additional Facts:
* At over 12 miles long and about a half-mile thick, the famous Aletsch valley glacier is the longest in the Alps, but due to climate change, the Aletsch is shrinking and predicted to completely melt by the end of this century.
* The Alps are Europe’s highest and most extensive mountain range.
* Mont Blanc is the highest mountain in the Alps, spanning 3 countries. Its granite ramparts distinguish it from other peaks. Mont Blanc’s ranges rose straight from the deep and are still rising, a phenomenon caused by glacial movement.
* The limestone Dolomites in Italy are half as high as Mont Blanc and were created as Africa’s collision with Europe pushed together an ancient tropical seafloor with the ancient skeletons of marine organisms into the sky.
* Humans have been living in the Alps since Paleolithic times, about 60,000 to 50,000 years ago. The Alpine region continues to have a strong cultural identity and its traditional culture of farming, cheesemaking, and woodworking still exists in Alpine villages. However, the tourism industry has been growing since the 20th century. While the region is home to 14 million people, it has 120 million annual visitors.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2678 2026-01-22 22:59:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2477) Photosysthesis
Gist
Photosynthesis is the vital process where plants, algae, and some bacteria use sunlight, water, and carbon dioxide to create their own food (glucose/sugars) and release oxygen, converting light energy into stored chemical energy, forming the base of most food webs and producing atmospheric oxygen. It involves light-dependent reactions (capturing energy in ATP/NADPH using chlorophyll) and light-independent reactions (Calvin Cycle) that use this energy to build sugars, occurring in chloroplasts.
Photosynthesis is the process where plants, algae, and some bacteria use sunlight, water, and carbon dioxide to create their own food (glucose/sugar) and release oxygen as a byproduct, converting light energy into stored chemical energy. This vital process, primarily occurring in chloroplasts, provides the energy and oxygen necessary for most life on Earth.
Summary
Photosynthesis is a system of biological processes by which photopigment-bearing autotrophic organisms, such as most plants, algae and cyanobacteria, convert light energy — typically from sunlight — into the chemical energy necessary to fuel their metabolism. The term photosynthesis usually refers to oxygenic photosynthesis, a process that releases oxygen as a byproduct of water splitting. Photosynthetic organisms store the converted chemical energy within the bonds of intracellular organic compounds (complex compounds containing carbon), typically carbohydrates like sugars (mainly glucose, fructose and sucrose), starches, phytoglycogen and cellulose. When needing to use this stored energy, an organism's cells then metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth.
Some organisms also perform anoxygenic photosynthesis, which does not produce oxygen. Some bacteria (e.g. purple bacteria) use bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, releasing sulfur instead of oxygen, which was a dominant form of photosynthesis in the euxinic Canfield oceans during the Boring Billion. Archaea such as Halobacterium also perform a type of non-carbon-fixing anoxygenic photosynthesis, where the simpler photopigment retinal and its microbial rhodopsin derivatives are used to absorb green light and produce a proton (hydron) gradient across the cell membrane, and the subsequent ion movement powers transmembrane proton pumps to directly synthesize adenosine triphosphate (ATP), the "energy currency" of cells. Such archaeal photosynthesis might have been the earliest form of photosynthesis that evolved on Earth, as far back as the Paleoarchean, preceding that of cyanobacteria (see Purple Earth hypothesis).
While the details may differ between species, the process always begins when light energy is absorbed by the reaction centers, proteins that contain photosynthetic pigments or chromophores. In plants, these pigments are chlorophylls (a porphyrin derivative that absorbs the red and blue spectra of light, thus reflecting green) held inside chloroplasts, abundant in leaf cells. In cyanobacteria, they are embedded in the plasma membrane. In these light-dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. The hydrogen freed by the splitting of water is used in the creation of two important molecules that participate in energetic processes: reduced nicotinamide adenine dinucleotide phosphate (NADPH) and ATP.
In plants, algae, and cyanobacteria, sugars are synthesized by a subsequent sequence of light-independent reactions called the Calvin cycle. In this process, atmospheric carbon dioxide is incorporated into already existing organic compounds, such as ribulose bisphosphate (RuBP). Using the ATP and NADPH produced by the light-dependent reactions, the resulting compounds are then reduced and removed to form further carbohydrates, such as glucose. In other bacteria, different mechanisms like the reverse Krebs cycle are used to achieve the same end.
The first photosynthetic organisms probably evolved early in the evolutionary history of life using reducing agents such as hydrogen or hydrogen sulfide, rather than water, as sources of electrons. Cyanobacteria appeared later; the excess oxygen they produced contributed directly to the oxygenation of the Earth, which rendered the evolution of complex life possible. The average rate of energy captured by global photosynthesis is approximately 130 terawatts, which is about eight times the total power consumption of human civilization. Photosynthetic organisms also convert around 100–115 billion tons (91–104 Pg petagrams, or billions of metric tons), of carbon into biomass per year. Photosynthesis was discovered in 1779 by Jan Ingenhousz who showed that plants need light, not just soil and water.
Details
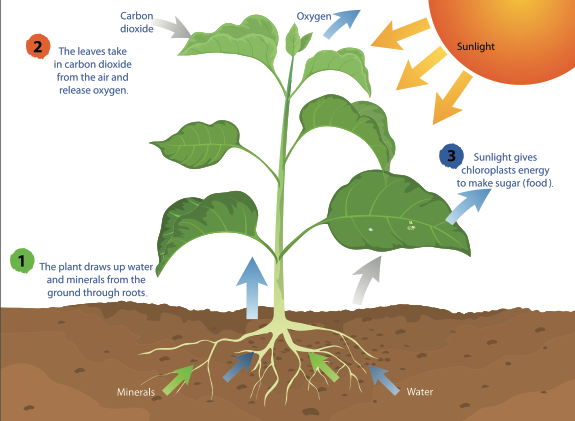
Photosynthesis is the process by which green plants and certain other organisms transform light energy into chemical energy. During photosynthesis in green plants, light energy is captured and used to convert water, carbon dioxide, and minerals into oxygen and energy-rich organic compounds.
Importance of photosynthesis
It would be impossible to overestimate the importance of photosynthesis in the maintenance of life on Earth. The Great Oxidation Event, which began about 2.4 billion years ago and was largely driven by the photosynthetic cyanobacteria, raised atmospheric oxygen to nearly 1 percent of present levels over a span of 600 million years, paving the way for the evolution of most forms of multicellular life. Photosynthesis completely transformed Earth’s environment and biosphere. The life-giving process continues to sustain biodiversity since autotrophs are foundational to nearly every food web on the planet. If photosynthesis ceased, there would soon be little food or other organic matter on Earth. Most organisms would disappear, and in time Earth’s atmosphere would become nearly devoid of gaseous oxygen. The only organisms able to exist under such conditions would be the chemosynthetic bacteria, which can utilize the chemical energy of certain inorganic compounds and thus are not dependent on the conversion of light energy.
Energy produced by photosynthesis carried out by plants millions of years ago is responsible for the fossil fuels (i.e., coal, oil, and natural gas) that power industrial society. In past ages, green plants and small organisms that fed on plants increased faster than they were consumed, and their remains were deposited in Earth’s crust by sedimentation and other geological processes. There, protected from oxidation, these organic remains were slowly converted to fossil fuels. These fuels not only provide much of the energy used in factories, homes, and transportation but also serve as the raw material for plastics and other synthetic products. Unfortunately, modern civilization is using up in a few centuries the excess of photosynthetic production accumulated over millions of years. Consequently, the carbon dioxide that has been removed from the air to make carbohydrates in photosynthesis over millions of years is being returned at an incredibly rapid rate. The carbon dioxide concentration in Earth’s atmosphere is rising the fastest it ever has in Earth’s history, and this phenomenon—known as global warming—is expected to have major implications on Earth’s climate.
Evolution of the process
Although life and the quality of the atmosphere today depend on photosynthesis, it is likely that green plants evolved long after the first living cells. When Earth was young, electrical storms and solar radiation probably provided the energy for the synthesis of complex molecules from abundant simpler ones, such as water, ammonia, and methane. The first living cells probably evolved from these complex molecules. For example, the accidental joining (condensation) of the amino acid glycine and the fatty acid acetate may have formed complex organic molecules known as porphyrins. These molecules, in turn, may have evolved further into colored molecules called pigments—e.g., chlorophylls of green plants, bacteriochlorophyll of photosynthetic bacteria, hemin (the red pigment of blood), and cytochromes, a group of pigment molecules essential in both photosynthesis and cellular respiration.
Primitive colored cells then had to evolve mechanisms for using the light energy absorbed by their pigments. At first, the energy may have been used immediately to initiate reactions useful to the cell. As the process for using light energy continued to evolve, however, a larger part of the absorbed light energy probably was stored as chemical energy, to be used to maintain life. Green plants, with their ability to use light energy to convert carbon dioxide and water to carbohydrates and oxygen, are the culmination of this evolutionary process.
The first oxygenic (oxygen-producing) cells probably were the cyanobacteria (blue-green algae), which appeared about two billion to three billion years ago. These microscopic organisms are believed to have greatly increased the oxygen content of the atmosphere, making possible the development of aerobic (oxygen-using) organisms. Cyanophytes are prokaryotic cells; that is, they contain no distinct membrane-enclosed subcellular particles (organelles), such as nuclei and chloroplasts. Green plants, by contrast, are composed of eukaryotic cells, in which the photosynthetic apparatus is contained within membrane-bound chloroplasts. The complete genome sequences of cyanobacteria and higher plants provide evidence that the first photosynthetic eukaryotes were likely the red algae that developed when nonphotosynthetic eukaryotic cells engulfed cyanobacteria. Within the host cells, these cyanobacteria evolved into chloroplasts.
There are a number of photosynthetic bacteria that are not oxygenic (e.g., the sulfur bacteria previously discussed). The evolutionary pathway that led to these bacteria diverged from the one that resulted in oxygenic organisms. In addition to the absence of oxygen production, nonoxygenic photosynthesis differs from oxygenic photosynthesis in two other ways: light of longer wavelengths is absorbed and used by pigments called bacteriochlorophylls, and reduced compounds other than water (such as hydrogen sulfide or organic molecules) provide the electrons needed for the reduction of carbon dioxide.
Additional Information
Photosynthesis is the process by which plants use sunlight, water, and carbon dioxide to create oxygen and energy in the form of sugar.
Most life on Earth depends on photosynthesis.The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O2) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating herbivores.
The process
During photosynthesis, plants take in carbon dioxide (CO2) and water (H2O) from the air and soil. Within the plant cell, the water is oxidized, meaning it loses electrons, while the carbon dioxide is reduced, meaning it gains electrons. This transforms the water into oxygen and the carbon dioxide into glucose. The plant then releases the oxygen back into the air, and stores energy within the glucose molecules.
Chlorophyll
Inside the plant cell are small organelles called chloroplasts, which store the energy of sunlight. Within the thylakoid membranes of the chloroplast is a light-absorbing pigment called chlorophyll, which is responsible for giving the plant its green color. During photosynthesis, chlorophyll absorbs energy from blue- and red-light waves, and reflects green-light waves, making the plant appear green.
Light-dependent Reactions vs. Light-independent Reactions
While there are many steps behind the process of photosynthesis, it can be broken down into two major stages: light-dependent reactions and light-independent reactions. The light-dependent reaction takes place within the thylakoid membrane and requires a steady stream of sunlight, hence the name light-dependent reaction. The chlorophyll absorbs energy from the light waves, which is converted into chemical energy in the form of the molecules ATP and NADPH. The light-independent stage, also known as the Calvin cycle, takes place in the stroma, the space between the thylakoid membranes and the chloroplast membranes, and does not require light, hence the name light-independent reaction. During this stage, energy from the ATP and NADPH molecules is used to assemble carbohydrate molecules, like glucose, from carbon dioxide.
C3 and C4 Photosynthesis
Not all forms of photosynthesis are created equal, however. There are different types of photosynthesis, including C3 photosynthesis and C4 photosynthesis. C3 photosynthesis is used by the majority of plants. It involves producing a three-carbon compound called 3-phosphoglyceric acid during the Calvin Cycle, which goes on to become glucose. C4 photosynthesis, on the other hand, produces a four-carbon intermediate compound, which splits into carbon dioxide and a three-carbon compound during the Calvin Cycle. A benefit of C4 photosynthesis is that by producing higher levels of carbon, it allows plants to thrive in environments without much light or water.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2679 2026-01-24 20:23:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2478) Channel Tunnel
Channel Tunnel
Gist
The Channel Tunnel (often called the 'Chunnel' for short) is an undersea tunnel linking southern England and northern France. It is operated by the company Getlink, who also run a railway shuttle (Le Shuttle) between Folkestone and Calais, carrying passengers in cars, vans and other vehicles.
Which country owns the Channel tunnel?
To be specific, LeShuttle is operated by Getlink, the company which owns and operates the Channel Tunnel, the infrastructure connecting the UK with France. Eurostar is a customer of Getlink and runs its passenger trains through the tunnel.
How long are you underwater in the chunnel?
How long are you underwater on the Eurostar? It takes around 35 minutes for the Eurostar to cross the 23-mile underwater stretch of the Channel Tunnel.
Summary
The Channel Tunnel (French: Tunnel sous la Manche, sometimes referred to as the Chunnel) is a 50.46-kilometre (31.35-mile) railway tunnel beneath the English Channel that connects Folkestone in the United Kingdom with Coquelles in northern France. Opened in 1994, it remains the only fixed link between Great Britain and the European mainland.
The tunnel has the longest underwater section of any tunnel in the world, at 37.9 km (23.5 miles), reaching a depth of 75 m (246 ft) below sea level and runs, on average, 45 m (148 ft) below the seabed. It is the third-longest railway tunnel in the world. Although the tunnel was designed for speeds up to 200 km/h (120 mph), trains are limited to a maximum speed of 160 km/h (99 mph) for safety reasons. It connects to high-speed railway lines on either end: the LGV Nord in France and High Speed 1 in the United Kingdom.
The tunnel is operated by Getlink (formerly Eurotunnel) and is used by Eurostar high-speed passenger trains, LeShuttle services for road vehicles, and freight trains. In 2017, Eurostar trains carried 10.3 million passengers, freight trains transported 1.2 million tonnes (2.6 billion pounds) of freight, and LeShuttle trains moved 10.4 million passengers in 2.6 million cars and 51,000 coaches, and 1.6 million heavy goods vehicles carrying 21.3 million tonnes (47 billion pounds) of freight. That compares with 11.7 million passengers, 2.2 million cars, and 2.6 million heavy goods vehicles transported by sea through the Port of Dover.
Proposals for a cross-Channel tunnel date back as early as 1802, but concerns over national security delayed development. The modern project was initiated by Eurotunnel in 1988 and completed in 1994, at a final cost of £4.65 billion (equivalent to £11.7 billion in 2023). An engineering marvel, the Channel Tunnel was, at the time of its opening, by far the longest tunnel in Europe, and has only been surpassed by the Gotthard Base Tunnel in Switzerland. However, despite its engineering significance, several economic assessments have found that it has had only a limited positive economic impact on the British economy. Additionally, the tunnel has also experienced occasional service disruptions due to technical faults, fires, severe weather, and unauthorised access by migrants around Calais seeking entry to the United Kingdom.
Details
The Channel Tunnel (often called the 'Chunnel' for short) is an undersea tunnel linking southern England and northern France. It is operated by the company Getlink, who also run a railway shuttle (Le Shuttle) between Folkestone and Calais, carrying passengers in cars, vans and other vehicles.
Eurostar is a totally separate company and is Getlink’s biggest customer, running high-speed passenger services through the Channel Tunnel between London and a number of other European cities on the continent, including Paris, Brussels, Lille, Amsterdam and Rotterdam.
The Chunnel actually comprises three tunnels: two rail tunnels, used for freight and passenger trains, and a service tunnel.
How long is the Channel Tunnel?
The Channel Tunnel is 31.5 miles long or 50.45 km. That's the equivalent of 169 Eiffel Towers stacked on top of each other.
23.5 miles (37.9 km) of the Channel Tunnel is under the English Channel, making it the world's longest undersea tunnel.
What year did the Channel Tunnel open?
The idea of a tunnel under the Channel was first proposed in 1802 but construction wasn't started until 1988. It was completed in 1993, and Eurostar services started in November 1994.
What does the Eurotunnel look like underwater?
This may be a disappointing answer, but you can't actually see the sea from the Eurostar. When you go through the tunnel and look out of the window, all you can see is your reflection in the glass because it's quite dark outside. You can catch glimpses of the walls of the tunnel, of course, which are made of reinforced concrete.
Where is the Channel Tunnel?
The Channel Tunnel runs between Calais in northern France and Folkestone in south Kent. Vehicle traffic for Le Shuttle gets on in Calais and gets off in Folkestone. Calais is about three hour's drive from Paris and Folkestone is about an hour and a half's drive from London.
Our passenger-only Eurostar trains leave from St Pancras International station in London and go directly to the centre of Paris, Brussels and other Eurostar destinations in Europe.
How deep is the Channel Tunnel?
At its deepest, the tunnel is 75 metres (246 feet) below the sea level. That's the same as 107 baguettes balancing on top of each other.
The English Channel is much deeper than the tunnel, with its deepest point measuring 175 meters (574 feet) below sea level.
How was the Channel Tunnel built?
The Channel Tunnel is made of three separate tunnels running parallel to each other. One train tunnel running south (UK to France), one train tunnel running north (France to UK) and one service tunnel. All three tunnels were drilled below the seabed and link Folkestone in Kent to Coquelles in Pas-de-Calais.
However, the idea of connecting the UK and France by tunnel is much older than people think – dating back to the early 1800s when its supporters included Napoleon Bonaparte.
Work on experimental tunnels started back in 1880 at Abbot’s Cliff near Folkestone, Kent. Many of the workers used hand tools, but a state-of-the-art boring machine was also used. Work was eventually abandoned until construction on the tunnel as we now know it began again in 1988.
Frequently Asked Questions:
Thinking of hopping on Eurostar for your next European adventure? Got a couple of need-to-know questions before you book your trip? Here we’ve answered a selection of the most frequently asked questions from our customers.
Who can travel through the Channel Tunnel?
Foot passengers can travel with Eurostar, between our UK stations London St Pancras International and our stations on the continent . People who want to travel with their own vehicle or on a coach can use the Eurotunnel Le Shuttle between Folkestone and Calais. Before travelling with either Eurostar or Eurotunnel you will need to go through security, border and ticket checks before going through the Tunnel.
How much did it cost to build the Channel Tunnel?
It took just under six years and 13,000 workers to build the Channel Tunnel. The total cost came at an eye-watering £4.65 billion which would be the equivalent of £12 billion in today's money.
Why travel with Eurostar rather than drive?
* Avoid the stress of driving, with direct high-speed journeys to top destinations, from city centre to city centre.
* Great value fares with no additional costs like fuel, road tolls and parking.
* Simple connections with other rail services in Europe, allowing you to go beyond our direct destinations on a single booking.
* Travel in style on our comfortable trains, including our new, state-of-the art trains with wi-fi.
How fast does the Eurostar go?
The Eurostar travels through the Channel Tunnel at a speed of 100 miles per hour (160kph) although when the train is outside the tunnel it reaches speed of 186 miles per hour (300 kph).
Additional Information
Channel Tunnel, rail tunnel between England and France that runs beneath the English Channel. The Channel Tunnel, 50 km (31 miles) long, consists of three tunnels: two for rail traffic and a central tunnel for services and security. The tunnel runs between Folkestone, England, and Sangatte (near Calais), France, and is used for both freight and passenger traffic. Passengers can travel either by ordinary rail coach or within their own motor vehicles, which are loaded onto special railcars. Trains can travel through the tunnel at speeds as high as 160 km (100 miles) per hour; the trip takes about 35 minutes. It has the longest undersea portion of any tunnel in the world (37.8 km [23.5 miles]).
The often-considered idea of constructing a tunnel under the English Channel was revived in 1986 by the United Kingdom and France. A rail tunnel was chosen over proposals for a very long suspension bridge, a bridge-and-tunnel link, and a combined rail-and-road link, and the project was privately financed by a consortium of British and French corporations and banks; the Anglo-French company operating the tunnel is called Eurotunnel. Digging began on both sides of the Strait of Dover in 1987–88 and was completed in 1991. The tunnel was officially opened on May 6, 1994.
In 2007 the Channel Tunnel Rail Link (CTRL), also called High Speed 1, was opened to connect the Channel Tunnel with London, facilitating even greater movement of international passenger traffic between mainland Europe and the United Kingdom. The high-speed railway runs 108 km (67 miles) and crosses under the Thames. Its trains can reach speeds of up to 300 km (186 miles) per hour.
In June–July 2015 the problem of migrants—many of them from eastern Africa—sneaking aboard vehicles on trains in an attempt to immigrate to the United Kingdom reached crisis proportions. During that period at least nine individuals were killed while trying to make their way to England via the tunnel. The United Kingdom and France stepped up security measures to try to deter migrants from attempting the crossing.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2680 2026-01-25 18:24:37
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2479) Greenland Shark
Greenland Shark
Gist
Greenland sharks (Somniosus microcephalus) are the longest-lived vertebrates on Earth, with lifespans estimated between 250 and 500 years. A 2016 study using radiocarbon dating of eye lens proteins indicated they live at least 272 years, with some individuals estimated to be roughly 400 years old, and possibly up to 512 years.
The Greenland shark has the longest known lifespan of all vertebrate species. It is estimated that the species has a lifespan of at least 272 years, with the oldest individual estimated to be 392 ± 120 years of age.
Summary
Greenland sharks are now the longest-living vertebrates known on Earth, scientists say.
Researchers used radiocarbon dating to determine the ages of 28 of the animals, and estimated that one female was about 400 years old.
The team found that the sharks grow at just 1cm a year, and reach sexual maturity at about the age of 150.
The research is published, external in the journal Science.
Lead author Julius Nielsen, a marine biologist from the University of Copenhagen, external, said: "We had our expectations that we were dealing with an unusual animal, but I think everyone doing this research was very surprised to learn the sharks were as old as they were."
The former vertebrate record-holder was a bowhead whale estimated to be 211 years old.
But if invertebrates are brought into the longevity competition, a 507-year-old clam called Ming, external holds the title of most aged animal.
Slow swimmers
Greenland sharks are huge beasts, that can grow up to 5m in length.
They can be found, swimming slowly, throughout the cold, deep waters of the North Atlantic.
With this leisurely pace of life and sluggish growth rate, the sharks were thought to live for a long time. But until now, determining any ages was difficult.
For some fish, scientists are able to examine ear bones called otoliths, which when sectioned, show a pattern of concentric rings that scientists can count as they would the rings in a tree.
Sharks are harder, but some species, such as the Great White, have calcified tissue that grows in layers on their back bones, that can also be used to age the animals.
"But the Greenland shark is a very, very soft shark - it has no hard body parts where growth layers are deposited. So it was believed that the age could not be investigated," Mr Nielsen told the BBC.
However the team found a clever way of working out the age.
"The Greenland shark's eye lens is composed of a specialised material - and it contains proteins that are metabolically inert," explained Mr Neilson.
"Which means after the proteins have been synthesised in the body, they are not renewed any more. So we can isolate the tissue that formed when the shark was a pup, and do radiocarbon dating."
The team looked at 28 sharks, most of which had died after being caught in fishing nets as by-catch.
Using this technique, they established that the largest shark - a 5m-long female - was extremely ancient.
Because radiocarbon dating does not produce exact dates, they believe that she could have been as "young" as 272 or as old as 512. But she was most likely somewhere in the middle, so about 400 years old.
It means she was born between the years of 1501 and 1744, but her most likely date of birth was in the 17th century.
"Even with the lowest part of this uncertainty, 272 years, even if that is the maximum age, it should still be considered the longest-living vertebrate," said Mr Nielsen.
Conversely, if her age is at the upper end of the scale, she will have out-lived Ming the clam - although her age has a greater probability of lying in the middle.
Details
The Greenland shark (Somniosus microcephalus), also known as the rubiks shark or gray shark, is a large shark of the family Somniosidae ("sleeper sharks"), closely related to the Pacific and southern sleeper sharks. Inhabiting the North Atlantic and Arctic Oceans, they are notable for their exceptional longevity, although they are poorly studied due to the depth and remoteness of their natural habitat.
Greenland sharks have the longest lifespan of any known vertebrate, estimated to be between 250 and 500 years. They are among the largest extant shark species, reaching a maximum confirmed length of 6.4 m (21 ft) and weighing more than 1,000 kg (2,200 lb). They reach sexual maturity around 150 years of age and their pups are born alive after an estimated gestation period of 8 to 18 years. The shark is a generalist feeder, consuming a variety of available foods, including carrion.
Greenland shark meat is toxic to mammals due to its high levels of trimethylamine N-oxide, although a treated form of it is eaten in Iceland as a delicacy known as kæstur hákarl. Because they live deep in remote parts of the northern oceans, Greenland sharks are not considered a threat to humans. A possible attack occurred in August 1936 on two British fishermen, but the species was never identified.
Description
The Greenland shark is one of the largest known existing species of shark, with adults growing to around 4 to 5 m (13 to 16 ft). The largest confirmed specimen measured up to 6.4 metres (21 ft) long and weighed around 1,023 kilograms (2,255 lb). The all-tackle International Game Fish Association (IGFA) record for this species is 775 kg (1,709 lb). It rivals the Pacific sleeper shark (possibly up to 7 m or 23 ft long) for the largest species in the family Somniosidae. Genetic data indicate that Greenland sharks diverged from ancestral sleeper sharks in the Canadian Arctic approximately 1–2.34 million years ago during the Pleistocene epoch, likely influenced by glacial fluctuations that periodically isolated marine populations. These ecological challenges may have driven physiological and metabolic adaptations for cold, deep waters.
The Greenland shark is a thickset species, with a short, rounded snout, small eyes, and small dorsal and pectoral fins. The gill openings are very small for the species's great size. Females are typically larger than males, with males reaching maturity at a smaller size than females.
Coloration can range from pale creamy-gray to blackish-brown and the body is typically uniform in color, though whitish spots or faint dark streaks are occasionally seen on the back.
The shark is often infested by the copepod Ommatokoita elongata, a crustacean that attaches itself to the shark's eyes. The copepod may display bioluminescence, thus attracting prey for the shark in a mutualistic relationship, but this hypothesis has not been verified. These parasites can cause multiple forms of damage to the sharks' eyes, such as ulceration, mineralization, and edema of the cornea, leading to almost complete blindness. This does not seem to reduce the life expectancy or predatory ability of Greenland sharks, due to their strong reliance on smell and hearing.
The genome of the Greenland shark was published in 2024. It is 6.45 billion base pairs in length.
Dentition
The dentition of a Greenland shark:
When feeding on large carcasses, the shark employs a rolling motion of its jaw. The 48 to 52 teeth of the upper jaw are very thin and pointed, lacking serrations. These upper jaw teeth act as an anchor, while the lower jaw proceeds to cut massive chunks out of the prey.
The 48 to 52 lower teeth interlock, are broad and square in shape, and contain short, smooth cusps that point outward. Teeth in the two halves of the lower jaw are strongly pitched in opposite directions.
Physiology
Like other Elasmobranchii, Greenland sharks have high concentrations of the two nitrogenous compounds urea and trimethylamine N-oxide (TMAO) in their tissues, which increase their buoyancy and function as osmoprotectants. TMAO also counteracts the protein-destabilizing tendencies of urea and deep-water pressure. Its presence in the tissues of both elasmobranch and teleost fish has been found to increase with depth.
The blood of Greenland sharks contains three major types of hemoglobin, made up of two copies of α globin combined with two copies of three very similar β subunits. These three types show very similar oxygenation and carbonylation properties, which are unaffected by urea, an important compound in marine Elasmobranchii physiology. They display identical electronic absorption and resonance in Raman spectroscopy, indicating that their heme-pocket structures are identical or highly similar. The hemoglobins also have a lower affinity for oxygen compared to those of temperate sharks. These characteristics are believed to be adaptations to living at great depths.
When hoisted upon deck, it beats so violently with its tail, that it is dangerous to be near it, and the seamen generally dispatch it, without much loss of time. The pieces that are cut off exhibit a contraction of their muscular fibres for some time after life is extinct. It is, therefore, extremely difficult to kill, and unsafe to trust the hand within its mouth, even when the head is cut off. And, if we are to believe Crantz, this motion is to be observed three days after, if the part is trod on or struck.
— Henry William Dewhurst, The Natural History of the Order Cetacea (1834).
Additional Information
Greenland shark, (Somniosus microcephalus), member of the sleeper shark family Somniosidae (order Squaliformes, which also includes the dogfish family, Squalidae) also known for being the world’s longest-lived vertebrate. The species is primarily found in the cold-water environments of the Arctic Ocean and North Atlantic, from Baffin Bay eastward to the Barents Sea, but its range also extends southward to the North Sea and the waters adjacent to the Eastern Seaboard of the United States. Bulky, with a rounded snout, small fins relative to body size, and gray to brown coloration, Greenland sharks are similar to spiny dogfish (Squalus acanthias), except that they lack a spine in front of the second dorsal fin and usually the one on the first dorsal fin.
Natural history
The Greenland shark is one of the largest cartilaginous fishes. It can reach a length of 7 metres (23 feet) and a weight of 1,025 kg (2,260 pounds) when fully grown, but most are between 2 and 4 metres (6.5 and 13 feet). Little is known, however, about how the species reproduces. Females are thought to reach sexual maturity when they surpass the 4-metre (13-foot) mark in length, which takes approximately 150 years to achieve. They are ovoviviparous (that is, eggs are retained within the body until they hatch) and produce an average of 10 offspring at a time. The type, amount, and duration of parental care the young receive are unknown, but scientists speculate that, like other shark species, Greenland sharks are independent from birth. No other vertebrate known has a life span as long as this species, and a 2016 study that used radiocarbon dating to examine isotopes in the shark’s eye-lens nuclei suggested that the oldest Greenland sharks may be more than 500 years old. Using this technique, scientists estimated that the largest Greenland shark in the study was likely between 272 and 512 years old.
Greenland sharks are rarely encountered by humans. They are thought to prefer colder, deeper environments but may be found anywhere between the sea surface and depths of 2,200 meters (about 7,200 feet). Greenland sharks are slow-moving, typically swimming at rates of less than 3 km (about 1.9 miles) per hour.
They are carnivorous, and their diet is often made up of several different types of fishes, including smaller sharks, eels, flounders, and sculpins. Crustaceans, seabirds, and carrion—as well as terrestrial mammals (such as horses and reindeer) that likely fell through the ice—have been found in stomach analyses of the species. Greenland sharks are not considered dangerous to humans, in part because they live in regions where people do not typically swim; the only known report of a possible attack by a Greenland shark on a person dates to 1859.
Conservation status
The International Union for Conservation of Nature has classified the Greenland shark as a vulnerable species since 2020. The fish was valued historically for its liver oil; about 114 litres (30 gallons) of liver oil can be obtained from a large specimen (see also fish oil). (Although the flesh of the Greenland shark may be eaten, it is toxic unless properly cleaned and dried or repeatedly boiled prior to consumption.) They were fished commercially from the 19th century until 1960. Norway persecuted Greenland sharks during the 1970s, because they were considered to be a nuisance that threatened other fisheries. In the early 1900s as many as 30,000 Greenland sharks were caught a year. In the present day the annual take is far smaller; small-scale subsistence fisheries in the Arctic harvest fewer than 100 individuals annually, and roughly 1,200 are caught accidentally in fishing trawls.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2681 2026-01-27 17:53:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2480) Boric Acid
Gist
What is boric acid? Boric acid and its sodium borate salts are pesticides that we can find in nature and many products. Borax is one of the most common products. Boric acid and its sodium salts each combine boron with other elements in a different way.
Boric acid is a versatile compound used in medicine (antiseptic for minor issues, treating yeast infections), pest control (insecticide for ants, roaches), cosmetics, and numerous industrial applications, including fireproofing wood, manufacturing glass/ceramics, controlling nuclear fission, and as a component in lubricants and cleaners, valued for its anti-fungal, anti-bacterial, and preservative properties.
Summary
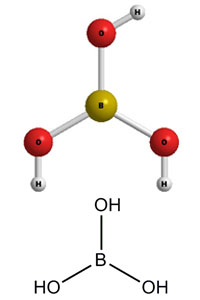
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula B(OH)3. It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters.
Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds.
The term "boric acid" is also used generically for any oxyacid of boron, such as metaboric acid HBO2 and tetraboric acid H2B4O7.
Details
Boric acid exists in natural deposits as a mineral, sassolite. It is also found in hot mineral water sources. The minerals are extracted with sulfuric acid and crystalline boric acid is separated. Borates have been used since antiquity for cleaning and as food preservatives among other things. Boric acid was first registered for use in the United States as an insecticide in 1948. It has also been used as an antiseptic and was found in numerous commercial products.
Uses
Boric acid is used as a fireproofing agent for wood, as a preservative, and as an antiseptic. It is used in the manufacture of glass, pottery, enamels, glazes, cosmetics, cements, porcelain, leather, carpets, hats, soaps, artificial gems, and in tanning, printing, dyeing, painting, and photography. It is a constituent of nickel plating baths and electric condensers, and it is used for impregnating wicks and hardening steel. In laboratory procedures, boric acid is used in the preparation of buffer solutions.
Boric acid is also used as a fungicide and as an insecticide powder. Domestic use may include its application as an insecticide for crawling insects such as roaches. In medicine, it had been widely used as a disinfectant and a constituent of baby powders, antiseptics, diaper rash ointments, eye washes, gargles, and a variety of other consumer products for its mild antiseptic property. Its routine medical use, however, has fallen out of favor because of its relatively weak antiseptic action and its potential for toxicity,
although it may still be used to treat recurrent vulvovaginitis.
Additional Information:
What is Boric Acid?
Boric acid or H3BO3 is a water-soluble acidic compound that consists of Hydrogen, Boron, and Oxygen. It is a Lewis acid of Boron that is weak and monobasic in nature. Other names for Boric acid include Hydrogen Borate or Orthoboric acid. Its molar mass is 61.83 g/mol. According to the pH scale, Boric acid consists of a pH of 5.1, thus indicating that it is a weak monobasic acid.
What Are The Uses Of Boric Acid?
There are various uses of Boric acid. It is helpful as an antiseptic for minor cuts and/or burns. It is used as an anti-fungal ointment or powder and removes any foul odor of the feet by applying it inside stockings. It is also used for treating vaginal infections. Boric acid plays an effective role in pesticides and is effective against pests like rats, houseflies, math, etc. It can also be used to remove odors and dirt from kitchens and bathrooms.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2682 2026-01-28 21:17:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2481) New Guinea
Gist
The New Guinea bioregion, located in the Australasia realm, is the world's second largest island (after Greenland) separated from Australia by the Torres Strait. New Guinea and its surrounding islands make up the northernmost grouping of islands in Melanesia.
Summary
New Guinea is a big island north of Australia. It is the second largest island in the world. Geologically and biologically it is related to Australia. It was first surveyed by Alfred Russel Wallace in the 19th century, who noted that it was, like Australia, on the eastern side of the Wallace line. That means it is closer to Australia than to Indonesia in its flora and fauna.
On the east side of New Guinea is the country Papua New Guinea. On the west side of the island are the Indonesian provinces of Papua, Central Papua, Highland Papua, South Papua, Southwest Papua, and West Papua. These provinces together are called Western New Guinea. The Indonesian "capture" of western Papua New Guinea was a modern act by dictator Suharto of Indonesia. The Australian "capture" of the eastern half was meant to make sure the peoples of this part of Indonesia were left alone.
An early BBC David Attenborough program showed that the country was wooded with many groups of humans speaking many languages. The most notable animals still alive were the birds of paradise. Of marsupials, the quolls are notable. They are small, solitary carnivores. There are six species surviving, two in New Guinea and four in Australia. Echidnas are mammals also known as spiny anteaters.
The number of people on New Guinea is unknown. Indonesia and Papua New Guinea estimated the number around 2020. They say there are about 15 million people. However, the real number could be 23 million or more.
New Guinea is important for its nature and biology. Its largest marsupial, the Diprotodon, is now extinct. Native humans live in sparsely wooded, hilly ground in small groups which speak different languages. New Guinea has over 1000 languages. This is more languages than anywhere else in the world.
Still surviving are the birds of paradise, with marsupials, including wallabies and possums, and the egg-laying monotreme, the echidna. Other than bats and some two dozen indigenous rodents, there are no pre-human indigenous placental mammals. Pigs, several additional species of rats, and the ancestor of the New Guinea singing dog were introduced with human colonization.
Details
New Guinea (also known as Papua or historically Irian) is the world's second-largest island, with an area of 785,753 sq km (303,381 sq mi). It has the third-largest remaining rainforest globally, and the highest plant biodiversity of any island. Located in Melanesia in the southwestern Pacific Ocean, the island is separated from Australia by the 150-kilometre (81-nautical-mile; 93-mile) wide Torres Strait, though both landmasses lie on the same continental shelf, and were united during episodes of low sea level in the Pleistocene glaciations as the combined landmass of Sahul. Numerous smaller islands are located to the west and east. The island's name was given by Spanish explorer Yñigo Ortiz de Retez during his maritime expedition of 1545 because of the perceived resemblance of the indigenous peoples of the island to those in the African region of Guinea.
The eastern half of the island is the major land mass of the nation of Papua New Guinea. The western half, known as Western New Guinea, forms a part of Indonesia and is organized as the provinces of Papua, Central Papua, Highland Papua, South Papua, Southwest Papua, and West Papua. The two major cities on the island are Port Moresby and Jayapura.
Names
The island has been known by various names:
The name Papua was used to refer to parts of the island before contact with the West. Its etymology is unclear; one theory states that it derived from Tidore, the language used by the Sultanate of Tidore. An expedition by the Sultan of Tidore, together with Sahmardan, the Sangaji of Patani, and the Papuan Gurabesi, managed to conquer some areas in New Guinea, which was then reorganised to form Korano Ngaruha ("Four Kings") or Raja Ampat, Papoua Gam Sio (lit. "The Papua Nine Negeri"), and Mafor Soa Raha (lit. The Mafor "Four Soa"). The name comes from the words papo ("to unite") and ua (negation), which means "not united", i.e. an outlying possession of Tidore.
Anton Ploeg reports that the word papua is often said to be derived from the Malay word papua or pua-pua, meaning "frizzly-haired", referring to the very curly hair of the island's inhabitants. However Sollewijn Gelpke in 1993 considered this unlikely as it had been used earlier, and he instead derived it from the Biak phrase sup i babwa, which means "the land below [the sunset]", and refers to the Raja Ampat Islands.
When Portuguese and Spanish explorers arrived via the Spice Islands, they also used the name Papua. However, Westerners, beginning with Spanish explorer Yñigo Ortiz de Retez in 1545, used the name New Guinea, due to the resemblance between the indigenous peoples of the island and Africans of the Guinea region. The name is one of several toponyms sharing similar etymologies, ultimately meaning "land of the blacks" or similar meanings.
The Dutch, who arrived later under Jacob Le Maire and Willem Schouten, called it Schouten island. They later used this name only to refer to islands off the north coast of Papua proper, the Schouten Islands or Biak Island. When the Dutch colonized the main island as part of the Dutch East Indies, they called it Nieuw Guinea.
The name Irian was used in the Indonesian language to refer to the island and Indonesian province, as Irian Barat (West Irian) Province and later Irian Jaya Province. The name Irian was suggested during a tribal committee meeting in Tobati, Jayapura, formed by Soegoro Atmoprasodjo under governor J. P. van Eechoed, to decide on a new name because of the negative association of Papua. Frans Kaisiepo, the committee leader, suggested the name from Mansren Koreri myths, Iri-an from the Biak language of Biak Island, meaning "hot land" (referring to the climate), but also from Iryan which means heated process as a metaphor for a land that is entering a new era. In Serui Iri-an (lit. "land-nation") means "pillar of nation", while in Merauke Iri-an (lit. "placed higher-nation") means "rising spirit" or "to rise". The name was promoted in 1945 by Marcus Kaisiepo, brother of Frans Kaisiepo. The name was politicized later by Corinus Krey, Marthen Indey, Silas Papare, and others with the Indonesian backronym Ikut Republik Indonesia Anti Nederland ("Join the Republic of Indonesia Oppose the Netherlands"). Irian was used somewhat in 1972. The name was used until 2001, when Papua was again used for the island and the province. The name Irian, which was originally favored by natives, is now considered to be a name imposed by the Indonesian government.
Geography
New Guinea is an island to the north of the Australian mainland, south of the equator. It is isolated by the Arafura Sea to the west, and the Torres Strait and Coral Sea to the east. Sometimes considered to be the easternmost island of the Indonesian archipelago, it lies north of Australia's Top End, the Gulf of Carpentaria and Cape York Peninsula, and west of the Bismarck Archipelago and the Solomon Islands archipelago. Biologically, New Guinea may also be considered part of Australia, as there is a continuity of flora and fauna between the two countries.
Politically, the western half of the island comprises six provinces of Indonesia: Papua, Central Papua, Highland Papua, South Papua, West Papua and Southwest Papua. The eastern half forms the mainland of the country of Papua New Guinea.
The shape of New Guinea is often compared to that of a bird-of-paradise (indigenous to the island), and this results in the usual names for the two extremes of the island: the Bird's Head Peninsula in the northwest (Vogelkop in Dutch, Kepala Burung in Indonesian; also known as the Doberai Peninsula), and the Bird's Tail Peninsula in the southeast (also known as the Papuan Peninsula).
A spine of east–west mountains, the New Guinea Highlands, dominates the geography of New Guinea, stretching over 1,600 km (1,000 mi) across the island, with many mountains over 4,000 m (13,100 ft). The western half of the island contains the highest mountains in Oceania, with its highest point, Puncak Jaya, reaching an elevation of 4,884 m (16,023 ft). The tree line is around 4,000 m (13,100 ft) elevation, and the tallest peaks contain equatorial glaciers—which have been retreating since at least 1936. Various other smaller mountain ranges occur both north and west of the central ranges. Except in high elevations, most areas possess a warm humid climate throughout the year, with some seasonal variation associated with the northeast monsoon season.
Another major habitat feature is the vast southern and northern lowlands. Stretching for hundreds of kilometres, these include lowland rainforests, extensive wetlands, savanna grasslands, and some of the largest expanses of mangrove forest in the world. The southern lowlands are the site of Lorentz National Park, a UNESCO World Heritage Site. The northern lowlands are drained principally by the Mamberamo River and its tributaries on the western side, and by the Sepik on the eastern side. The more extensive southern lowlands are drained by a larger number of rivers, principally the Digul in the west and the Fly in the east. The largest island offshore, Dolak, lies near the Digul estuary, separated by a strait so narrow it has been named a "creek".
New Guinea contains many of the world's ecosystem types: glacial, alpine tundra, savanna, montane and lowland rainforest, mangroves, wetlands, lake and river ecosystems, seagrasses, and some of the richest coral reefs on the planet.
The entire length of the New Guinea Highlands system passes through New Guinea as a vast watershed. The northern rivers flow into the Pacific Ocean, the southern rivers into the Arafura Sea and the Gulf of Papua. On the north side, the largest rivers are the Mamberamo, Sepik and Ramu. Mamberamo was born from the confluence of two large inland rivers. Tariku comes from the west to the east and Taritatu from the east. These rivers meander through swamps with huge internal descents and then merge. The Mamberamo thus formed reaches the ocean by breaking through the Coastal Mountains. Mamberamo River is navigable to Marine Falls. The Sepik is a much more important river. Similarly, it collects water from a spacious pool. It is 1,100 kilometers from the Victor Emanuel Range to the estuary, making it the longest river in New Guinea. The winding, muddy, sluggish river can be navigated for 500 km. Ramu is a 650 km long river. Its lower section is navigable, but its upper flow is high-falling, fast-flowing. The energy of the river is used by a power plant near the city of Kainantu.
On the south side, the most significant rivers are Pulau, Digul, Fly, Kikori and Purari. The largest river in the western part of the island is Digul. It originates from the Star Mountains, which rise to an altitude of 4,700 m. The coastal plain is bordered by a swamp world hundreds of kilometers wide. Digul is the main transport route to the fertile hills and mountains within the island. The river Fly is born near the eastern branches of the Digul. It is named after one of the ships of the English Royal Fleet, which first sailed into the mouth of the river in 1845. The total length of the river is 1,050 km. Smaller boats can sail 900 km on the river. The estuary section, which decomposes into islands, is 70 km wide. The tide of the sea can have an effect of up to 300 kilometers. Strickland, a tributary of the Fly, reaches the Papuan Plain through wild gorges. Fly and Strickland together form the largest river in New Guinea. The many rivers flowing into the Gulf of Papua form a single delta complex. The rivers of the island are extremely rich in water due to the annual rainfall of 2,000–10,000 mm. According to a modest calculation, the New Guinea River carries about 1,500 {km}^{3}/a/(48,000 {m}^{3}/s) of water into the sea. Fly alone carries more water 238 {km}^{3}/a (7,500 {m}^{3}/s) than all the rivers in Australia combined.
Additional Information
New Guinea is an island of the eastern Malay Archipelago, in the western Pacific Ocean, north of Australia. It is bounded by the Pacific Ocean to the north, the Bismarck and Solomon seas to the east, the Coral Sea and Torres Strait to the south, and the Arafura Sea to the southwest. New Guinea is administratively divided into two parts: its western half comprises the Indonesian propinsi (or provinsi; provinces) of Papua and West Papua (collectively, formerly called Irian Jaya); and its eastern half comprises the major part of Papua New Guinea, an independent country since 1975.
New Guinea is the second largest island in the world, exceeded in size only by Greenland. It is about 1,500 miles (2,400 km) long (from northwest to southeast) and about 400 miles (650 km) wide at its widest (north to south) part. Area island, 317,150 square miles (821,400 square km). Pop. (2010) Papua and West Papua provinces including nearby islands, 3,593,803; (2011) Papua New Guinea excluding islands, 6,178,781.
Land
An unbroken chain of mountains with peaks above 13,000 feet (4,000 metres) in elevation extends across New Guinea from the northwest to the southeast, rising to 16,024 feet (4,884 metres) at Jaya Peak in western Papua province—the highest point in Indonesia. The summits of the chain are glaciated, and the mountains incorporate extinct volcanoes and elongated, fertile highland basins usually above 4,900 feet (1,490 metres) in elevation. To the north of the mountain chain is a deep structural trench occupied by the valleys of the Mamberamo, Sepik, Ramu, and Markham rivers. Fronting the north-central coastal plains of New Guinea is a series of fault-rimmed mountains that generally lie below 11,500 feet (3,500 metres). The mostly lowland Bomberai Peninsula and the more mountainous Doberai Peninsula constitute the extreme northwestern part of the island.
South of the central mountain chain is the Fly-Digul shelf, a vast swampy plain crossed by numerous rivers including the Fly, Bian, Digul, Mapi, Pulau, and Lorentz. To the southeast the Owen Stanley Range extends about 200 miles (320 km) and forms a wide peninsula, separating the Solomon Sea to the north from the Coral Sea to the south.
The climate of New Guinea is basically tropical, with mean annual maximum temperatures ranging between 86 and 90 °F (30 and 32 °C) in the lowlands; daytime temperatures in the highlands generally exceed 72 °F (22 °C) year-round. The southeast trade winds blow for about seven months each year, and rainfall on the southward-facing slopes of the central highlands frequently exceeds 300 inches (7,620 mm) annually. Consequently, the Fly-Digul shelf and bordering highlands are one of the world’s wettest places and also one of the least-inhabited. The central highlands receive rain throughout the year totaling between 100 and 160 inches (2,540 and 4,065 mm). Port Moresby on the southeastern coast receives only about 40 inches (1,000 mm) of rain per year.
New Guinea has a rich variety of plant life, including orchids, figs, and species of false beech. Mangrove swamps are found along the coastline almost everywhere. Farther inland mangroves are succeeded by nipa palms. Large stands of sago palms are found along the deltas and rivers of the southern coast. Primary lowland rainforest covers much of New Guinea up to about 3,300 feet (1,000 metres) above sea level. In the central highlands above 3,300 feet, stands of oak, beech, and pine are common. Wildlife includes many reptiles and such marsupials as the tree kangaroo and phalanger. Notable birds are the cassowary (a large, flightless bird), the spectacular birds of paradise, and parrots.
People
Almost the whole of New Guinea is occupied by speakers of Papuan languages, the original settlers of the island, who live mainly in the interior and southern sections. Papuan societies are characterized by leadership invested in local “big men” and by reciprocal gift giving. Ethnic composition is complex among the Papuans, who speak some 700 different languages. Constituting a small minority are communities of speakers of Austronesian (Melanesian) languages on the western, northern, and eastern coasts and on many of the affiliated offshore islands. There are also some Polynesians, Chinese, and Europeans. Several pidgins, such as Tok Pisin and Hiri Motu, are widely spoken; Indonesian (the official language of Indonesia) and English (the official language of Papua New Guinea) are also spoken. Although about two-thirds of Papua New Guinea’s population is Christian, traditional religious beliefs and rituals are still widely practiced.
Throughout most of the highland basins, much of the natural vegetation has been removed by the relatively intensive agriculture of the highlanders. The central highlands are the most densely populated part of the island. Swidden (slash-and-burn, or shifting) cultivation is practiced in the forested foothills to the north of the central highlands and in the grasslands of the Mamberamo and Sepik river basins, where the population is sparse. The north coast is also generally well populated.
Economy
Copper and gold are the main mineral resources of the island. One of the largest concentrations of copper in the world is at Tembagapura, about 25 miles (40 km) southwest of Mount Jaya in Papua. Another major deposit of copper has been developed just inside Papua New Guinea among the headwaters of the Fly River at Ok Tedi. Large amounts of gold also are produced at Ok Tedi. Petroleum is extracted in the Doberai Peninsula area of Papua and near Lake Kutubu in the central highlands of Papua New Guinea. Natural gas has been discovered in the Fly-Kikori area in Papua New Guinea.
The bulk of New Guinea’s population are subsistence farmers. Yams, taro, sago, and bananas are the lowland staple foods, and the sweet potato is the main highland food; pig husbandry is widespread. Cash crops in Papua New Guinea include coffee, cacao, copra, palm oil, tea, and rubber; skyjack tuna, prawns, and timber are also exported. Internal transport consists of a few secondary coastal roads, riverboats, and airways, with the latter becoming increasingly important.
History
New Guinea was possibly occupied as early as 50,000 years ago. By about 7000 bce sedentary agriculture with extensive swamp drainage and irrigation was practiced in the highland basins. The island, especially the western half, was known to Indonesian and Asian seafarers centuries before it was known to Europeans. The Portuguese in 1511 were the first Europeans to sight the island but made no landing until 1527.
The Dutch claimed the western half of the island in 1828 as part of the Dutch East Indies. In the 1870s Captain John Moresby of Great Britain surveyed the southeastern coast, and by 1884 the southeastern quadrant of New Guinea had been annexed by Great Britain. The German New Guinea Company took over administration of the northeast quadrant in the same year. The administration of British New Guinea was passed to Australia in 1904, and its name was changed to the Territory of Papua.
Following World War I, German New Guinea was taken over by Australia as a mandated territory of the League of Nations in 1921. After Japan temporarily occupied large parts of the island during the early years of World War II, Australia combined its administration of the Territory of Papua and the New Guinea mandate into the Territory of Papua and New Guinea. Also after the war, the western half of the island, then known as Irian Barat, was returned to Dutch control. Indonesia became independent in 1949, and a plebiscite was held in 1969 to decide Irian Barat’s future; as a result it was annexed to Indonesia. Papua New Guinea was granted independence within the British Commonwealth in 1975.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2683 2026-01-31 19:04:04
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2482) Skeletal Muscle
Gist
Skeletal muscle is a type of muscle tissue attached to bones by tendons, responsible for voluntary movements like walking, lifting, and maintaining posture, and is characterized by its striped (striated) appearance and control by the nervous system for conscious action. It's a vital organ, making up a significant portion of body weight, and also generates heat, protects organs, and stores nutrients.
People with problems of skeletal muscle usually present with complaints of pain, stiffness or weakness. Muscle pain and pain caused by other structures in the body can be difficult to identify because muscle overlies many different types of structures including the bursa , joints, and bone.
Summary
Skeletal muscle, in vertebrates, is the most common of the three types of muscle in the body. Skeletal muscles are attached to bones by tendons, and they produce all the movements of body parts in relation to each other. Unlike smooth muscle and cardiac muscle, skeletal muscle is under voluntary control. Similar to cardiac muscle, however, skeletal muscle is striated; its long, thin, multinucleated fibres are crossed with a regular pattern of fine red and white lines, giving the muscle a distinctive appearance. Skeletal muscle fibres are bound together by connective tissue and communicate with nerves and blood vessels.
Details
Skeletal muscle (commonly referred to as muscle) is one of the three types of vertebrate muscle tissue, the others being cardiac muscle and smooth muscle. They are part of the voluntary muscular system and typically are attached by tendons to bones of a skeleton. The skeletal muscle cells are much longer than in the other types of muscle tissue, and are also known as muscle fibers. The tissue of a skeletal muscle is striated – having a striped appearance due to the arrangement of the sarcomeres.
A skeletal muscle contains multiple fascicles – bundles of muscle fibers. Each individual fiber and each muscle is surrounded by a type of connective tissue layer of fascia. Muscle fibers are formed from the fusion of developmental myoblasts in a process known as myogenesis resulting in long multinucleated cells. In these cells, the nuclei, termed myonuclei, are located along the inside of the cell membrane. Muscle fibers also have multiple mitochondria to meet energy needs.
Muscle fibers are in turn composed of myofibrils. The myofibrils are composed of actin and myosin filaments called myofilaments, repeated in units called sarcomeres, which are the basic functional, contractile units of the muscle fiber necessary for muscle contraction. Muscles are predominantly powered by the oxidation of fats and carbohydrates, but anaerobic chemical reactions are also used, particularly by fast twitch fibers. These chemical reactions produce adenosine triphosphate (ATP) molecules that are used to power the movement of the myosin heads.
Skeletal muscle comprises about 35% of the body of humans by weight. The functions of skeletal muscle include producing movement, maintaining body posture, controlling body temperature, and stabilizing joints. Skeletal muscle is also an endocrine organ. Under different physiological conditions, subsets of 654 different proteins as well as lipids, amino acids, metabolites and small RNAs are found in the secretome of skeletal muscles.
Skeletal muscles are substantially composed of multinucleated contractile muscle fibers (myocytes). However, considerable numbers of resident and infiltrating mononuclear cells are also present in skeletal muscles. In terms of volume, myocytes make up the great majority of skeletal muscle. Skeletal muscle myocytes are usually very large, being about 2–3 cm long and 100 μm in diameter. By comparison, the mononuclear cells in muscles are much smaller. Some of the mononuclear cells in muscles are endothelial cells (which are about 50–70 μm long, 10–30 μm wide and 0.1–10 μm thick), macrophages (21 μm in diameter) and neutrophils (12-15 μm in diameter). However, in terms of nuclei present in skeletal muscle, myocyte nuclei may be only half of the nuclei present, while nuclei from resident and infiltrating mononuclear cells make up the other half.
Additional Information
Skeletal muscles comprise 30% to 40% of your total body mass. They’re the muscles that connect to your bones and allow you to perform a wide range of movements and functions. Skeletal muscles are voluntary, meaning you control how and when they work. It’s important to keep your skeletal muscles as strong and healthy as possible.
Overview:
What is skeletal muscle?
The majority of the muscles in your body are skeletal muscles (striated muscles). They make up between 30% and 40% of your total body mass. Tendons (tough bands of connective tissue) attach skeletal muscle tissue to bones throughout your body. Your shoulder muscles, hamstring muscles and abdominal muscles are all examples of skeletal muscles.
There are three types of muscles in your body: skeletal, cardiac and smooth muscle. Skeletal muscles are voluntary muscles, meaning you control how and when they move and work. Nerves in your somatic nervous system send signals to make them function. If you reach for a book on a shelf, you’re using skeletal muscles in your neck, arm and shoulder.
Cardiac and smooth muscle are involuntary muscles that your autonomic nervous system controls. That means they work without you having to think about it. For example, muscles in your urinary system help rid your body of waste and toxins.
Skeletal muscle mass varies from person to person. Males have more skeletal muscle mass than females. People who are tall or have overweight also tend to have higher muscle mass. Muscle mass decreases with age.
Function:
What do skeletal muscles do?
Your striated muscles are a vital part of your musculoskeletal system. They serve a variety of functions, including:
* Chewing and swallowing, which are the first parts of digestion
* Expanding and contracting your chest cavity so you can inhale and exhale
* Maintaining body posture
* Moving the bones in different parts of your body
* Protecting your joints and holding them in place
* Storing nutrients
* Sustaining body temperature
Anatomy:
Where are the skeletal muscles located?
Your skeletal muscles are located between the bones (skeletal system) throughout your body. They consist of flexible muscle fibers that range from less than half an inch to just over 3 inches in diameter. These fibers usually span the length of the muscle. The fibers contract (tighten), which allows the muscles to move bones so you can perform lots of different movements.
What are the parts of skeletal muscles?
Each muscle can contain thousands of fibers. Different types of sheaths, or coverings, surround the fibers:
* Epimysium. The outermost layer of tissue surrounding the entire muscle.
* Perimysium. The middle layer surrounding bundles of muscle fibers.
* Endomysium. The innermost layer surrounding individual muscle fibers.
What do skeletal muscles look like?
Skeletal muscle fibers are red and white. They look striated, or striped, so they’re often called striated muscles. Cardiac muscles are also striated, but smooth muscles aren’t.
Conditions and Disorders:
What are the common conditions and disorders that affect striated muscles?
A wide range of conditions can affect skeletal muscles, from mild injuries to serious or even life-threatening myopathies (diseases that affect skeletal muscles). A few are:
* Muscular dystrophies
* Myasthenia gravis (MG)
* Rhabdomyolysis
* Sarcopenia
* Strains
* Tendonitis.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2684 2026-02-01 18:28:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2483) Mauritius
Gist
Mauritius is geographically an African island nation in the Indian Ocean, but its majority population (around two-thirds) has Indian ancestry, making it a unique blend of African geography and significant Indian cultural/ethnic influence, with Hinduism being the dominant religion, unlike other African nations.
Mauritius is famous for its stunning tropical scenery with beaches, lagoons, and volcanic mountains, making it a top honeymoon and luxury travel spot, but it's also known for its unique history as the only home of the extinct dodo bird, its rich multicultural heritage (Indian, African, Chinese, European), diverse cuisine, vibrant underwater life for diving, rare endemic wildlife (like the Mauritius kestrel), and UNESCO World Heritage sites like Aapravasi Ghat and Le Morne.
Summary
Mauritius, island country in the Indian Ocean, located off the eastern coast of Africa. Physiographically, it is part of the Mascarene Islands. The capital is Port Louis.
Land
Mauritius lies about 500 miles (800 km) east of Madagascar in the Indian Ocean. Its outlying territories are Rodrigues Island, situated about 340 miles (550 km) eastward, the Cargados Carajos Shoals, 250 miles (400 km) northeastward, and the Agalega Islands, 580 miles (930 km) northward from the main island. Mauritius also claims sovereignty over the Chagos Archipelago (including Diego Garcia), some 1,250 miles (2,000 km) to the northeast, although this claim was long disputed by Britain. A tentative agreement between Mauritius and Britain to resolve the competing claims was announced in October 2024, and a final agreement to cede sovereignty to Mauritius was signed in May 2025.
Capital: Port Louis
Population: (2025 est.) 1,232,000
Currency Exchange Rate: 1 USD equals 45.660 Mauritian rupee
Relief and drainage
The island of Mauritius is volcanic in origin and is almost entirely surrounded by coral reefs. The northern part is a plain that rises to a central plateau, varying in elevation from about 900 to 2,400 feet (270 to 730 meters) above sea level. The plateau is bordered by small mountains that may have formed the rim of an ancient volcano; the highest point (2,717 feet [828 meters]) is Piton de la Petite Rivière Noire in the southwest. The two major rivers, the Grand River South East and the Black River, are the primary sources of hydroelectric power. Lake Vacoas, one of the main reservoirs, is the chief source of water.
Soils and climate
More than half of the country’s area is arable, and it is almost entirely planted in sugarcane, the major export crop. Vegetables and tea for local consumption are also grown.
The climate is maritime subtropical, with fairly uniform temperature throughout the year. Mean temperatures vary from the mid-70s F (low to mid-20s C) at sea level to the upper 60s F (upper 10s C) on the high plateau. Two seasons are recognized: hot (December to April) and cool (June to September). Annual rainfall varies from around 35 inches (900 mm) on the west coast to 60 inches (1,525 mm) on the southeast coast and about 200 inches (5,080 mm) on the central plateau.
Plant and animal life
The vegetation includes some 600 indigenous species, even though little original forest is left. The fauna includes the samber (a long-tailed, dark brown deer), tenrec (a spiny insectivore), and mongoose, as well as a variety of birds and insects. The island was once home to the dodo, a flightless bird that was extinct by 1681. Efforts began in the late 20th century to save several other species of endemic birds that were close to extinction.
Details
Mauritius, officially the Republic of Mauritius, is an island nation in the Indian Ocean, about 2,000 kilometres (1,100 nautical miles) off the southeastern coast of East Africa, east of Madagascar. It includes the main island (also called Mauritius), as well as Rodrigues, Agaléga, St. Brandon (Cargados Carajos shoals). The islands of Mauritius and Rodrigues, along with nearby Réunion (a French overseas department), are part of the Mascarene Islands. The main island of Mauritius, where the population is concentrated, hosts the capital and largest city, Port Louis. The country spans 2,040 square kilometres (790 sq mi) and has an exclusive economic zone covering approximately 2,000,000 square kilometres (580,000 square nautical miles).
The 1502 Portuguese Cantino planisphere has led some historians to speculate that Arab sailors were the first to discover the uninhabited island around 975, naming it Dina Arobi. Called Ilha do Cirne or Ilha do Cerne on early Portuguese maps, the island was visited by Portuguese sailors in 1507. A Dutch fleet, under the command of Admiral Van Warwyck, landed at what is now the Grand Port District and took possession of the island in 1598, renaming it after Maurice, Prince of Orange. Short-lived Dutch attempts at permanent settlement took place over a century aimed at exploiting the local ebony forests, establishing sugar and arrack production using cane plant cuttings from Java together with over three hundred Malagasy slaves, all in vain. When French colonisation began in 1715, the island was renamed "Isle de France". In 1810, the United Kingdom seized the island and under the Treaty of Paris, France ceded Mauritius and its dependencies to the United Kingdom. The British colony of Mauritius now included Rodrigues, Agaléga, St. Brandon, the Chagos Archipelago, and, until 1906, the Seychelles. Mauritius and France dispute sovereignty over the island of Tromelin, the treaty failing to mention it specifically. Mauritius became the British Empire's main sugar-producing colony and remained a primarily sugar-dominated plantation-based colony until independence, in 1968. In 1992, the country abolished the monarchy, replacing it with the president.
In 1965, three years before the independence of Mauritius, the United Kingdom split the Chagos Archipelago away from Mauritius, and the islands of Aldabra, Farquhar, and Desroches from the Seychelles, to form the British Indian Ocean Territory (BIOT). The local population was forcibly expelled and the largest island, Diego Garcia, was leased to the United States restricting access to the archipelago. Ruling on the sovereignty dispute, the International Court of Justice has ordered the return of the Chagos Islands to Mauritius leading to a 2025 bilateral agreement on the recognition of its sovereignty on the islands, signed in May 2025.
Given its geographic location and colonial past, the people of Mauritius are diverse in ethnicity, culture, language and faith. It is the only country in Africa where Hinduism is the most practised religion. Indo-Mauritians make up the bulk of the population with significant Creole, Sino-Mauritian and Franco-Mauritian minorities. The island's government is closely modelled on the Westminster parliamentary system with Mauritius highly ranked for economic and political freedom. The Economist Democracy Index ranks Mauritius as the only country in Africa with full democracy while the V-Dem Democracy Indices classified it as an electoral autocracy. Mauritius ranks 73rd (very high) in the Human Development Index and the World Bank classifies it as a high-income economy. It is amongst the most competitive and most developed economies in the African region. The country is a welfare state. The government provides free universal health care, free education up through the tertiary level, and free public transportation for students, senior citizens, and the disabled. Mauritius is consistently ranked as the most peaceful country in Africa.
Along with the other Mascarene Islands, Mauritius is known for its biodiverse flora and fauna with many unique species endemic to the country. The main island was the only known home of the dodo, which, along with several other avian species, became extinct soon after human settlement. Other endemic animals, such as the echo parakeet, the Mauritius kestrel and the pink pigeon, have survived and are subject to intensive and successful ongoing conservation efforts.
Additional Information
Mauritius is a stable and prosperous Indian Ocean archipelago.
Once dependent on sugar exports, the island has built up a strong outsourcing and financial services sector, as well as an important tourism industry, and now boasts one of Africa's highest per capita incomes.
In the 1960s, hundreds of inhabitants of the Chagos Islands, over which Mauritius claimed sovereignty, were deported to make way for a US military base on the island of Diego Garcia.
The islands had been administered as part of Mauritius from the 18th Century onwards. In 1965, shortly before Mauritian independence the UK separated them along with Aldabra, Farquhar and Desroches in the Seychelles to form the British Indian Ocean Territory.
The latter three islands were returned to Seychelles in 1976 on its independence.
In 2024, after years of negotiations, the UK announced it would hand sovereignty of the Chagos Islands back to Mauritius.
This included the tropical atoll of Diego Garcia, used by the US government as a military base for its navy ships and long-range bomber aircraft.
The US-UK base remains on Diego Garcia - this was a key factor enabling the deal to go forward at a time of growing geopolitical rivalries in the region between Western countries, India, and China.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2685 2026-02-02 17:23:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2484) Maldives
Gist
The official language of the Maldives is Dhivehi (Maldivian), an Indo-Aryan language, but English is widely spoken, especially in tourist areas and resorts, making communication easy for visitors, while Arabic serves as the religious language for Islam.
Summary
Maldives, officially the Republic of Maldives, and historically known as the Maldive Islands, is an archipelagic country in South Asia, located in the eastern Arabian Sea, within the northern Indian Ocean. Maldives is southwest of Sri Lanka and India, about 750 kilometres (470 miles; 400 nautical miles) from the Asian continent's mainland. Maldives' chain of 26 atolls stretches across the equator from Ihavandhippolhu Atoll in the north to Addu Atoll in the south.
Maldives is the smallest country in Asia. Its land area is only 298 square kilometres (115 sq mi), but this is spread over roughly 90,000 square kilometres (35,000 sq mi) of the sea, making it one of the world's most spatially dispersed sovereign states. With a population of 515,132 in the 2022 census, it is the second least populous country in Asia and the ninth-smallest country by area, but also one of the most densely populated countries. Maldives has an average ground-level elevation of around 1.5 metres (4 ft 11 in) above sea level, and a highest natural point of only 2.4 metres (7 ft 10 in), making it the world's lowest-lying country. Some sources state the highest point, Mount Villingili, as 5.1 metres or 17 feet.
Malé is the capital and the most populated city, traditionally called the "King's Island", where the ancient royal dynasties ruled from its central location. Maldives has been inhabited for over 2,500 years. Documented contact with the outside world began around 947 AD when Arab travellers began visiting the islands. In the 12th century, partly due to the importance of the Arabs and Persians as traders in the Indian Ocean, Islam reached the Maldivian Archipelago. Maldives was soon consolidated as a sultanate, developing strong commercial and cultural ties with Asia and Africa. From the mid-16th century, the region came under the increasing influence of European colonial powers, with Maldives becoming a British protectorate in 1887. Independence from the United Kingdom came in 1965, and a presidential republic was established in 1968 with an elected People's Majlis. The ensuing decades have seen political instability, efforts at democratic reform, and environmental challenges posed by climate change and rising sea levels. Maldives became a founding member of the South Asian Association for Regional Cooperation (SAARC).
Fishing has historically been the dominant economic activity, and remains the second largest sector, behind the rapidly growing tourism industry. Maldives rates "high" on the Human Development Index, with a per capita income significantly higher than other SAARC nations. The World Bank classifies Maldives as having an upper-middle income economy.
Maldives is a member of the United Nations, the Commonwealth of Nations, the Organisation of Islamic Cooperation, and the Non-Aligned Movement, and is a Dialogue Partner of the Shanghai Cooperation Organisation. It temporarily withdrew from the Commonwealth in October 2016 after being threatened with expulsion from the organisation for its human rights infringements and democratic backsliding. It was readmitted to the Commonwealth on 1 February 2020 after showing evidence of reform and functioning democratic processes.
Details
Maldives is an independent island country in the north-central Indian Ocean. It consists of a chain of about 1,200 small coral islands and sandbanks (some 200 of which are inhabited), grouped in clusters, or atolls.
The islands extend more than 510 miles (820 km) from north to south and 80 miles (130 km) from east to west. The northernmost atoll is about 370 miles (600 km) south-southwest of the Indian mainland, and the central area, including the capital island of Male (Male’), is about 400 miles (645 km) southwest of Sri Lanka.
Land
The Maldive Islands are a series of coral atolls built up from the crowns of a submerged ancient volcanic mountain range. All the islands are low-lying, none rising to more than 6 feet (1.8 metres) above sea level. Barrier reefs protect the islands from the destructive effects of monsoons. The rainy season, from May to August, is brought by the southwest monsoon; from December to March the northeast monsoon brings dry and mild winds. The average annual temperature varies from 76 to 86 °F (24 to 30 °C). Rainfall averages about 84 inches (2,130 mm) per year. The atolls have sandy beaches, lagoons, and a luxuriant growth of coconut palms, together with breadfruit trees and tropical bushes. Fish abound in the reefs, lagoons, and seas adjoining the islands; sea turtles are caught for food and for their oil, a traditional medicine.
People
The population of Maldives belongs almost entirely to the Maldivian ethnic group, which is the result of various peoples settling in the islands successively through the country’s history. The first settlers, it is generally believed, were Tamil and Sinhalese peoples from southern India and Sri Lanka. Traders from Arab countries, Malaya, Madagascar, Indonesia, and China visited the islands through the centuries. The official language is an Indo-European language called Dhivehi (or Maldivian); Arabic, Hindi, and English are also spoken. Islam is the state religion.
More than half of the population is considered rural. With the exception of those living in Male, the only relatively large settlement in the country, the inhabitants of the Maldives live in villages on small islands in scattered atolls. Only about 20 of the islands have more than 1,000 inhabitants, and the southern islands are more densely populated than the northern ones. The birth rate for the Maldives is somewhat higher than the world average, but the death rate is lower. More than one-fifth of the total population is under 15 years of age. Life expectancy is about 74 years for men and 79 for women.
Economy
Since the 1970s the economy of the Maldives has developed rapidly. Annual growth of gross domestic product (GDP) has been high, averaging about 6 percent in the 2010s, and the gross national income (GNI) per capita—among the lowest in the world in the 1970s—reached the level of upper middle-income countries by the late 2010s. The economy is based on tourism, fishing, boatbuilding, and boat repairing, with the tourism sector driving the rapid growth.
Agriculture, forestry, and fishing
Fishing, long the traditional base of the economy, has been far surpassed by tourism as the main source of gross domestic product (GDP). While the sector still produces the bulk of the country’s exports and continues to grow (albeit at a slower pace than the tourism industry), it employs less than one-fifth of the labour force and contributes less than one-tenth of the GDP. Tuna is the predominant fish caught, traditionally by the pole-and-line method, although a good deal of the fishing fleet has been mechanized. Most of the fish catch is sold to foreign companies for processing and export.
Although formal businesses have seen rapid growth in the country, especially on the main islands, much of the population subsists outside the money economy on fishing, coconut collecting, and the growing of vegetables and melons, roots and tubers (cassava, sweet potatoes, and yams), and tropical fruits. Cropland, scattered over many small islands, is minimal, and nearly all the staple foods must be imported.
Manufacturing
Industries are largely of the handicraft or cottage type, including the making of coir (coconut-husk fibre) and coir products, fish canning, and boatbuilding. Textile and garment manufacturing was among the more lucrative industries from the mid-1990s until the 2005 expiry of an import quota regime in international textile trade left factories in the Maldives unable to compete. Construction makes up the bulk of the industrial sector.
Trade
Maldives entered the South Asian Free Trade Area (SAFTA) in 2006 and signed a free trade agreement with China in 2017. Imports include consumer goods such as food (principally rice), textiles, medicines, and petroleum products. Fish—mostly dried, frozen, or canned skipjack tuna—accounts for the bulk of exports. China, India, the United Arab Emirates, Thailand, Sri Lanka, and Singapore are among the main trading partners.
Services
The tourism industry, although nonexistent before 1972, underpins the services sector. Annually, more than 1.5 million tourists visit the Maldives. Its more than 130 resort islands include high-end hotel brands, and its marine geography offers unique diving and water sports opportunities as well as undersea accommodations, restaurants, and spas. By the mid-2010s the service sector accounted for four-fifths of the country’s GDP.
Labour and taxation
The rapid growth of the tourism industry left its mark on the labour market, which saw a dramatic shift away from agriculture and toward services. In part because of lack of training among the general population, a number of foreign workers from South Asia provide skills needed to help develop businesses. As businesses on resort islands away from the general populace demanded an increasing share of the total labour force, the participation rate of women, who are discouraged by the culture from living away from their families, fell substantially. About three-fifths of women participated in the labour force in the 1970s, but the rate dipped as low as one-fifth of women in the mid-1990s. By the 2010s, however, the participation rate had recovered to about half of women.
Beginning in 2011, the Maldives collected taxes primarily on the profits of businesses and financial institutions and on goods and services within the tourism sector. An income tax was implemented in 2020.
Transportation
Transportation between islands and atolls is vital for the country. China and India, competing for influence over the strategically located Maldive Islands, have provided significant foreign direct investment to develop infrastructure that would further connect the islands. Boats provide the principal means of transport between the atolls, and scheduled shipping services link the country with Sri Lanka, Singapore, and India. The national airline carries passengers between several airports in the country as well as internationally. The airport at Male handles most international traffic, although there are other airports that serve limited international travel.
Additional Information
Capital: Male
Area: 300 sq km
Population: 392,000
Languages: Dhivehi, English
The Maldives is a republic lies south-west of the Indian sub-continent. It is made up of a chain of nearly 1,200 islands, most of them uninhabited.
None of the coral islands stand more than 1.8 metres (six feet) above sea level, making the country vulnerable to any rise in sea levels associated with global warming.
The economy revolves around tourism, and scores of islands have been developed for the top end of the tourist market.
Its political history has been unsettled since the electoral defeat of long-serving President Maumoon Abdul Gayoom in 2008.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2686 2026-02-03 18:43:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2485) Seychelles
Gist
Seychelles is considered a rich country in Africa, often ranking highest in GDP per capita on the continent due to its strong tourism and fisheries-based economy, leading to high-income status with good social programs, though some inequality exists, note WorldAtlas, Global Finance Magazine, Seychelles News Agency, ISS African Futures, U.S. Department of State (.gov), and YouTube.
Summary
Seychelles, officially the Republic of Seychelles (French: République des Seychelles; Seychellois Creole: Repiblik Sesel), is an island country and archipelagic state consisting of 115 islands in the Indian Ocean. Its capital and largest city, Victoria, is 1,500 kilometres (800 nautical miles) east of mainland Africa. Nearby island countries and territories include the Maldives, Comoros, Madagascar, Mauritius, and the French overseas departments of Mayotte and Réunion to the south; and the Chagos Archipelago to the east. Seychelles is the smallest country in Africa as well as the least populated sovereign African country, with an estimated population of 100,600 in 2022.
The Seychelles archipelago was uninhabited prior to sustained external contact. Although Arab and Swahili sailors likely knew of the islands earlier through Indian Ocean trade routes, there is no evidence of permanent settlement before European involvement.
The islands were first recorded by Europeans in the 16th century, but were not settled until the 18th century, when France formally claimed them. During the period of French colonization, enslaved Africans—many of whom had already been captured through existing African, Arab slave trade, and Indian Ocean slave trade networks—were brought to the islands for plantation labor.
It faced competing French and British interests until it came under full British control in the early 19th century. After Britain assumed control in the early 19th century, slavery was abolished and later replaced in part by indentured laborers from India. Since proclaiming independence from the United Kingdom in 1976, it has developed from a largely agricultural society to a market-based diversified economy, characterized by service, public sector, and tourism activities. From 1976 to 2015, nominal GDP grew nearly 700%, and purchasing power parity nearly 1600%. Since the late 2010s, the government has taken steps to encourage foreign investment.
As of the early 21st century, Seychelles has the highest nominal per capita GDP and the highest Human Development Index ranking of any African country. According to the 2024 V-Dem Democracy indices, Seychelles is the 43rd-ranked electoral democracy worldwide, the 1st-ranked liberal democracy in Africa, and the 2nd-ranked electoral democracy on the continent.
Seychellois culture and society is an eclectic mix of French, British, Indian and African influences, with infusions of Chinese elements. The country is a member of the United Nations, the African Union, the Southern African Development Community, and the Commonwealth of Nations.
Details:
Ethnic groups, languages, and religion
The original French colonists on the previously uninhabited islands, along with their black slaves, were joined in the 19th century by deportees from France. Asians from China, India, and Malaya (Peninsular Malaysia) arrived later in smaller numbers. Widespread intermarriage has resulted in a population of mixed descent.
Creole, also called Seselwa, is the mother tongue of most Seychellois. Under the constitution, Creole, English, and French are recognized as national languages.
More than three-fourths of the population are Roman Catholics. There are also Anglicans, Christians of other denominations, Hindus, and Muslims.
Settlement patterns and demographic trends
More than four-fifths of the population lives on Mahé, many in the capital city, Victoria. The birth and death rates, as well as the annual population growth rate, are below the global average. Some one-fifth of the population is younger than age 15, and an additional one-sixth is under age 30. Life expectancy for both men and women is significantly higher than the global average.
Economy
Seychelles has a mixed developing economy that is heavily dependent upon the service sector in general and the tourism industry in particular. Despite continued visible trade deficits, the economy has experienced steady growth. The gross domestic product (GDP) is growing more rapidly than the population. The gross national income (GNI) per capita is significantly higher than those found in most nearby continental African countries.
Agriculture, forestry, and fishing
Agriculture accounts for only a fraction of the GDP and employs an equally modest proportion of the workforce. Arable land is limited and the soil is generally poor—and the country remains dependent upon imported foodstuffs—but copra (from coconuts), cinnamon bark, vanilla, tea, limes, and essential oils are exported. Seychelles has a modern fishing industry that supplies both domestic and foreign markets; canned tuna is a particularly important product. The extraction of guano for export is also an established economic activity.
Manufacturing, finance, and trade
The country’s growing manufacturing sector—which has expanded to account for almost one-sixth of the total GDP—is composed largely of food-processing plants; production of alcoholic beverages and of soft drinks is particularly significant. Animal feed, paint, and other goods are also produced.
Seychelles’ sizable trade deficit is offset by income from the tourism industry and from aid and investment. Although the country’s relative prosperity has not made it a preferred aid recipient, it does receive assistance from the World Bank, the European Union, the African Development Bank, and a variety of contributing countries, and aid obtained per capita is relatively high. The Central Bank of Seychelles, located in Victoria, issues the official currency, the Seychelles rupee.
Seychelles’ main imports are petroleum products, machinery, and foodstuffs. Canned tuna, copra, frozen fish, and cinnamon are the most important exports, together with the reexport of petroleum products. Significant trade partners include France, the United Kingdom, the United Arab Emirates, and Italy.
Services
The service sector accounts for nearly four-fifths of the GDP and employs the largest proportion of the workforce, almost three-fourths of all laborers. After the opening of an international airport on Mahé in 1971, the tourism industry grew rapidly, and at the beginning of the 21st century it provided almost one-fourth of the total GDP. Each year Seychelles draws thousands of tourists, many attracted by the islands’ magnificent venues for scuba diving, surfing, windsurfing, fishing, swimming, and sunbathing. The warm southeasterly trade winds offer ideal conditions for sailing, and the waters around Mahé and the other islands are afloat with small boats.
Transportation and telecommunications
The majority of Seychelles’ roadways are paved, most of which are on the islands of Mahé and Praslin; there are no railroads. Ferry services operate between the islands—for example, linking Victoria with destinations that include Praslin and La Digue. Air service is centered on Seychelles International Airport, located near Victoria on Mahé, and the smaller airports and airstrips found on several islands. Seychelles has air connections with a number of foreign cities and direct flights to major centers that include London, Paris, Frankfurt, Rome, and Bangkok. Scheduled domestic flights, provided by Air Seychelles, chiefly offer service between Mahé and Praslin, although chartered flights elsewhere are also available. The tsunami that reached Seychelles in 2004 damaged portions of the transportation infrastructure, including the road linking Victoria with the international airport.
Telecommunications infrastructure in Seychelles is quite developed. The country has a high rate of cellular telephone use—among the highest in sub-Saharan Africa—and, at the beginning of the 21st century, the use of personal computers in Seychelles was several times the average for the region.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2687 2026-02-04 17:39:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2486) Automative Industry
Gist
An automobile engine is defined as a mechanical system that generates power through the combustion of fuel, producing heat energy that must be effectively dissipated to prevent overheating, typically using coolants and radiators for heat transfer.
"Automative" refers to processes, systems, or mechanisms that are automated or self-acting, often relating to machinery that operates without direct human intervention. It is distinct from "automotive" (related to motor vehicles), though they share similar roots in mechanics. The term is sometimes used to describe robotic or AI-driven automation.
Summary
Automotive industry is all those companies and activities involved in the manufacture of motor vehicles, including most components, such as engines and bodies, but excluding tires, batteries, and fuel. The industry’s principal products are passenger automobiles and light trucks, including pickups, vans, and sport utility vehicles. Commercial vehicles (i.e., delivery trucks and large transport trucks, often called semis), though important to the industry, are secondary. The design of modern automotive vehicles is discussed in the articles automobile, truck, bus, and motorcycle; automotive engines are described in gasoline engine and diesel engine.
The modern industry
The modern automotive industry is huge. In the United States it is the largest single manufacturing enterprise in terms of total value of products, value added by manufacture, and number of wage earners employed. One of every six American businesses is dependent on the manufacture, distribution, servicing, or use of motor vehicles; sales and receipts of automotive firms represent more than one-fifth of the country’s wholesale business and more than one-fourth of its retail trade. For other countries these proportions are somewhat smaller, but Japan, South Korea, and the countries of western Europe have been rapidly approaching the level in the United States.
Consolidation
The trend toward consolidation in the industry has already been traced. In each of the major producing countries the output of motor vehicles is in the hands of a few very large firms, and small independent producers have virtually disappeared. The fundamental cause of this trend is mass production, which requires a heavy investment in equipment and tooling and is therefore feasible only for a large organization. Once the technique is instituted, the resulting economies of scale give the large firm a commanding advantage, provided of course that the market can absorb the number of vehicles that must be built to justify the investment. Although the precise numbers required are difficult to determine, the best calculations, considering both the assembly operation and the stamping of body panels, place the optimum output at between 200,000 and 400,000 cars per year for a single plant. Increasingly stringent and costly regulations aimed at correcting environmental damage due to the rising number of vehicles on the road also have been a factor in the move toward consolidation.
The structural organization of these giant enterprises, despite individual variation, resembles the pattern first adopted by General Motors in the 1920s. There is a central organization with an executive committee responsible for overall policy and planning. The operating divisions are semiautonomous, each reporting directly to the central authority but responsible for its own internal management. In some situations the operating divisions even compete with each other. The Ford Motor Company was consciously reorganized on the GM pattern after World War II; other American automotive firms have similar structures.
In addition, the largest producers decentralize their manufacturing operations by means of regional assembly plants. These permit the central factory to ship frames and components rather than complete automobiles to the areas served by the assembly plants, effecting substantial savings in transportation costs. This system was developed for the Ford company in 1911.
Some alteration of that principle took place in the 1980s and ’90s as Japanese firms built new plants around the world and American and European manufacturers adopted, to varying degrees, the Japanese “just-in-time” inventory method. Rather than stockpiling a large number of parts at the assembly plant or shipping all the parts from central locations, automakers have yielded the manufacture of many noncritical components (such as seats and wheel assemblies) to independent suppliers to make the pieces at small facilities close to the assembly plants. The components are often assembled into larger groups of parts or modules (a complete instrument panel, for example) and sent to the assembly plant in the exact sequence and at the exact time needed.
Diversity of products
The automotive industry’s immense resources in production facilities and technical and managerial skills have been devoted predominantly to the building of motor vehicles, but there has been a consistent and strong incentive to extend into related products and occasionally into operations whose relationship to automobiles is remote. The Ford Motor Company, for example, once manufactured tractors and made the famous Ford Trimotor all-metal transport airplane in the late 1920s and early ’30s. GM manufactured refrigerators and diesel-powered railway locomotives. By the end of the 20th century, however, Ford and GM had divested themselves of most of their nonautomotive operations and had spun off the majority of their automotive component-making divisions into separate stock companies—Delphi Automotive Systems in the case of General Motors and Visteon Automotive in the case of Ford.
In Europe, but to a lesser extent, automakers also divested noncore operations, while depressed economic conditions in Japan forced auto companies there to begin divorcing themselves from nonautomotive and components companies in which they had long held interests. By the late 1990s the trend was toward more international consolidation of core automotive operations.
New car development
The process of putting a new car on the market has become largely standardized. If a completely new model is contemplated, the first step is a market survey. Since there may be an interval of five years between this survey and the appearance of the new car in the dealers’ showrooms, there is a distinct element of risk, as illustrated by the Ford Motor Company’s Edsel of the late 1950s. (Market research had indicated a demand for a car in a relatively high price range, but, by the time the Edsel appeared, both public taste and economic conditions had changed.) Conferences then follow for engineers, stylists, and executives to agree on the basic design. The next stage is a mock-up of the car, on which revisions and refinements can be worked out.
Because of the increasingly competitive and international nature of the industry, manufacturers have employed various means to shorten the time from conception to production to less than three years in many cases. This has been done at GM, for example, by incorporating vehicle engineers, designers, manufacturing engineers, and marketing managers into a single team responsible for the design, engineering, and marketing launch of the new model. Automakers also involve component manufacturers in the design process to eliminate costly time-consuming reengineering later. Often the component maker is given full responsibility for the design and engineering of a part as well as for its manufacture.
Manufacturing processes
The bulk of the world’s new cars come from the moving assembly line introduced by Ford, but the process is much more refined and elaborated today. The first requisite of this process is an accurately controlled flow of materials into the assembly plants. No company can afford either the money or the space to stockpile the parts and components needed for any extended period of production. Interruption or confusion in the flow of materials quickly stops production. Ford envisioned an organization in which no item was ever at rest from the time the raw material was extracted until the vehicle was completed—a dream that has not yet been realized.
The need for careful control over the flow of materials is an incentive for automobile firms to manufacture their own components, sometimes directly but more often through subsidiaries. Yet complete integration does not exist, nor is it desirable. Tires, batteries, and dashboard instruments are generally procured from outside sources. In addition, and for the same reasons, the largest companies support outside suppliers even for items of in-house manufacture. First, it may be more economical to buy externally than to provide additional internal facilities for the purpose. Second, the supplier firm may have special equipment and capability. Third, the outside supplier provides a check on the costs of the in-house operation. American companies rely more than others on independent suppliers.
Production of a new model also calls for elaborate tooling, and the larger the output, the more highly specialized the tools in which the manufacturer is willing to invest. For example, it is expensive to install a stamping press exclusively to make a single body panel for a single model, but, if the model run reaches several hundred thousand, the cost is amply justified.
The assembly process itself has a quite uniform pattern throughout the world. As a rule, there are two main assembly lines, body and chassis. On the first the body panels are welded together, the doors and windows are installed, and the body is painted and trimmed (with upholstery, interior hardware, and wiring). On the second line the frame has the springs, wheels, steering gear, and power train (engine, transmission, drive shaft, and differential) installed, plus the brakes and exhaust system. The two lines merge at the point at which the car is finished except for minor items and necessary testing and inspection. A variation on this process is “unitized” construction, whereby the body and frame are assembled as a unit. In this system the undercarriage still goes down the chassis line for the power train, front suspension, and rear axle, to be supported on pedestals until they are joined to the unitized body structure. Most passenger vehicles today are manufactured by the unitized method, and most trucks and commercial vehicles still employ a separate frame.
Assembly lines have been elaborately refined by automatic control systems, transfer machines, computer-guided welding robots, and other automated equipment, which have replaced many manual operations when volume is high. Austin Motors in Britain pioneered with its automatic transfer machines in 1950. The first large-scale automated installation in the United States was a Ford Motor Company engine plant that went into production in 1951. A universal form of automatic control has used computers to schedule assembly operations so that a variety of styles can be programmed along the same assembly line. Customers can be offered wide choices in body styles, wheel patterns, and colour combinations.
Sales and service organization
Mass production implies mass consumption, which in turn requires an elaborate distributive organization to sell the cars and to develop confidence among customers that adequate service will be available. In the early days of the industry, cars were sold directly from the factory or through independent dealers, who might handle several different makes. Many bicycle manufacturers simply used their existing sales outlets when they added horseless carriages to their line. When sales in large quantities became the objective, however, more elaborate and better organized techniques of distribution became essential.
In the United States the restricted franchise dealership became the uniform and almost exclusive method of selling new cars. In this system, dealers may sell only the particular make of new car specified in their franchise, must accept a quota of cars specified by the manufacturer, and must pay cash on delivery. In return the dealers receive some guarantee of sales territory and may be assisted in various ways by the manufacturer—financing or aid in advertising, for example. Contracts also specify that dealers must maintain service facilities according to standards approved by the manufacturer.
Seemingly weighted in favour of the manufacturer, the system has been subjected to periodic dealer complaints, producing state legislation and a federal statute in 1956 to protect dealers from arbitrary actions by manufacturers. Yet dealers have never been united in these attitudes, and no effective substitute for the restricted franchise has yet been found. On the contrary, it is becoming the general practice in other parts of the world where large-scale markets for motor vehicles have developed.
Attempts by automakers in the 1990s to move away from the traditional franchised dealer network to direct selling via the Internet met strong resistance in the United States. American dealers enlisted the help of state governments in enacting prohibitions of this practice (and in blocking attempts by automakers to own dealers through subsidiary corporations). In markets outside the United States, principally in Europe and South America, manufacturers sell directly to consumers via the Internet in limited quantities.
The market in used cars is an important part of the distribution system for motor vehicles in all countries with a substantial motor vehicle industry because it affects the sale and styling of new cars. The institution of the annual model was adopted in the United States during the 1920s to promote new-car sales in the face of used-car competition. The new model must have enough changes in styling or engineering to persuade prospective buyers that it is indeed an improvement. At the same time, it must not be so radically different from its predecessors as to give the buyer doubts about its resale potential.
Like all machinery, motor vehicles wear out. Some become scrap metal to feed steel furnaces; some go to wrecking yards where usable parts are salvaged. Throughout the world, however, the disposal of discarded motor vehicles has become a problem without a completely satisfactory solution. In many areas, landscapes are disfigured by abandoned wrecks or unsightly automobile graveyards. Spurred by European legislation requiring automakers to take back all of their end-of-life-cycle vehicles beginning in 2007, manufacturers worldwide have begun engineering new products with the complete recycling of components in mind. At the same time, they have used more and different recycled material in new vehicles. For example, old bumper covers have been recycled into fender liners or battery trays for new cars.
Details
There are a wide variety of propulsion systems available or potentially available for automobiles and other vehicles. Options included internal combustion engines fueled by petrol, diesel, propane, or natural gas; hybrid vehicles, plug-in hybrids, fuel cell vehicles fueled by hydrogen and all electric cars. Fueled vehicles seem to have the advantage due to the limited range and high cost of batteries. Some options required construction of a network of fueling or charging stations. With no compelling advantage for any particular option, car makers pursued parallel development tracks using a variety of options. Reducing the weight of vehicles was one strategy being employed.
Recent developments
The use of high-technology (such as electronic engine control units) in advanced designs resulting from substantial investments in development research by European countries and Japan seemed to give an advantage to them over Chinese automakers and parts suppliers who, as of 2013, had low development budgets and lacked capacity to produce parts for high-tech engine and power train designs.
Characteristics
The chief characteristic of an automotive engine (compared to a stationary engine or a marine engine) is a high power-to-weight ratio. This is achieved by using a high rotational speed. However, automotive engines are sometimes modified for marine use, forming a marine automobile engine.
History
In the early years, steam engines and electric motors were tried, but with limited success. In the 20th century, the internal combustion engine (ICE), became dominant. In 2015, the internal combustion engine remains the most widely used but a resurgence of electricity seems likely because of increasing concern about ICE engine exhaust gas emissions.
As of 2017, the majority of the cars in the United States are gasoline powered. In the early 1900s, the internal combustion engines faced competition from steam engines and electric motors. The internal combustion engines of the time were powered by gasoline. Internal combustion engines function with the concept of a piston being pushed by the pressure of a certain explosion. This explosion is burning the hydrocarbon within the cylinder of an engine. Out of all the cars manufactured during the time, only around one fourth are actually considered internal combustion. Within the next couple of years, the internal combustion engine came out to become the most popular automotive engine. Sometime within the 19th century, Rudolf Diesel invented a new form of internal combustion power, using a concept of injecting liquid fuel into air heated solely by compression. This is the predecessor to the modern diesel engine used in automobiles, but more specifically, heavy duty vehicles such as semi-trucks.
Engine types:
Internal combustion engines
Petrol engines quickly became the choice of manufacturers and consumers alike. Despite the rough start, noisy and dirty engine, and the difficult gear shifting, new technologies such as the production line and the advancement of the engine allowed the standard production of the gas automobiles. This is the start, from the invention of the gas automobile in 1876, to the beginning of mass production in the 1890s. Henry Ford's Model T drove down the price of cars to a more affordable price. At the same time, Charles Kettering invented an electric starter, allowing the engine to be started without the need for a mechanical hand crank. The abundance of fuel propelled gas automobiles to be highly capable and affordable. The demand of gasoline rose from 3 billion barrels in 1919 to around 15 billion in 1929.
An internal combustion engine is powered by the expansion of gas which is created by the combustion of hydrocarbon gases fuels. To elaborate, an internal combustion used the heat of a combustion created by the injected hydrocarbon fuel to create mechanical motion. At the time of the early 1900s, wood alcohol was a popular fuel for French and German automobiles, but as governments imposed large taxes on the production, the price of wood alcohol rose above that of gasoline. Gasoline engines became popular as a result of this, as internal combustion engines were commonly known as gasoline engines. Although gasoline engines became popular, they were not particularly desirable due to the dangers of fuel leaks that may cause explosions. Therefore, many inventors attempted to create a kerosene burning engine as a result. This was not a successful venture applying it for automotive usage. There are many different types of fuels for internal combustion engines. These include diesel, gasoline, and ethanol.
Steam engines
The steam engine was invented in the late 1700s, and the primary method of powering engines and soon, locomotives. One of the most popular steam automobiles was the “Stanley Steamer,” offering low pollution, power, and speed. The downside of these steam automobiles is the unreliability, complexity, and the frequent accidents that occurred with them. The startup time for a steam car may take up to 45 minutes, defeating the purpose of faster transportation. By the time the steam automobile was improved, the complexity of manufacturing relative to the gas automobiles made steam automobiles unprofitable.
A steam engine is a device which transforms heat into mechanical motion. This is provided with the usage of boilers, which create steam by boiling water. In the early 1900s, Abner Doble introduced a steam-powered car in the United States which had capabilities that could potentially overpower Ford's Model T in efficiency. Steam has been known to have very efficient fuel economy with a high power source. That is why half the world was powered by steam for almost the entirety of the 19th century and almost half the 20th century. The main drawback of the steam engine in automobiles was that operators were required to have full knowledge of boilers and steam engines before operating, as it was detrimental to the engine itself if the operator neglected it.
Electric motors
Electric vehicles seemed to be the most viable option, similar to the steam automobiles. They were first invented in the early 1800s, and became a viable option of transportation around 1890, when William Morrison created the first electric car that traveled 14 miles per hour. The electric cars offered low pollution and a soundless ride, unlike their gasoline counterparts. The greatest downside of electric cars was the range. The typical electric car could reach around 20 miles before requiring a recharge. Manufacturers could not increase the number of batteries, due to the bulkiness of the batteries at the time. Without an incentive to purchase the electric automobiles, gas automobiles were the most viable option at the time.
Electric cars use batteries to store electricity which is used to power electric motors. The battery delivers the power to the motor, which is either Alternating Current (AC) or Direct Current (DC). The difference between AC and DC motors is the sort of system that is required to run it in an electric vehicle. An AC motor is generally cheaper but the components required to run it in an electric vehicle such as the controller and inverter makes it more expensive than the DC motor. A unique feature of electric vehicles compared to its gasoline counterparts, the electric vehicle is more simple than the gasoline vehicle. The electric vehicle bypasses the gasoline car components such as the crankshaft which allows it to generate power much faster than gasoline. Because of the faster transfer of power, the electric vehicle is able to accelerate faster than gasoline cars.
In the 1970s, the electric vehicle made its reappearance because of the 1973 OPEC Oil Embargo. Previously, the abundant gasoline had become the prime source of fuel for vehicles. But after the shortage, manufacturers began looking towards electric vehicles again. Despite the improved technology from the 1800s, the electric vehicles faced similar technological flaws such as limited mileage and speed. They could only travel up to 45 miles per hour and had a range of approximately 40 miles.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2688 2026-02-05 21:21:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2487) Skyscraper
Skyscraper
Gist
A skyscraper is a very tall, multi-story building, typically found in cities, that is supported by a metal framework and often used for offices, hotels, or residences, with modern definitions generally considering buildings over 100 or 150 meters (330-490 ft) as skyscrapers. The term emerged in the late 1880s with early high-rises in the U.S., evolving to describe exceptionally tall structures.
A skyscraper typically has over 40 floors, though the definition has evolved from early buildings of 10-20 stories to today's supertalls that often exceed 100 floors, with no strict minimum, but generally defined by being very tall and continuously habitable. Modern skyscrapers leverage steel frames and elevators, allowing for extreme heights, like the 163-story Burj Khalifa.
Summary
A skyscraper is a tall building with many habitable floors. Most modern sources define skyscrapers as being at least 100 metres (330 ft) or 150 metres (490 ft) in height, though there is no universally accepted definition, other than being very tall high-rise buildings. Skyscrapers may host offices, hotels, residential spaces, and retail spaces. Skyscrapers are a common feature of large cities, often due to a high demand for space and limited availability of land.
One common feature of skyscrapers is having a steel frame that supports curtain walls. These curtain walls either bear on the framework below or are suspended from the framework above, rather than resting on load-bearing walls of conventional construction. Some early skyscrapers have a steel frame that enables the construction of load-bearing walls taller than those made of reinforced concrete. Modern skyscraper walls are not load-bearing, and most skyscrapers are characterized by large surface areas of windows made possible by steel frames and curtain walls. However, skyscrapers can have curtain walls that mimic conventional walls with a small surface area of windows. Modern skyscrapers often have a tubular structure, and are designed to act like a hollow cylinder to resist wind, seismic, and other lateral loads. To appear more slender, allow less wind exposure and transmit more daylight to the ground, many skyscrapers have a design with setbacks, which in some cases is also structurally required.
Skyscrapers first appeared in the United States at the end of the 19th century, especially in the cities of Chicago and New York City. Following a building boom across the western world in the early 20th century, skyscraper development was halted in the 1930s by the Great Depression, and did not resume until the 1950s. A skyscraper boom in the downtowns of many American cities took place during the 1960s to 1980s. Towards the second half of the 20th century, skyscrapers began to be built more frequently outside the United States, particularly in East Asia and Southeast Asia during the 1990s. China has since overtaken the United States as the country with the most skyscrapers. Skyscrapers are an increasingly global phenomenon, and can be found in over 70 countries.
There are over 7 thousand skyscrapers over 150 m (490 ft) in height worldwide, most of which were built in the 21st century. Over three-quarters of skyscrapers taller than 150 m (492 ft) are located in Asia. Eighteen cities in the world have more than 100 skyscrapers that are taller than 150 m (492 ft), most recently Toronto and Singapore in 2025. The city with the most skyscrapers in the world is Hong Kong, with 569 skyscrapers, followed by Shenzhen in China with 444, New York City with 317, and Dubai in the United Arab Emirates with 270. Dubai is home to the tallest skyscraper in the world, the Burj Khalifa.
Details
Skyscraper is a very tall multistoried building. The name first came into use during the 1880s, shortly after the first skyscrapers were built, in the United States. The development of skyscrapers came as a result of the coincidence of several technological and social developments. The term skyscraper originally applied to buildings of 10 to 20 stories, but by the late 20th century the term was used to describe high-rise buildings of unusual height, generally greater than 40 or 50 stories.
The increase in urban commerce in the United States in the second half of the 19th century augmented the need for city business space, and the installation of the first safe passenger elevator (in the Haughwout Department Store, New York City) in 1857 made practical the erection of buildings more than four or five stories tall. Although the earliest skyscrapers rested on extremely thick masonry walls at the ground level, architects soon turned to the use of a cast-iron and wrought-iron framework to support the weight of the upper floors, allowing for more floor space on the lower stories. James Bogardus built the Cast Iron Building (1848, New York City) with a rigid frame of iron providing the main support for upper-floor and roof loads.
It was, however, the refinement of the Bessemer process, first used in the United States in the 1860s, that allowed for the major advance in skyscraper construction. As steel is stronger and lighter in weight than iron, the use of a steel frame made possible the construction of truly tall buildings. William Le Baron Jenney’s 10-story Home Insurance Company Building (1884–85) in Chicago was the first to use steel-girder construction. Jenney’s skyscrapers also first employed the curtain wall, an outer covering of masonry or other material that bears only its own weight and is affixed to and supported by the steel skeleton. Structurally, skyscrapers consist of a substructure of piers beneath the ground, a superstructure of columns and girders above the ground, and a curtain wall hung on the girders.
As the population density of urban areas has increased, so has the need for buildings that rise rather than spread. The skyscraper, which was originally a form of commercial architecture, has increasingly been used for residential purposes as well.
The design and decoration of skyscrapers have passed through several stages. Jenney and his protégé Louis Sullivan styled their buildings to accentuate verticality, with delineated columns rising from base to cornice. There was, however, some retention of, and regression to, earlier styles as well. As part of the Neoclassical revival, for instance, skyscrapers such as those designed by the firm of McKim, Mead, and White were modeled after Classical Greek columns. The Metropolitan Life Insurance Building in New York City (1909) was modeled by Napoleon Le Brun after the Campanile of St. Mark’s in Venice, and the Woolworth Building (1913), by Cass Gilbert, is a prime example of neo-Gothic decoration. Even the Art Deco carvings on such towers as the Chrysler Building (1930), the Empire State Building (1931), and the RCA Building (1931) in New York City, which were then considered as modern as the new technology, are now viewed as more related to the old ornate decorations than to truly modern lines.
The International Style with its total simplicity seemed ideally suited to skyscraper design, and, during the decades following World War II, it dominated the field, notable early examples being the Seagram Building (1958) in New York City and the Lake Shore Drive Apartments (1951) in Chicago. The stark verticality and glass curtain walls of this style became a hallmark of ultramodern urban life in many countries. During the 1970s, however, attempts were made to redefine the human element in urban architecture. Zoning ordinances encouraged the incorporation of plazas and parks into and around the bases of even the tallest skyscrapers, just as zoning laws in the first decades of the 20th century were passed to prevent city streets from becoming sunless canyons and led to the shorter, stepped skyscraper. Office towers, such as those of the World Trade Center (1972) in New York City and the Sears Tower (1973; now called Willis Tower) in Chicago, continued to be built, but most of them, such as the Citicorp Center (1978) in New York City, featured lively and innovative space for shopping and entertainment at street level.
Another factor influencing skyscraper design and construction in the late 20th and early 21st centuries was the need for energy conservation. Earlier, sealed windows that made necessary continuous forced-air circulation or cooling, for instance, gave way in mid-rise buildings to operable windows and glass walls that were tinted to reflect the sun’s rays. Also, perhaps in reaction to the austerity of the International Style, the 1980s saw the beginnings of a return to more classical ornamentation, such as that of Philip Johnson’s AT&T Building (1984) in New York City.
Additional Information
A skyscraper is a very tall high-rise building, usually more than 152 metres (500 feet) in height. Most skyscrapers are built in urban areas such as cities, and they are very common in the central business district (also called downtown) areas of many large cities including New York City, Chicago, London, Paris, Sydney, Beijing, Berlin, Toronto, Moscow, Hong Kong and Tokyo. Skyscrapers are common in downtown districts where it's more economical to build up instead of out.
History
Originally, the word skyscraper meant a tall sail on a sailing ship. Over time, the word's meaning has changed, and today it means a tall building. Until the nineteenth century, buildings taller than six stories tall were not common. Tall buildings made of weak materials would fall down. In addition, people did not like walking up many steps and running water could only be brought up to fifty feet (15m) high.
Better technology helped make skyscrapers more common. Stronger building materials such as steel and reinforced concrete were developed, so stronger buildings could be made. Water pumps brought water up to heights above fifty feet.
The first building to be considered a skyscraper, the Home Insurance Building, was built in Chicago, Illinois in the United States, and was designed by William LeBaron Jenney. The building, ten stories high, was built from 1884 to 1885. It was destroyed in 1931 because they wanted to build another building in its previous place.
In the same year the Home Insurance Building was destroyed, one of the oldest and most famous skyscrapers, the Empire State Building, opened in New York City. Later in the 20th century, people started building skyscrapers in cities that did not have many tall buildings in the past. In 1973, the then-called Sears Tower in Chicago was finished and became the world's tallest building until the late 1990s. It took the record from the World Trade Center in New York City, which opened in 1970 but was destroyed in the September 11, 2001 attacks.
Many taller buildings have been built since then, including Taipei 101 in Taipei. This building was the world's tallest from 2004 until 2008, when the Burj Khalifa in Dubai opened. Burj Khalifa is at this time the tallest building and man-made structure ever made, but the Jeddah Tower in Jeddah, which is still being built, will be even taller.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2689 2026-02-06 17:09:05
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2488) Mass
Gist
In physics, mass is the fundamental property of matter that quantifies the amount of "stuff" in an object and its resistance to acceleration (inertia), measured in kilograms (kg). It's a scalar quantity, meaning it has magnitude but no direction, and remains constant regardless of location, unlike weight, which is a force dependent on gravity. Mass determines how much force is needed to change an object's motion.
The simple definition of mass is the quantity of matter within a given object. Mass is determined by the atomic and molecular makeups of objects. All objects are made up of protons, neutrons, and electrons, and each of these has different makeups and masses.
Summary
Mass is an intrinsic property of a body. In modern physics, it is generally defined as the strength of an object's gravitational attraction to other bodies - as measured by an observer moving along at the same speed.
It was traditionally believed to be related to the quantity of matter in a body, until the discovery of the atom and particle physics. It was found that different atoms and different elementary particles, theoretically with the same amount of matter, have nonetheless different masses. Mass in modern physics has multiple definitions which are conceptually distinct, but physically equivalent.
Mass can be experimentally defined as a measure of the body's inertia, meaning the resistance to acceleration (change of velocity) when a net force is applied.
The SI base unit of mass is the kilogram (kg). In physics, mass is not the same as weight, even though mass is often determined by measuring the object's weight using a spring scale, rather than balance scale comparing it directly with known masses. An object on the Moon would weigh less than it does on Earth because of the lower gravity, but it would still have the same mass. This is because weight is a force, while mass is the property that (along with gravity) determines the strength of this force.
In the Standard Model of physics, the mass of elementary particles is believed to be a result of their coupling with the Higgs boson in what is known as the Brout–Englert–Higgs mechanism.
Details
Mass, in physics, is a quantitative measure of inertia, a fundamental property of all matter. It is, in effect, the resistance that a body of matter offers to a change in its speed or position upon the application of a force. The greater the mass of a body, the smaller the change produced by an applied force. The unit of mass in the International System of Units (SI) is the kilogram, which is defined in terms of Planck’s constant, which is defined as equal to 6.62607015 × {10}^{-34} joule second. One joule is equal to one kilogram times metre squared per second squared. With the second and the metre already defined in terms of other physical constants, the kilogram is determined by accurate measurements of Planck’s constant. (Until 2019 the kilogram was defined by a platinum-iridium cylinder called the International Prototype Kilogram kept at the International Bureau of Weights and Measures in Sèvres, France.) In the English system of measurement, the unit of mass is the slug, a mass whose weight at sea level is 32.17 pounds.
Weight, though related to mass, nonetheless differs from the latter. Weight essentially constitutes the force exerted on matter by the gravitational attraction of Earth, and so it varies slightly from place to place. In contrast, mass remains constant regardless of its location under ordinary circumstances. A satellite launched into space, for example, weighs increasingly less the farther it travels away from Earth. Its mass, however, stays the same.
According to the principle of conservation of mass, the mass of an object or collection of objects never changes, no matter how the constituent parts rearrange themselves. If a body split into pieces, the mass divides with the pieces, so that the sum of the masses of the individual pieces is equal to the original mass. Or, if particles are joined together, the mass of the composite is equal to the sum of the masses of the constituent particles. However, this principle is not always correct.
With the advent of the special theory of relativity by Einstein in 1905, the notion of mass underwent a radical revision. Mass lost its absoluteness. The mass of an object was seen to be equivalent to energy, to be interconvertible with energy, and to increase significantly at exceedingly high speeds near that of light (about 3 × {10}^{8} metres per second, or 186,000 miles per second). The total energy of an object was understood to comprise its rest mass as well as its increase of mass caused by high speed. The rest mass of an atomic nucleus was discovered to be measurably smaller than the sum of the rest masses of its constituent neutrons and protons. Mass was no longer considered constant, or unchangeable. In both chemical and nuclear reactions, some conversion between mass and energy occurs, so that the products generally have smaller or greater mass than the reactants. The difference in mass is so slight for ordinary chemical reactions that mass conservation may be invoked as a practical principle for predicting the mass of products. Mass conservation is invalid, however, for the behaviour of masses actively involved in nuclear reactors, in particle accelerators, and in the thermonuclear reactions in the Sun and stars. The new conservation principle is the conservation of mass-energy.
Additional Information
Key takeaways
* Mass is the quantity of matter that makes up a given object
* Mass and weight are not the same thing. Mass deals with matter. Weight deals with the gravitational pull the Earth has on an object.
* The formula for calculating mass is Mass = Density x Volume. You can also find an object’s inertial and gravitational mass.
Mass definition
Mass is everywhere you look. Everything that is made up of something has mass. Even the oxygen we breathe has mass!
Understanding mass gives you a greater understanding of the way the world around you works. The screen you’re reading off of right now has mass! The keyboard you type with has mass! The food you eat, the bed you sleep on, and the bus you ride to school are all made of mass.
That’s because mass is made up of atoms, and everything we see contains atoms.
Mass is all around you, but what is mass exactly?
Mass definition
The simple definition of mass is the quantity of matter within a given object.
Mass is determined by the atomic and molecular makeups of objects. All objects are made up of protons, neutrons, and electrons, and each of these has different makeups and masses.
Units of mass
In 1875, scientists at the International Metric Convention created the International Prototype Kilogram which would, from then on, be used as the basis for mass measurement. The prototype kilogram was used to standardise one kilogram for every country in the world and therefore standardise the units of mass.
Part of the metric system, the kilogram, kg, then became what is called an SI unit of mass, or a part of the International System of Units.
Even though the physical prototype kilogram was replaced with a more practical definition in 2019, the kg and the SI unit are still used to measure mass today.
The mass unit of measure is the kilograms and multiples or fractions of the kilogram. The chart of mass units below demonstrates the most common units used to measure mass.
Mass vs weight
Mass and weight often get confused because in many cases, they both deal with the concept of heaviness and lightness. However, the two are very different!
First, let’s define mass again.
According to the mass definition, mass is a measurement of the quantity of matter. Mass is measured in grams and related units.
Weight is the measurement of the Earth’s gravitational pull on an object. Weight is measured in ounces, pounds, and tons.
Weight is the combination of mass and gravity: Weight = Mass x Gravity
Your weight depends on the gravitational pull of the Earth. If you were standing on Mars, your weight would depend on the gravitational pull of Mars, and therefore, you would have a different weight. Your mass, on the other hand, does not depend on gravity and will not change no matter where in the universe you are.
On Earth, it just so happens that weight and mass tend to be very similar. This is why the two are so easily confused with each other.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2690 2026-02-07 18:20:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2489) Specific Gravity
Gist
Specific gravity (SG) is the ratio of a substance's density to the density of a reference material, usually water at 4°C for liquids/solids and air for gases, telling you how much denser or lighter a substance is compared to the reference. It's a dimensionless number (no units), where SG > 1 means the substance sinks (denser than water) and SG < 1 means it floats (lighter). It's used in industries to check purity, identify materials, and determine buoyancy.
Specific gravity tells you whether something is floating or sinking in water. A specific gravity below 1 means that the sample is less dense (lighter) than water and will, therefore, float. For example, an oil with a specific gravity of 0.825 will float on water.
Summary
Relative density, also called specific gravity, is a dimensionless quantity defined as the ratio of the density (mass divided by volume) of a substance to the density of a given reference material. Specific gravity for solids and liquids is nearly always measured with respect to water at its densest (at 4 °C or 39.2 °F); for gases, the reference is air at room temperature (20 °C or 68 °F). The term "relative density" (abbreviated r.d. or RD) is preferred in SI, whereas the term "specific gravity" is gradually being abandoned.
If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance with a relative density greater than 1 will sink.
Temperature and pressure must be specified for both the sample and the reference. Pressure is nearly always 1 atm (101.325 kPa). Where it is not, it is more usual to specify the density directly. For specific gravity, the reference temperature for water is often 4 °C (39.2 °F) because it's the point where water is densest (1 g/cc), but 15 °C (59 °F), 15.6 °C (60 °F), or 20 °C (68 °F) are also common standards, depending on the industry (like brewing or petroleum). In British brewing practice, the specific gravity, as specified above, is multiplied by 1000. Specific gravity is commonly used in industry as a simple means of obtaining information about the concentration of solutions of various materials such as brines, must weight (syrups, juices, honeys, brewers wort, must, etc.) and acids.
Details
Specific gravity, also known as relative density, measures the density of a substance compared to the density of water.
Specific gravity is related to density, as both are physical properties used to determine how "dense" a particular material is. This material can be a gas, liquid, or solid.
Specific gravity and density are used to identify a material, determine the concentration of a liquid solution e.g., alcohol or sugar in a drink), or test whether a product is within specifications.
1. What Are Density and Specific Gravity?
The density of a sample is defined as its mass divided by its volume.
Specific gravity, also known as relative density, is used to describe the density of a substance compared to the density of water. To calculate specific gravity, divide the sample's density by the density of water.
2. What Is the Difference Between Specific Gravity and Density?
The main difference between specific gravity and density is that specific gravity is dimensionless, meaning it has no units, while density has a unit g/cc.
Specific gravity tells you whether something is floating or sinking in water. A specific gravity below 1 means that the sample is less dense (lighter) than water and will, therefore, float. For example, an oil with a specific gravity of 0.825 will float on water.
3. How to Convert Specific Gravity to Density?
Density of the Substance = Specific Gravity of the Substance x Density of Water.
4. How Does the Temperature of Samples Affect the Specific Gravity and Density?
The temperature of a sample affects both the density and the specific gravity. The higher the temperature, the higher the volume and the lower the density. If the temperature increases, the volume increases, and the density decreases. However, the mass of the substance does not change with temperature.
The most notable exception to this rule is liquid water, which reaches its maximum density at 3.98 ºC; above this point, the volume of water increases, and it becomes less dense. The opposite is true when water is cooled.
Since specific gravity is the density of the sample divided by the density of water, both densities will decrease with increasing temperature, but not by the same magnitude. The effect of the temperature will usually be slightly less important for specific gravity than for density.
5. Which Instruments Are Used to Measure Specific Gravity and Density?
There are different types of instruments to measure both specific gravity and density:
Hydrometer
A hydrometer is a cost-effective instrument used to determine the specific gravity/density of liquids. Made of blown glass, it consists of a bulbous bottom weighted with lead or steel shot and a long, narrow stem with a scale. The hydrometer is immersed into the sample liquid until it floats. The density reading is taken by looking at the scale, where the level of the sample liquid aligns with a marking on the hydrometer scale. Most hydrometers measure the specific gravity of samples: in simple terms, a hydrometer tells the user if a liquid is denser or less dense than water. It will float higher in a liquid with a greater specific gravity, such as water with sugar dissolved, compared to one with a lower specific gravity, such as pure water or alcohol.
When using a hydrometer, the user has two options:
a) Use the hydrometer at its calibration temperature (usually 16 °C or 20 °C). Depending on the sample volume, it can take some time for the sample to reach this temperature.
b) Simply record the measurement value at the surrounding temperature. Both measurement and temperature values must be recorded. If needed, a correction factor can be applied later to obtain the temperature-corrected measurement value.
Pycnometer
Typically made of glass, a pycnometer is a flask of a pre-defined volume used to measure the specific gravity/density of a liquid. It can also determine the specific gravity/density of dispersions, solids, and even gases.
A thermometer is also required to measure the temperature. User training is required to guarantee accurate measurements with the pycnometer.
Portable digital density meter
Portable digital density meters are used to quickly and accurately determine the specific gravity/density of liquids. Determination of density or specific gravity using digital meters is based on two factors:
a) The oscillation, or vibration, of a U-shaped glass tube (U-tube).
b) The relationship between the liquid sample mass and the frequency of oscillation of the U-tube. Filling the U-tube with sample liquid affects its frequency of oscillation: due to factory adjustment with samples of known densities, this frequency of oscillation can be directly correlated with the density of any liquid sample with an accuracy of 0.001 g/{cm}^{3} or a specific gravity with an accuracy of 0.001. Handheld digital density meters measure the sample at ambient temperature. If a result is needed at a certain temperature, the digital density meter can apply a correction factor to the measured result to compensate the result to a defined temperature. Each measurement takes only a few seconds, allowing users to move on to the next sample quickly. The measured density can be automatically converted into other units and concentrations for specific applications, such as specific gravity, API, alcohol%, °Brix, etc.
Benchtop digital density meter
Benchtop digital density meters use the same technology as portable digital density meters, the oscillation of a U-shaped glass tube (U-tube). In addition, they feature a built-in Peltier temperature control, which brings the sample to the selected temperature (e.g., 20°C). The temperature control can range from 0 °C to 95 °C. These density meters can reach an accuracy of 0.000005 g/{m}^{3} for density, or 0.000005 for specific gravity.
Some benchtop digital density meters can be connected to sample automation solutions for single or multiple samples, which offer automated sampling, rinsing, and drying. These density meters can often be upgraded into a dedicated automated multi-parameter system combining density, refractive index, pH, color, conductivity, and more to save time, increase data quality, and prevent any alteration of samples between individual analyses.
One of the benefits of digital density meters using the U-shaped glass tube is the small volume of sample required (typically 1.5 mL), which allows for a faster temperature equilibrium of the sample.
Additional Information
Relative density is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material (i.e., water). It is usually measured at room temperature (20 Celsius degrees) and standard atmosphere (101.325kPa). It is unitless. You can often find it in the section 9 of a safety data sheet (SDS).
Regulatory Implications of Relative Density
Relative density is often used to calculate the volume or weight of samples needed for preparing a solution with a specified concentration. It also helps us understand the environmental distribution of insoluble substances (i.e, oil spill) in aquatic eco-system (on water surface or bottom sediment) if the substance is released to water.
Relative density test is not required for every chemical. Under REACH, the study does not need to be conducted if:
* the substance is only stable in solution in a particular solvent and the solution density is similar to that of the solvent. In such cases, an indication of whether the solution density is higher or lower than the solvent density is sufficient, or
* the substance is a gas. In this case, an estimation based on calculation shall be made from its molecular weight and the Ideal Gas Laws.
(REACH: REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals, a comprehensive European Union regulation that governs the production and use of chemical substances to protect human health and the environment. It requires companies to register chemical substances, assess their risks, and manage them safely, placing the responsibility on industry to ensure chemical safety throughout the supply chain.)

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2691 2026-02-08 17:23:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2490) Refractory
Gist
Refractory materials are specialized, non-metallic substances engineered to withstand extreme temperatures, (often >1000 Degrees Fahrenheit or 538 Degrees Centigrade), high pressure, and corrosion without softening or deformation. They are critical for lining furnaces, kilns, and reactors. Key properties include high thermal stability, low thermal expansion, and resistance to molten slag and thermal shock.
Refractory materials are inorganic (not from living material), non-metal substances that can withstand extremely high temperatures without any loss of strength or shape. They are used in devices, such as furnaces, that heat substances and in tanks and other storage devices that hold hot materials.
Summary:
What Are Refractories?
Refractories are ceramic materials designed to withstand the very high temperatures (in excess of 1,000°F [538°C]) encountered in modern manufacturing. More heat-resistant than metals, they are used to line the hot surfaces found inside many industrial processes.
In addition to being resistant to thermal stress and other physical phenomena induced by heat, refractories can withstand physical wear and corrosion caused by chemical agents. Thus, they are essential to the manufacture of petrochemical products and the refining of gasoline.
Refractory products generally fall into one of two broad categories: preformed shapes or unformed compositions, often called specialty or monolithic refractories. Then, there are refractory ceramic fibers, which resemble residential insulation, but insulate at much higher temperatures. Bricks and shapes are the more traditional form of refractories and historically have accounted for the majority of refractory production.
Refractories come in all shapes and sizes. They can be pressed or molded for use in floors and walls, produced in interlocking shapes and wedges, or curved to fit the insides of boilers and ladles. Some refractory parts are small and possess a complex and delicate geometry; others, in the form of precast or fusion-cast blocks, are massive and may weigh several tons.
What Are Refractories Made Of?
Refractories are produced from natural and synthetic materials, usually nonmetallic, or combinations of compounds and minerals such as alumina, fireclays, bauxite, chromite, dolomite, magnesite, silicon carbide, and zirconia.
What Are Refractories Used For?
From the simple (e.g., fireplace brick linings) to the sophisticated (e.g., reentry heat shields for the space shuttle), refractories are used to contain heat and protect processing equipment from intense temperatures. In industry, they are used to line boilers and furnaces of all types (reactors, ladles, stills, kilns, etc.).
It is a tribute to refractory engineers, scientists, technicians, and plant personnel that more than 5,000 brand name products in the United States are listed in the latest “Product Directory of the Refractories Industry.”
Details
In materials science, a refractory (or refractory material) is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.
Refractories are defined by ASTM C71 as "non-metallic materials having those chemical and physical properties that make them applicable for structures, or as components of systems, that are exposed to environments above 1,000 °F (811 K; 538 °C)". Refractory materials are used in furnaces, kilns, incinerators, and reactors. Refractories are also used to make crucibles and molds for casting glass and metals. The iron and steel industry and metal casting sectors use approximately 70% of all refractories produced.
Refractory materials
Refractory materials must be chemically and physically stable at high temperatures. Depending on the operating environment, they must be resistant to thermal shock, be chemically inert, and/or have specific ranges of thermal conductivity and of the coefficient of thermal expansion.
The oxides of aluminium (alumina), silicon (silica) and magnesium (magnesia) are the most important materials used in the manufacturing of refractories. Another oxide usually found in refractories is the oxide of calcium (lime). Fire clays are also widely used in the manufacture of refractories.
Refractories must be chosen according to the conditions they face. Some applications require special refractory materials. Zirconia is used when the material must withstand extremely high temperatures. Silicon carbide and carbon (graphite) are two other refractory materials used in some very severe temperature conditions, but they cannot be used in contact with oxygen, as they would oxidize and burn.
Binary compounds such as tungsten carbide or boron nitride can be very refractory. Hafnium carbide is the most refractory binary compound known, with a melting point of 3890 °C. The ternary compound tantalum hafnium carbide has one of the highest melting points of all known compounds (4215 °C).
Molybdenum disilicide has a high melting point of 2030 °C and is often used as a heating element.
Uses
Refractory materials are useful for the following functions:
* Serving as a thermal barrier between a hot medium and the wall of a containing vessel
* Withstanding physical stresses and preventing erosion of vessel walls due to the hot medium
* Protecting against corrosion
* Providing thermal insulation
Refractories have multiple useful applications. In the metallurgy industry, refractories are used for lining furnaces, kilns, reactors, and other vessels which hold and transport hot media such as metal and slag. Refractories have other high temperature applications such as fired heaters, hydrogen reformers, ammonia primary and secondary reformers, cracking furnaces, utility boilers, catalytic cracking units, air heaters, and sulfur furnaces. They are used for surfacing flame deflectors in rocket launch structures.
Additional Information
A refractory is any material that has an unusually high melting point and that maintains its structural properties at very high temperatures. Composed principally of ceramics, refractories are employed in great quantities in the metallurgical, glassmaking, and ceramics industries, where they are formed into a variety of shapes to line the interiors of furnaces, kilns, and other devices that process materials at high temperatures.
In this article the essential properties of ceramic refractories are reviewed, as are the principal refractory materials and their applications. At certain points in the article reference is made to the processing techniques employed in the manufacture of ceramic refractories; more detailed description of these processes can be found in the articles traditional ceramics and advanced ceramics. The connection between the properties of ceramic refractories and their chemistry and microstructure is explained in ceramic composition and properties.
Properties
Because of the high strengths exhibited by their primary chemical bonds, many ceramics possess unusually good combinations of high melting point and chemical inertness. This makes them useful as refractories. (The word refractory comes from the French réfractaire, meaning “high-melting.”) The property of chemical inertness is of special importance in metallurgy and glassmaking, where the furnaces are exposed to extremely corrosive molten materials and gases. In addition to temperature and corrosion resistance, refractories must possess superior physical wear or abrasion resistance, and they also must be resistant to thermal shock. Thermal shock occurs when an object is rapidly cooled from high temperature. The surface layers contract against the inner layers, leading to the development of tensile stress and the propagation of cracks. Ceramics, in spite of their well-known brittleness, can be made resistant to thermal shock by adjusting their microstructure during processing. The microstructure of ceramic refractories is quite coarse when compared with whitewares such as porcelain or even with less finely textured structural clay products such as brick. The size of filler grains can be on the scale of millimetres, instead of the micrometre scale seen in whiteware ceramics. In addition, most ceramic refractory products are quite porous, with large amounts of air spaces of varying size incorporated into the material. The presence of large grains and pores can reduce the load-bearing strength of the product, but it also can blunt cracks and thereby reduce susceptibility to thermal shock. However, in cases where a refractory will come into contact with corrosive substances (for example, in glass-melting furnaces), a porous structure is undesirable. The ceramic material can then be made with a higher density, incorporating smaller amounts of pores.
Composition and processing
The composition and processing of ceramic refractories vary widely according to the application and the type of refractory. Most refractories can be classified on the basis of composition as either clay-based or nonclay-based. In addition, they can be classified as either acidic (containing silica [SiO2] or zirconia [ZrO2]) or basic (containing alumina [Al2O3] or alkaline-earth oxides such as lime [CaO] or magnesia [MgO]). Among the clay-based refractories are fireclay, high-alumina, and mullite ceramics. There is a wide range of nonclay refractories, including basic, extra-high alumina, silica, silicon carbide, and zircon materials. Most clay-based products are processed in a manner similar to other traditional ceramics such as structural clay products; e.g., stiff-mud processes such as press forming or extrusion are employed to form the ware, which is subsequently dried and passed through long tunnel kilns for firing. Firing, as described in the article traditional ceramics, induces partial vitrification, or glass formation, which is a liquid-sintering process that binds particles together. Nonclay-based refractories, on the other hand, are bonded using techniques reserved for advanced ceramic materials. For instance, extra-high alumina and zircon ceramics are bonded by transient-liquid or solid-state sintering, basic bricks are bonded by chemical reactions between constituents, and silicon carbide is reaction-bonded from silica sand and coke. These processes are described in the article advanced ceramics.
Clay-based refractories
In this section the composition and properties of the clay-based refractories are described. Most are produced as preformed brick. Much of the remaining products are so-called monolithics, materials that can be formed and solidified on-site. This category includes mortars for cementing bricks and mixes for ramming or gunning (spraying from a pressure gun) into place. In addition, lightweight refractory insulation can be made in the form of fibreboards, blankets, and vacuum-cast shapes.
Fireclay
The workhorse of the clay-based refractories are the so-called fireclay materials. These are made from clays containing the aluminosilicate mineral kaolinite (Al2[Si2O5][OH]4) plus impurities such as alkalis and iron oxides. The alumina content ranges from 25 to 45 percent. Depending upon the impurity content and the alumina-to-silica ratio, fireclays are classified as low-duty, medium-duty, high-duty, and super-duty, with use temperature rising as alumina content increases. Fireclay bricks, or firebricks, exhibit relatively low expansion upon heating and are therefore moderately resistant against thermal shock. They are fairly inert in acidic environments but are quite reactive in basic environments. Fireclay bricks are used to line portions of the interiors of blast furnaces, blast-furnace stoves, and coke ovens.
High alumina
High-alumina refractories are made from bauxite, a naturally occurring material containing aluminum hydroxide (Al[OH]3) and kaolinitic clays. These raw materials are roasted to produce a mixture of synthetic alumina and mullite (an aluminosilicate mineral with the chemical formula 3Al2O3 · 2SiO2). By definition high-alumina refractories contain between 50 and 87.5 percent alumina. They are much more robust than fireclay refractories at high temperatures and in basic environments. In addition, they exhibit better volume stability and abrasion resistance. High-alumina bricks are used in blast furnaces, blast-furnace stoves, and liquid-steel ladles.
Mullite
Mullite is an aluminosilicate compound with the specific formula 3Al2O3 · 2SiO3 and an alumina content of approximately 70 percent. It has a melting point of 1,850° C (3,360° F). Various clays are mixed with bauxite in order to achieve this composition. Mullite refractories are solidified by sintering in electric furnaces at high temperatures. They are the most stable of the aluminosilicate refractories and have excellent resistance to high-temperature loading. Mullite bricks are used in blast-furnace stoves and in the forehearth roofs of glass-melting furnaces.
Non-clay-based refractories
Nonclay refractories such as those described below are produced almost exclusively as bricks and pressed shapes, though some magnesite-chrome and alumina materials are fuse-cast into molds. The usual starting materials for these products are carbonates or oxides of metals such as magnesium, aluminum, and zirconium.
Basic
Basic refractories include magnesia, dolomite, chrome, and combinations of these materials. Magnesia brick is made from periclase, the mineral form of magnesia (MgO). Periclase is produced from magnesite (a magnesium carbonate, MgCO3), or it is produced from magnesium hydroxide (Mg[OH]2), which in turn is derived from seawater or underground brine solutions. Magnesia bricks can be chemically bonded, pitch-bonded, burned, or burned and then pitch-impregnated.
Dolomite refractories take their name from the dolomite ore, a combination of calcium and magnesium carbonates (CaCO3 · MgCO3), from which they are produced. After burning they must be impregnated with tar or pitch to prevent rehydration of lime (CaO). Chrome brick is made from chromium ores, which are complex solid solutions of the spinel type (a series of oxide minerals including chromite and magnetite) plus silicate gangue, or impurity phases.
All the basic refractories exhibit outstanding resistance to iron oxides and the basic slags associated with steelmaking—especially when they incorporate carbon additions either as flakes or as residual carbon from pitch-bonding or tar-impregnation. For this reason they find wide employment in the linings of basic oxygen furnaces, electric furnaces, and open-hearth furnaces. They also are used to line the insides of copper converters.
Extra-high alumina
Extra-high alumina refractories are classified as having between 87.5 and 100 percent Al2O3 content. The alumina grains are fused or densely sintered together to obtain high density. Extra-high alumina refractories exhibit excellent volume stability to over 1,800° C (3,275° F).
Silica
Silica refractories are made from quartzites and silica gravel deposits with low alumina and alkali contents. They are chemically bonded with 3–3.5 percent lime. Silica refractories have good load resistance at high temperatures, are abrasion-resistant, and are particularly suited to containing acidic slags. Of the various grades—coke-oven quality, conventional, and super-duty—the super-duty, which has particularly low impurity contents, is used in the superstructures of glass-melting furnaces.
Zircon
Refractories made of zircon (a zirconium silicate, ZrSiO4) also are used in glass tanks because of their good resistance to the corrosive action of molten glasses. They possess good volume stability for extended periods at elevated temperatures, and they also show good creep resistance (i.e., low deformation under hot loading).
Silicon carbide
Silicon carbide (SiC) ceramics are made by a process referred to as reaction bonding, invented by the American Edward G. Acheson in 1891. In the Acheson process, pure silica sand and finely divided carbon (coke) are reacted in an electric furnace at temperatures in the range of 2,200°–2,480° C (4,000°–4,500° F). SiC ceramics have outstanding high-temperature load-bearing strength and dimensional stability. They also exhibit great thermal shock resistance because of their high thermal conductivity. (In this case, high thermal conductivity prevents the formation of extreme temperature differences between inner and outer layers of a material, which frequently are a source of thermal expansion stresses.) Therefore, SiC makes good kiln furniture for supporting other ceramics during their firing.
Other non-clay-based refractories
Other refractories produced in smaller quantities for special applications include graphite (a layered, multicrystalline form of carbon), zirconia (ZrO2), forsterite (Mg2SiO4), and combinations such as magnesia-alumina, magnesite-chrome, chrome-alumina, and alumina-zirconia-silica. Alumina-zirconia-silica (AZS), which is melted and cast into molds or directly into the melting tanks of glass furnaces, is an excellent corrosion-resistant refractory that does not release impurities into the glass melt. AZS is also poured to make tank blocks (also called soldier blocks or sidewall blocks) used in the construction and repair of glass furnaces.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2692 2026-02-09 17:48:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2491) Red Sea
Gist
The Red Sea is a vital 2,250 km long, 355 km wide, and up to 2,730 m deep, narrow, and hypersaline inlet of the Indian Ocean, positioned between Northeast Africa and the Arabian Peninsula. As a crucial, warm-water maritime trade route (carrying 12%) of global trade) connected via the Suez Canal and Bab-el-Mandeb Strait, it separates countries like Egypt, Sudan, and Eritrea from Saudi Arabia and Yemen.
The Red Sea is known for its vital trade route (Suez Canal), incredibly rich biodiversity with vibrant coral reefs and unique marine life, extremely warm and salty water, year-round sunshine, and significant historical/religious importance, particularly the biblical story of Moses. It's a major global hotspot for scuba diving and snorkeling due to its clear waters and abundant fish species.
Summary
The Red Sea is a sea inlet of the Indian Ocean, lying between Africa and Asia. Its connection to the ocean is in the south, through the Bab-el-Mandeb Strait and the Gulf of Aden. To the north of the Red Sea lies the Sinai Peninsula, the Gulf of Aqaba, and the Gulf of Suez, which leads to the Suez Canal. It is underlain by the Red Sea Rift, which is part of the Great Rift Valley.
The Red Sea has a surface area of roughly 438,000 sq km (169,000 sq mi), is about 2,250 km (1,400 mi) long, and 355 km (221 mi) across at its widest point. It has an average depth of 490 m (1,610 ft), and in the central Suakin Trough, it reaches its maximum depth of 2,730 m (8,960 ft).
The Red Sea is quite shallow, with approximately 40% of its area being less than 100 m (330 ft) deep, and approximately 25% being less than 50 m (160 ft) deep. The extensive shallow shelves are noted for their marine life and corals. More than 1,000 invertebrate species and 200 types of soft and hard coral live in the sea. The Red Sea is the world's northernmost tropical sea and has been designated a Global 200 ecoregion.
Details
Red Sea is a narrow strip of water extending southeastward from Suez, Egypt, for about 1,200 miles (1,930 km) to the Bab el-Mandeb Strait, which connects with the Gulf of Aden and thence with the Arabian Sea. Geologically, the Gulfs of Suez and Aqaba (Elat) must be considered as the northern extension of the same structure. The sea separates the coasts of Egypt, Sudan, and Eritrea to the west from those of Saudi Arabia and Yemen to the east. Its maximum width is 190 miles, its greatest depth 9,974 feet (3,040 metres), and its area approximately 174,000 square miles (450,000 square km).
The Red Sea contains some of the world’s hottest and saltiest seawater. With its connection to the Mediterranean Sea via the Suez Canal, it is one of the most heavily traveled waterways in the world, carrying maritime traffic between Europe and Asia. Its name is derived from the colour changes observed in its waters. Normally, the Red Sea is an intense blue-green; occasionally, however, it is populated by extensive blooms of the algae Trichodesmium erythraeum, which, upon dying off, turn the sea a reddish brown colour.
The following discussion focuses on the Red Sea and the Gulfs of Suez and Aqaba.
Physical features:
Physiography and submarine morphology
The Red Sea lies in a fault depression that separates two great blocks of Earth’s crust—Arabia and North Africa. The land on either side, inland from the coastal plains, reaches heights of more than 6,560 feet above sea level, with the highest land in the south.
At its northern end the Red Sea splits into two parts, the Gulf of Suez to the northwest and the Gulf of Aqaba to the northeast. The Gulf of Suez is shallow—approximately 180 to 210 feet deep—and it is bordered by a broad coastal plain. The Gulf of Aqaba, on the other hand, is bordered by a narrow plain, and it reaches a depth of 5,500 feet. From approximately 28° N, where the Gulfs of Suez and Aqaba converge, south to a latitude near 25° N, the Red Sea’s coasts parallel each other at a distance of roughly 100 miles apart. There the seafloor consists of a main trough, with a maximum depth of some 4,000 feet, running parallel to the shorelines.
South of this point and continuing southeast to latitude 16° N, the main trough becomes sinuous, following the irregularities of the shoreline. About halfway down this section, roughly between 20° and 21° N, the topography of the trough becomes more rugged, and several sharp clefts appear in the seafloor. Because of an extensive growth of coral banks, only a shallow narrow channel remains south of 16° N. The sill (submarine ridge) separating the Red Sea and the Gulf of Aden at the Bab el-Mandeb Strait is affected by this growth; therefore, the depth of the water is only about 380 feet, and the main channel becomes narrow.
The clefts within the deeper part of the trough are unusual seafloor areas in which hot brine concentrates are found. These patches apparently form distinct and separated deeps within the trough and have a north-south trend, whereas the general trend of the trough is from northwest to southeast. At the bottom of these areas are unique sediments, containing deposits of heavy metal oxides from 30 to 60 feet thick.
Most of the islands of the Red Sea are merely exposed reefs. There is, however, a group of active volcanoes just south of the Dahlak Archipelago (15° 50′ N), as well as a recently extinct volcano on the island of Jabal Al-Ṭāʾir.
Geology
The Red Sea occupies part of a large rift valley in the continental crust of Africa and Arabia. This break in the crust is part of a complex rift system that includes the East African Rift System, which extends southward through Ethiopia, Kenya, and Tanzania for almost 2,200 miles and northward for more than 280 miles from the Gulf of Aqaba to form the great Wadi Aqaba–Dead Sea–Jordan Rift; the system also extends eastward for 600 miles from the southern end of the Red Sea to form the Gulf of Aden.
The Red Sea valley cuts through the Arabian-Nubian Massif, which was a continuous central mass of Precambrian igneous and metamorphic rocks (i.e., formed deep within the Earth under heat and pressure more than 540 million years ago), the outcrops of which form the rugged mountains of the adjoining region. The massif is surrounded by these Precambrian rocks overlain by Paleozoic marine sediments (542 to 251 million years old). These sediments were affected by the folding and faulting that began late in the Paleozoic; the laying down of deposits, however, continued to occur during this time and apparently continued into the Mesozoic Era (251 to 65.5 million years ago). The Mesozoic sediments appear to surround and overlap those of the Paleozoic and are in turn surrounded by early Cenozoic sediments (i.e., between 65.5 and 55.8 million years old). In many places large remnants of Mesozoic sediments are found overlying the Precambrian rocks, suggesting that a fairly continuous cover of deposits once existed above the older massif.
The Red Sea is considered a relatively new sea, whose development probably resembles that of the Atlantic Ocean in its early stages. The Red Sea’s trough apparently formed in at least two complex phases of land motion. The movement of Africa away from Arabia began about 55 million years ago. The Gulf of Suez opened up about 30 million years ago, and the northern part of the Red Sea about 20 million years ago. The second phase began about 3 to 4 million years ago, creating the trough in the Gulf of Aqaba and also in the southern half of the Red Sea valley. This motion, estimated as amounting to 0.59 to 0.62 inch (15.0 to 15.7 mm) per year, is still proceeding, as indicated by the extensive volcanism of the past 10,000 years, by seismic activity, and by the flow of hot brines in the trough.
Climate
The Red Sea region receives very little precipitation in any form, although prehistoric artifacts indicate that there were periods with greater amounts of rainfall. In general, the climate is conducive to outdoor activity in fall, winter, and spring—except during windstorms—with temperatures varying between 46 and 82 °F (8 and 28 °C). Summer temperatures, however, are much higher, up to 104 °F (40 °C), and relative humidity is high, rendering vigorous activity unpleasant. In the northern part of the Red Sea area, extending down to 19° N, the prevailing winds are north to northwest. Best known are the occasional westerly, or “Egyptian,” winds, which blow with some violence during the winter months and generally are accompanied by fog and blowing sand. From latitude 14° to 16° N the winds are variable, but from June through August strong northwest winds move down from the north, sometimes extending as far south as the Bab el-Mandeb Strait; by September, however, this wind pattern retreats to a position north of 16° N. South of 14° N the prevailing winds are south to southeast.
Hydrology
No water enters the Red Sea from rivers, and rainfall is scant; but the evaporation loss—in excess of 80 inches per year—is made up by an inflow through the eastern channel of the Bab el-Mandeb Strait from the Gulf of Aden. This inflow is driven toward the north by prevailing winds and generates a circulation pattern in which these low-salinity waters (the average salinity is about 36 parts per thousand) move northward. Water from the Gulf of Suez has a salinity of about 40 parts per thousand, owing in part to evaporation, and consequently a high density. This dense water moves toward the south and sinks below the less dense inflowing waters from the Red Sea. Below a transition zone, which extends from depths of about 300 to 1,300 feet, the water conditions are stabilized at about 72 °F (22 °C), with a salinity of almost 41 parts per thousand. This south-flowing bottom water, displaced from the north, spills over the sill at Bab el-Mandeb, mostly through the eastern channel. It is estimated that there is a complete renewal of water in the Red Sea every 20 years.
Below this southward-flowing water, in the deepest portions of the trough, there is another transition layer, only 80 feet thick, below which, at some 6,400 feet, lie pools of hot brine. The brine in the Atlantis II Deep has an average temperature of almost 140 °F (60 °C), a salinity of 257 parts per thousand, and no oxygen. There are similar pools of water in the Discovery Deep and in the Chain Deep (at about 21°18′ N). Heating from below renders these pools unstable, so that their contents mix with the overlying waters; they thus become part of the general circulation system of the sea.
Economic aspects:
Resources
Five major types of mineral resources are found in the Red Sea region: petroleum deposits, evaporite deposits (sediments laid down as a result of evaporation, such as halite, sylvite, gypsum, and dolomite), sulfur, phosphates, and the heavy-metal deposits in the bottom oozes of the Atlantis II, Discovery, and other deeps. The oil and natural gas deposits have been exploited to varying degrees by the nations adjoining the sea; of note are the deposits near Jamsah (Gemsa) Promontory (in Egypt) at the juncture of the Gulf of Suez and the Red Sea. Despite their ready availability, the evaporites have been exploited only slightly, primarily on a local basis. Sulfur has been mined extensively since the early 20th century, particularly from deposits at Jamsah Promontory. Phosphate deposits are present on both sides of the sea, but the grade of the ore has been too low to warrant exploitation with existing techniques.
None of the heavy metal deposits have been exploited, although the sediments of the Atlantis II Deep alone have been estimated to be of considerable economic value. The average analysis of the Atlantis II Deep deposit has revealed an iron content of 29 percent; zinc 3.4 percent; copper 1.3 percent; and trace quantities of lead, silver, and gold. The total brine-free sediment estimated to be present in the upper 30 feet of the Atlantis II Deep is about 50 million tons. These deposits appear to extend to a depth of 60 feet below the present sediment surface, but the quality of the deposits below 30 feet is unknown. The sediments of the Discovery Deep and of several other deposits also have significant metalliferous content but at lower concentrations than that in the Atlantis II Deep, and thus they have not been of as much economic interest. The recovery of sediment located beneath 5,700 to 6,400 feet of water poses problems. But since most of these metalliferous deposits are fluid oozes, it is thought to be possible to pump them to the surface in much the same way as oil. There also are numerous proposals for drying and beneficiating (treating for smelting) these deposits after recovery.
Navigation
Navigation in the Red Sea is difficult. The unindented shorelines of the sea’s northern half provide few natural harbours, and in the southern half the growth of coral reefs has restricted the navigable channel and blocked some harbour facilities. At Bab el-Mandeb Strait, the channel is kept open to shipping by blasting and dredging. Atmospheric distortion (heat shimmer), sandstorms, and highly irregular water currents add to the navigational hazards.
Study and exploration
The Red Sea is one of the first large bodies of water mentioned in recorded history. It was important in early Egyptian maritime commerce (2000 bce) and was used as a water route to India by about 1000 bce. It is believed that it was reasonably well-charted by 1500 bce, because at that time Queen Hatshepsut of Egypt sailed its length. Later the Phoenicians explored its shores during their circumnavigatory exploration of Africa in about 600 bce. Shallow canals were dug between the Nile and the Red Sea before the 1st century ce but were later abandoned. A deep canal between the Mediterranean and Red seas was first suggested about 800 ce by the caliph Hārūn al-Rashīd, but it was not until 1869 that the French diplomat Ferdinand de Lesseps oversaw the completion of the Suez Canal connecting the two seas.
The Red Sea was subject to substantial scientific research in the 20th century, particularly since World War II. Notable cruises included those of the Swedish research vessel Albatross (1948) and the American Glomar Challenger (1972). In addition to studying the sea’s chemical and biological properties, researchers focused considerable attention on understanding its geologic structure. Much of the geologic study was in conjunction with oil exploration.
Additional Information
When my friends and family ask me what I am doing in my research, I respond that “I am investigating the winds and currents of the Red Sea in the Middle East.” Scary faces pop up. All they see are the winds of wars—the ever-present terrorist attacks, fighting, and killings in the region. “Are you crazy?” they say.
I get this same question (and sometimes the same reaction) from my oceanography colleagues. Since I began my postdoctoral research at Woods Hole Oceanographic Institution, working with WHOI physical oceanographers Amy Bower and Tom Farrar, I have learned two things: first, that few people realize how beautiful the Middle East is, and second, that the seas there have fascinating and unusual characteristics and far-reaching impacts on life in and around them. These seas furnish moisture for the arid Middle Eastern atmosphere and allowed great civilizations to flourish thousands of years ago around these seas.
For an oceanographer like myself, the Red Sea can be viewed as a mini-ocean, like a toy model ocean. Most of the oceanic features in a big ocean such as the Atlantic, we can also find there.
But the Red Sea also has its own curious characteristics that are not seen in other oceans. It is extremely warm—temperatures in its surface waters reach than 30° Celsius (86° Fahrenheit)—and water evaporates from it at a prodigious rate, making it extremely salty. Because of its narrow confines and constricted connection to the global ocean and because it is subject to seasonal flip-flopping wind patterns governed by the monsoons, it has odd circulation patterns. Its currents change in summer and winter.
The Red Sea is one of the few places on Earth that has what is known as a poleward-flowing eastern boundary current. Eastern boundary currents are so called because they hug the eastern coasts of continents. But all other such eastern boundary currents head south in the northern hemisphere. But the Red Sea Eastern Boundary Current, unlike all others, flows in the direction of the North Pole.
Unravelling the intricate tapestry that creates this rare eastern boundary current in the Red Sea was a goal of my postdoctoral research. But I have found that the Red Sea is far more mesmerizing and complex than I initially imagined. A variety of exotic threads are woven into the tapestry that produce the Red Sea’s unusual oceanographic phenomena: seasonal monsoons, desert sandstorms, wind jets through narrow mountain gaps, the Strait of Bab Al Mandeb that squeezes passage in and out of the sea—even locust swarms.
The politics of the nations surrounding the Red Sea are also complex and make it among the more difficult places to collect data. That explains why many Red Sea phenomena have remained unknown. But unexplored regions are the juiciest for scientists, because they are the ripest places to make new discoveries.
Gateway to the Red Sea
In the Red Sea, the water evaporates at one of the highest rates in the world. Like a bathtub in a steam room, you would have to add water from the tap to keep its water level stable.
The Red Sea compensates for the large water volume it loses each year through evaporation by importing water from the Gulf of Aden—through the narrow Strait of Bab Al Mandeb between Yemen on the Arabian Peninsula and Djibouti and Eritrea on the Horn of Africa.
The Strait of Bab Al Mandeb works as a gate. All waters in and out of the sea must pass through it. No other gates exist, making the Red Sea what is known as a semi-enclosed marginal sea.
In winter, incoming surface waters from the Gulf of Aden flow in a typical western boundary current, hugging the western side of the Red Sea along the coasts of Eritrea and Sudan. The current transports the waters northward. But in the central part of the Red Sea, this current veers sharply to the right. When it reaches the eastern side, it continues its convoluted journey to the north, but now it hugs the eastern side of the sea along coast of Saudi Arabia.
Here’s where the mystery deepens. The Red Sea Eastern Boundary Current exists only in winter. In summer, it’s not there. I wanted to find out how it forms, how it changes, and why it seasonally disappears.
Detectives and pirates
To unravel the complex tapestry that makes the Red Sea Eastern Boundary Current, I am like a CSI (Crime Scene Investigator) agent, sifting through as much data as I can get and putting them together to solve a mystery.
But it’s hard to obtain data from the Red Sea. Its narrow confines mean that its waters are restricted by countries around it that are often in conflict. It’s hard for researchers to get permission to enter them.
In addition, many waters in and enroute to the Red Sea have been beset by piracy. In the spring of 2018, I was aboard of the NOAA ship Ronald H. Brown in the Arabian Sea. It was the first time in more than a decade that the U.S. Navy allowed an American research vessel to go to the Arabian Sea. We were allowed to go only on the eastern side, and we couldn’t go anywhere beyond 17.5° N, because it wasn’t safe. On board, we conducted many safety drills, learning how to hide from pirates.
The Red Sea is also hard for satellites. Its width is small compared with the spatial resolution of most oceanographic satellites. Altimeter and wind satellites have a spatial resolution around 30 to 50 kilometers. The maximum Red Sea width is only 355 kilometers. In addition, satellites can give us information about what happens at the sea surface, but they can’t reveal the mixing and other processes that go on beneath the surface.
That’s why my research was kidnapped and carried off into an unanticipated direction, and my focus shifted from sea to air.
A plague of locusts
In the Red Sea, evaporation is a critical factor driving how the sea operates, and to determine how much water evaporates, we need to know about the winds. Why? Because evaporation rates depend on the winds. If the winds are stronger, the evaporation is stronger; if the winds are weaker, evaporation is weaker.
To complicate the situation a bit more, evaporation depends not only on the strength of the winds but where the winds are coming from. If the winds are coming over the sea, the air humidity in the winds will be higher, and evaporation will be lower; if the winds are coming from the desert, the air will be dry, and evaporation will be higher.
So, to unravel the Eastern Boundary Current, we needed to have a pretty good picture of how the winds blow in winter. When I started my postdoctoral research, I was really surprised to see that this important factor—the wind variability of the Red Sea—wasn’t well-known, even though interest on it goes way back!
Pioneering studies about winds in the Red Sea were motivated by a desire to determine the northward migration of desert locust swarms that invade areas and voraciously consume all the vegetation in it. This plague has been described in the Old Testament of the Bible and has tormented countries bordering the Red Sea since times immemorial.
The locusts breed along the shores of the Red Sea. Summer monsoon rains spur locust eggs to hatch. When enough rains fall to create plenty of water and vegetation for food, large numbers of locusts hatch and form swarms. Winds determine where the swarms will be carried off to infest neighboring regions.
Lining both sides of the Red Sea are tall mountains that create a kind of tunnel, so that winds blow predominantly along, not across, the Red Sea. In summer, the winds blow from north to south. In winter, however, the monsoon flips the wind direction in the southern part of the Red Sea, andtwo opposing airstreams meet at some point in the central Red Sea called the Red Sea Convergence Zone. It acts as a conduit for migrating locust swarms, and where it is positioned determines where the swarms go.
Mountain-gap wind jets
The mountains along Red Sea coasts affect the winds in another way. The mountains aren’t entirely compact; there are several gaps in them. The tunnel surrounding the Red Sea has a few holes in both sides. Sometimes the winds blow through one of these holes and cross the tunnel. These are the mountain-gap wind jets.
The mountain-gap winds in summer blow from Africa to Saudi Arabia through the Tokar Gap near the Sudanese coast. In winter, the mountain-gap winds blow in the opposite direction, from Saudi Arabia to Africa, through many nameless gaps in the northern part of the Red Sea.
These jets stir up frequent sandstorms carrying sand and dirt from surrounding deserts into the Red Sea. The sandstorms carry fertilizing nutrients that promote life in the Red Sea. The sands also block incoming sunlight and cool the sea surface.
But do these overlooked jets also affect the Red Sea in other ways?
Blasts of dry air
We decided to put together lots of different data to find out the fundamental characteristics of these mountain-gap jet events. Our data came from satellites and from a heavily instrumented mooring that measured winds and humidity in the air and temperatures and salinity in the sea below. WHOI maintained the mooring for two-years in the Red Sea when it collaborated with King Abdullah University of Science and Technology.
The satellite images revealed that these events weren’t rare. We learned that in most winters, there are typically two to three events in December and January in which the winds blow west across the northern part of the Red Sea. In satellite images, they are impressive and beautiful.
The mountain-gap events typically last three to eight days. We observed large year-to-year differences, with an increasing number of events in the last decade.
We discovered that the wintertime mountain-gap wind events blast the Red Sea with dry air. They are like the cold-air outbreaks that hit the U.S. East Coast in winter. Of course, for the Red Sea, it would be better to name them as dry-air outbreaks!
The dry-air blasts abruptly increase evaporation on the surface of the sea. This colossal evaporation removes a large amount of heat and water vapor out of the sea, leaving it much saltier. The mountain-gap winds also stir up deeper, cooler waters that mix with surface waters.
The waters become saltier and colder. This disrupts the Eastern Boundary Current. During most wind-jet events, it seems to fade away.
Connecting the oceans
We are still looking for answers about how the Eastern Boundary Current forms and why it flows north. But we have learned much about the wind-jet events that cause it to disappear periodically in winter.
The large-scale evaporation from these wind-jet events may also drive waters in the northern Red Sea to become cooler, saltier, and dense enough to sink the depths and flow all the way south and back out of the Strait of Bab Al Mandeb.
These salty Red Sea waters escape to the Gulf Aden, where they start a long journey through the Indian Ocean. They cross the Equator. Some may travel into the Atlantic Ocean. Some may flow toward Western Australia.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2693 2026-02-10 18:43:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2492) Himalayan Mountains
Gist
The Himalayas are a massive mountain range in Asia, forming a crescent-shaped arc that separates the Indian subcontinent from the Tibetan Plateau and stretches across five countries: India, Nepal, Bhutan, China (Tibet), and Pakistan. Known as the "abode of snow," they contain Earth's highest peaks, including Mount Everest, and influence the climate and rivers of South Asia.
The Himalayas consists of four parallel mountain ranges from south to north: the Sivalik Hills on the south; the Lower Himalayan Range; the Great Himalayas, which is the highest and central range; and the Tibetan Himalayas on the north. The Karakoram are generally considered separate from the Himalayas.
Summary
The Himalayas, or Himalaya, is a mountain range in Asia separating the plains of the Indian subcontinent from the Tibetan Plateau. The range has some of the Earth's highest peaks, including the highest, Mount Everest. More than 100 peaks exceeding elevations of 7,200 m (23,600 ft) above sea level lie in the Himalayas.
The Himalayas abut on or cross territories of five countries: Nepal, India, China, Bhutan and Pakistan. The sovereignty of the range in the Kashmir region is disputed among India, Pakistan, and China. The Himalayan range is bordered on the northwest by the Karakoram and Hindu Kush ranges, on the north by the Tibetan Plateau, and on the south by the Indo-Gangetic Plain. Some of the world's major rivers, the Indus, the Ganges, and the Tsangpo–Brahmaputra, rise in the vicinity of the Himalayas, and their combined drainage basin is home to some 600 million people; 53 million people live in the Himalayas. The Himalayas have profoundly shaped the cultures of South Asia and Tibet. Many Himalayan peaks are sacred in Hinduism and Buddhism. The summits of several—Kangchenjunga (from the Indian side), Gangkhar Puensum, Machapuchare, Nanda Devi, and Kailash in the Tibetan Transhimalaya—are off-limits to climbers.
The Himalayas were uplifted after the collision of the Indian tectonic plate with the Eurasian plate, specifically, by the folding, or nappe-formation of the uppermost Indian crust, even as a lower layer continued to push on into Tibet and add thickness to its plateau; the still lower crust, along with the mantle, however, subducted under Eurasia. The Himalayan mountain range runs west-northwest to east-southeast in an arc 2,400 km (1,500 mi) long. Its western anchor, Nanga Parbat, lies just south of the northernmost bend of the Indus river. Its eastern anchor, Namcha Barwa, lies immediately west of the great bend of the Yarlung Tsangpo River. The Indus-Yarlung suture zone, along which the headwaters of these two rivers flow, separates the Himalayas from the Tibetan plateau; the rivers also separate the Himalayas from the Karakorams, the Hindu Kush, and the Transhimalaya. The range varies in width from 350 km (220 mi) in the west to 151 km (94 mi) in the east.
Details
Himalayas are great mountain system of Asia forming a barrier between the Plateau of Tibet to the north and the alluvial plains of the Indian subcontinent to the south. The Himalayas include the highest mountains in the world, with more than 110 peaks rising to elevations of 24,000 feet (7,300 meters) or more above sea level. One of those peaks is Mount Everest (Tibetan: Chomolungma; Chinese: Qomolangma Feng; Nepali: Sagarmatha), the world’s highest, with an elevation of 29,032 feet (8,849 meters).
For thousands of years the Himalayas have held a profound significance for the peoples of South Asia, as their literature, mythologies, and religions reflect. Since ancient times the vast glaciated heights have attracted the attention of the pilgrim mountaineers of India, who coined the Sanskrit name Himalaya—from hima (“snow”) and alaya (“abode”)—for that great mountain system. In contemporary times the Himalayas have offered the greatest attraction and the greatest challenge to mountaineers throughout the world.
The ranges, which form the northern border of the Indian subcontinent and an almost impassable barrier between it and the lands to the north, are part of a vast mountain belt that stretches halfway around the world from North Africa to the Pacific Ocean coast of Southeast Asia. The Himalayas themselves stretch uninterruptedly for about 1,550 miles (2,500 km) from west to east between Nanga Parbat (26,660 feet [8,126 meters]), in the Pakistani-administered portion of the Kashmir region, and Namjagbarwa (Namcha Barwa) Peak (25,445 feet [7,756 meters]), in the Tibet Autonomous Region of China. Between those western and eastern extremities lie the two Himalayan countries of Nepal and Bhutan. The Himalayas are bordered to the northwest by the mountain ranges of the Hindu Kush and the Karakoram and to the north by the high and vast Plateau of Tibet. The width of the Himalayas from south to north varies between 125 and 250 miles (200 and 400 km). Their total area amounts to about 230,000 square miles (595,000 square km).
Though India, Nepal, and Bhutan have sovereignty over most of the Himalayas, Pakistan and China also occupy parts of them. In the disputed Kashmir region, Pakistan has administrative control of some 32,400 square miles (83,900 square km) of the range lying north and west of the “line of control” established between India and Pakistan in 1972. China administers some 14,000 square miles (36,000 square km) in the Ladakh region and has claimed territory at the eastern end of the Himalayas within the Indian state of Arunachal Pradesh. Those disputes accentuate the boundary problems faced by India and its neighbors in the Himalayan region.
Physical features
The most characteristic features of the Himalayas are their soaring heights, steep-sided jagged peaks, valley and alpine glaciers often of stupendous size, topography deeply cut by erosion, seemingly unfathomable river gorges, complex geologic structure, and series of elevational belts (or zones) that display different ecological associations of flora, fauna, and climate. Viewed from the south, the Himalayas appear as a gigantic crescent with the main axis rising above the snow line, where snowfields, alpine glaciers, and avalanches all feed lower-valley glaciers that in turn constitute the sources of most of the Himalayan rivers. The greater part of the Himalayas, however, lies below the snow line. The mountain-building process that created the range is still active. As the bedrock is lifted, considerable stream erosion and gigantic landslides occur.
The Himalayan ranges can be grouped into four parallel longitudinal mountain belts of varying width, each having distinct physiographic features and its own geologic history. They are designated, from south to north, as the Outer, or Sub-, Himalayas (also called the Siwalik Range); the Lesser, or Lower, Himalayas; the Great Himalaya Range (Great Himalayas); and the Tethys, or Tibetan, Himalayas. Farther north lie the Trans-Himalayas in Tibet proper. From west to east the Himalayas are divided broadly into three mountainous regions: western, central, and eastern.
Geologic history
Over the past 65 million years, powerful global plate-tectonic forces have moved Earth’s crust to form the band of Eurasian mountain ranges—including the Himalayas—that stretch from the Alps to the mountains of Southeast Asia.
During the Jurassic Period (about 201 to 145 million years ago), a deep crustal downwarp—the Tethys Ocean—bordered the entire southern fringe of Eurasia, then excluding the Arabian Peninsula and the Indian subcontinent. About 180 million years ago, the old supercontinent of Gondwana (or Gondwanaland) began to break up. One of Gondwana’s fragments, the lithospheric plate that included the Indian subcontinent, pursued a northward collision course toward the Eurasian Plate during the ensuing 130 to 140 million years. The Indian-Australian Plate gradually confined the Tethys trench within a giant pincer between itself and the Eurasian Plate. As the Tethys trench narrowed, increasing compressive forces bent the layers of rock beneath it and created interlacing faults in its marine sediments. Masses of granites and basalts intruded from the depth of the mantle into that weakened sedimentary crust. Between about 40 and 50 million years ago, the Indian subcontinent finally collided with Eurasia. The plate containing India was sheared downward, or subducted, beneath the Tethys trench at an ever-increasing pitch.
During the next 30 million years, shallow parts of the Tethys Ocean gradually drained as its sea bottom was pushed up by the plunging Indian-Australian Plate; that action formed the Plateau of Tibet. On the plateau’s southern edge, marginal mountains—the Trans-Himalayan ranges of today—became the region’s first major watershed and rose high enough to become a climatic barrier. As heavier rains fell on the steepening southern slopes, the major southern rivers eroded northward toward the headwaters with increasing force along old transverse faults and captured the streams flowing onto the plateau, thus laying the foundation for the drainage patterns for a large portion of Asia. To the south the northern reaches of the Arabian Sea and the Bay of Bengal rapidly filled with debris carried down by the ancestral Indus, Ganges (Ganga), and Brahmaputra rivers. The extensive erosion and deposition continue even now as those rivers carry immense quantities of material every day.
Finally, some 20 million years ago, during the early Miocene Epoch, the tempo of the crunching union between the two plates increased sharply, and Himalayan mountain building began in earnest. As the Indian subcontinental plate continued to plunge beneath the former Tethys trench, the topmost layers of old Gondwana metamorphic rocks peeled back over themselves for a long horizontal distance to the south, forming nappes. Wave after wave of nappes thrust southward over the Indian landmass for as far as 60 miles (about 100 km). Each new nappe consisted of Gondwana rocks older than the last. In time those nappes became folded, contracting the former trench by some 250 to 500 horizontal miles (400 to 800 km). All the while, downcutting rivers matched the rate of uplift, carrying vast amounts of eroded material from the rising Himalayas to the plains where it was dumped by the Indus, Ganges, and Brahmaputra rivers. The weight of that sediment created depressions, which in turn could hold more sediment. In some places the alluvium beneath the Indo-Gangetic Plain now exceeds 25,000 feet (7,600 meters) in depth.
Probably only within the past 600,000 years, during the Pleistocene Epoch (roughly 2,600,000 to 11,700 years ago), did the Himalayas become the highest mountains on Earth. If strong horizontal thrusting characterized the Miocene and the succeeding Pliocene Epoch (about 23 to 2.6 million years ago), intense uplift epitomized the Pleistocene. Along the core zone of the northernmost nappes—and just beyond—crystalline rocks containing new gneiss and granite intrusions emerged to produce the staggering crests seen today. On a few peaks, such as Mount Everest, the crystalline rocks carried old fossil-bearing Tethys sediments from the north piggyback to the summits.
Once the Great Himalayas had risen high enough, they became a climatic barrier: the marginal mountains to the north were deprived of rain and became as parched as the Plateau of Tibet. In contrast, on the wet southern flanks the rivers surged with such erosive energy that they forced the crest line to migrate slowly northward.
Simultaneously, the great transverse rivers breaching the Himalayas continued their downcutting in pace with the uplift. Changes in the landscape, however, compelled all but those major rivers to reroute their lower courses because, as the northern crests rose, so also did the southern edge of the extensive nappes. The formations of the Siwalik Series were overthrust and folded, and in between the Lesser Himalayas downwarped to shape the midlands. Now barred from flowing due south, most minor rivers ran east or west through structural weaknesses in the midlands until they could break through the new southern barrier or join a major torrent.
In some valleys, such as the Vale of Kashmir and the Kathmandu Valley of Nepal, lakes formed temporarily and then filled with Pleistocene deposits. After drying up some 200,000 years ago, the Kathmandu Valley rose at least 650 feet (200 meters), an indication of localized uplift within the Lesser Himalayas.
Physiography of the Himalayas
The Outer Himalayas comprise flat-floored structural valleys and the Siwalik Range, which borders the Himalayan mountain system to the south. Except for small gaps in the east, the Siwaliks run for the entire length of the Himalayas, with a maximum width of 62 miles (100 km) in the northern Indian state of Himachal Pradesh. In general, the 900-foot (275-meter) elevation contour line marks their southern boundary; they rise an additional 2,500 feet (760 meters) to the north. The main Siwalik Range has steeper southern slopes facing the Indian plains and descends gently northward to flat-floored basins, called duns. The best-known of those is the Dehra Dun, in southern Uttarakhand state, just north of the border with northwestern Uttar Pradesh state.
To the north the Siwalik Range abuts a massive mountainous tract, the Lesser Himalayas. In that range, 50 miles (80 km) in width, mountains rising to 15,000 feet (4,500 meters) and valleys with elevations of 3,000 feet (900 meters) run in varying directions. Neighboring summits share similar elevations, creating the appearance of a highly dissected plateau. The three principal ranges of the Lesser Himalayas—the Nag Tibba, the Dhaola Dhar, and the Pir Panjal—have branched off from the Great Himalaya Range lying farther north. The Nag Tibba, the most easterly of the three ranges, reaches an elevation of some 26,800 feet (8,200 meters) near its eastern end, in Nepal, and forms the watershed between the Ganges and Yamuna rivers in Uttarakhand.
To the west is the picturesque Vale of Kashmir, in Jammu and Kashmir union territory (the Indian-administered portion of Kashmir). A structural basin (i.e., an elliptical basin in which the rock strata are inclined toward a central point), the vale forms an important section of the Lesser Himalayas. It extends from southeast to northwest for 100 miles (160 km), with a width of 50 miles (80 km), and has an average elevation of 5,100 feet (1,600 meters). The basin is traversed by the meandering Jhelum River, which runs through Wular Lake, a large freshwater lake in Jammu and Kashmir northwest of Srinagar.
The backbone of the entire mountain system is the Great Himalaya Range, rising into the zone of perpetual snow. The range reaches its maximum height in Nepal; among its peaks are 10 of the 13 highest in the world, each of which exceeds 26,250 feet (8,000 meters) in elevation. From west to east those peaks are Nanga Parbat, Dhaulagiri 1, Annapurna 1, Manaslu 1, Xixabangma (Gosainthan), Cho Oyu, Mount Everest, Lhotse, Makalu 1, and Kanchenjunga 1.
The range trends northwest-southeast from Jammu and Kashmir to Sikkim, an old Himalayan kingdom that is now a state of India. East of Sikkim it runs east-west for another 260 miles (420 km) through Bhutan and the eastern part of Arunachal Pradesh as far as the peak of Kangto (23,260 feet [7090 meters]) and finally bends northeast, terminating at Namcha Barwa.
There is no sharp boundary between the Great Himalayas and the ranges, plateaus, and basins lying to the north of the Great Himalayas. Those are generally grouped together under the names of the Tethys, or Tibetan, Himalayas and the Trans-Himalayas, which extend far northward into Tibet. In Kashmir and in the Indian state of Himachal Pradesh, the Tethys are at their widest, forming the Spiti Basin and the Zanskar Range.
Drainage of the Himalayas
The Himalayas are drained by 19 major rivers, of which the Indus and the Brahmaputra are the largest, each having catchment basins in the mountains of about 100,000 square miles (260,000 square km) in extent. Five of the 19 rivers, with a total catchment area of about 51,000 square miles (132,000 square km), belong to the Indus system—the Jhelum, the Chenab, the Ravi, the Beas, and the Sutlej—and collectively define the vast region divided between Punjab state in India and Punjab province in Pakistan. Of the remaining rivers, nine belong to the Ganges system—the Ganges, Yamuna, Ramganga, Kali (Kali Gandak), Karnali, Rapti, Gandak, Baghmati, and Kosi rivers—draining roughly 84,000 square miles (218,000 square km) in the mountains, and three belong to the Brahmaputra system—the Tista, the Raidak, and the Manas—draining another 71,000 square miles (184,000 square km) in the Himalayas.
The major Himalayan rivers rise north of the mountain ranges and flow through deep gorges that generally reflect some geologic structural control, such as a fault line. The rivers of the Indus system as a rule follow northwesterly courses, whereas those of the Ganges-Brahmaputra systems generally take easterly courses while flowing through the mountain region.
To the north of India, the Karakoram Range, with the Hindu Kush range on the west and the Ladakh Range on the east, forms the great water divide, shutting off the Indus system from the rivers of Central Asia. The counterpart of that divide on the east is formed by the Kailas Range and its eastward continuation, the Nyainqêntanglha (Nyenchen Tangla) Mountains, which prevent the Brahmaputra from draining the area to the north. South of that divide, the Brahmaputra flows to the east for about 900 miles (1,450 km) before cutting across the Great Himalaya Range in a deep transverse gorge, although many of its Tibetan tributaries flow in an opposite direction, as the Brahmaputra may once have done.
The Great Himalayas, which normally would form the main water divide throughout their entire length, function as such only in limited areas. That situation exists because the major Himalayan rivers, such as the Indus, the Brahmaputra, the Sutlej, and at least two headwaters of the Ganges—the Alaknanda and the Bhagirathi—are probably older than the mountains they traverse. It is believed that the Himalayas were uplifted so slowly that the old rivers had no difficulty in continuing to flow through their channels and, with the rise of the Himalayas, acquired an even greater momentum, which enabled them to cut their valleys more rapidly. The elevation of the Himalayas and the deepening of the valleys thus proceeded simultaneously. As a result, the mountain ranges emerged with a completely developed river system cut into deep transverse gorges that range in depth from 5,000 to 16,000 feet (1,500 to 5,000 meters) and in width from 6 to 30 miles (10 to 50 km). The earlier origin of the drainage system explains the peculiarity that the major rivers drain not only the southern slopes of the Great Himalayas but, to a large extent, its northern slopes as well, the water divide being north of the crest line.
The role of the Great Himalaya Range as a watershed, nevertheless, can be seen between the Sutlej and Indus valleys for 360 miles (580 km); the drainage of the northern slopes is carried by the north-flowing Zanskar and Dras rivers, which drain into the Indus. Glaciers also play an important role in draining the higher elevations and in feeding the Himalayan rivers. Several glaciers occur in Uttarakhand, of which the largest, the Gangotri, is 20 miles (32 km) long and is one of the sources of the Ganges. The Khumbu Glacier drains the Everest region in Nepal and is one of the most popular routes for the ascent of the mountain. The rate of movement of the Himalayan glaciers varies considerably; in the neighboring Karakoram Range, for example, the Baltoro Glacier moves about 6 feet (2 meters) per day, while others, such as the Khumbu, move only about 1 foot (30 cm) daily. Most of the Himalayan glaciers are in retreat, at least in part because of climate change.
Soils
The north-facing slopes generally have a fairly thick soil cover, supporting dense forests at lower elevations and grasses higher up. The forest soils are dark brown in color and silt loam in texture; they are ideally suited for growing fruit trees. The mountain meadow soils are well developed but vary in thickness and in their chemical properties. Some of the wet deep upland soils of that type in the eastern Himalayas—for example, in the Darjeeling (Darjiling) Hills and in the Assam valley—have a high humus content that is good for growing tea. Podzolic soils (infertile acidic forest soils) occur in a belt some 400 miles (640 km) long in the valleys of the Indus and its tributary the Shyok River, to the north of the Great Himalaya Range, and in patches in Himachal Pradesh. Farther east, saline soils occur in the dry high plains of the Ladakh region. Of the soils that are not restricted to any particular area, alluvial soils (deposited by running water) are the most productive, though they occur in limited areas, such as the Vale of Kashmir, the Dehra Dun, and the high terraces flanking the Himalayan valleys. Lithosols, consisting of imperfectly weathered rock fragments that are deficient in humus content, cover many large areas at high elevations and are the least-productive soils.
Climate of the Himalayas
The Himalayas, as a great climatic divide affecting large systems of air and water circulation, help determine meteorological conditions in the Indian subcontinent to the south and in the Central Asian highlands to the north. By virtue of its location and stupendous height, the Great Himalaya Range obstructs the passage of cold continental air from the north into India in winter and also forces the southwesterly monsoon (rain-bearing) winds to give up most of their moisture before crossing the range northward. The result is heavy precipitation (both rain and snow) on the Indian side but arid conditions in Tibet. The average annual rainfall on the south slopes varies between 60 inches (1,530 mm) at Shimla, Himachal Pradesh, and Mussoorie, Uttarakhand, in the western Himalayas and 120 inches (3,050 mm) at Darjeeling, West Bengal state, in the eastern Himalayas. North of the Great Himalayas, at places such as Skardu, Gilgit, and Leh in the Ladakh portion of the Indus valley, only 3 to 6 inches (75 to 150 mm) of precipitation occur.
Local relief and location determine climatic variation not only in different parts of the Himalayas but even on different slopes of the same range. Because of its favorable location on top of the Mussoorie Range facing the Dehra Dun, the town of Mussoorie, for example, at an elevation of about 6,100 feet (1,900 meters), receives 92 inches (2,335 mm) of precipitation annually, compared with 62 inches (1,575 mm) in the town of Shimla, which lies some 90 miles (145 km) to the northwest behind a series of ridges reaching 6,600 feet (2,000 meters). The eastern Himalayas, which are at a lower latitude than the western Himalayas, are relatively warmer. The average minimum temperature for the month of May, recorded in Darjeeling at an elevation of 6,380 feet (1,945 meters), is 52 °F (11 °C). In the same month, at an elevation of 16,500 feet (5,000 meters) in the neighborhood of Mount Everest, the minimum temperature is about 17 °F (−8 °C); at 19,500 feet (6,000 meters) it falls to −8 °F (−22 °C), the lowest minimum having been −21 °F (−29 °C); during the day, in areas sheltered from strong winds that often blow at more than 100 miles (160 km) per hour, the sun is often pleasantly warm, even at high elevations.
There are two periods of precipitation: the moderate amounts brought by winter storms and the heavier precipitation of summer, with its southwesterly monsoon winds. During winter, low-pressure weather systems advance into the Himalayas from the west and cause heavy snowfall. Within the regions where western disturbances are felt, condensation occurs in upper air levels, and, as a result, precipitation is much greater over the high mountains. During that season snow accumulates around the Himalayan high peaks, and precipitation is greater in the west than the east. In January, for example, Mussoorie in the west receives almost 3 inches (75 mm), whereas Darjeeling to the east receives less than 1 inch (25 mm). By the end of May the meteorological conditions have reversed. Southwesterly monsoon currents channel moist air toward the eastern Himalayas, where the moisture rising over the steep terrain cools and condenses to fall as rain or snow; in June, therefore, Darjeeling receives about 24 inches (600 mm) and Mussoorie less than 8 inches (200 mm). The rain and snow cease in September, after which the finest weather in the Himalayas prevails until the beginning of winter in December.
Plant life
Himalayan vegetation can be broadly classified into four types—tropical, subtropical, temperate, and alpine—each of which prevails in a zone determined mainly by elevation and precipitation. Local differences in relief and climate, as well as exposure to sunlight and wind, cause considerable variation in the species present within each zone. Tropical evergreen rainforest is confined to the humid foothills of the eastern and central Himalayas. The evergreen dipterocarps—a group of timber- and resin-producing trees—are common; their different species grow on different soils and on hill slopes of varying steepness. Ceylon ironwood (Mesua ferrea) is found on porous soils at elevations between 600 and 2,400 feet (180 and 720 meters); bamboos grow on steep slopes; oaks (genus Quercus) and Indian horse chestnuts (Aesculus indica) grow on the lithosol (shallow soils consisting of imperfectly weathered rock fragments), covering sandstones from Arunachal Pradesh westward to central Nepal at elevations from 3,600 to 5,700 feet (1,100 to 1,700 meters). Alder trees (genus Alnus) are found along the watercourses on the steeper slopes. At higher elevations those species give way to mountain forests in which the typical evergreen is the Himalayan screw pine (Pandanus furcatus). Besides those trees, some 4,000 species of flowering plants, of which 20 are palms, are estimated to occur in the eastern Himalayas.
With decreasing precipitation and increasing elevation westward, the rainforests give way to tropical deciduous forests, where the valuable timber tree sal (Shorea robusta) is the dominant species. Wet sal forests thrive on high plateaus at elevations of about 3,000 feet (900 meters), while dry sal forests prevail higher up, at 4,500 feet (1,400 meters). Farther west, steppe forest (i.e., expanse of grassland dotted with trees), steppe, subtropical thorn steppe, and subtropical semidesert vegetation occur successively. Temperate mixed forests extend from about 4,500 to roughly 11,000 feet (1,400 to 3,400 meters) and contain conifers and broad-leaved temperate trees. Evergreen forests of oaks and conifers have their westernmost outpost on the hills above Murree, some 30 miles (50 km) northwest of Rawalpindi, in Pakistan; those forests are typical of the Lesser Himalayas, being conspicuous on the outer slopes of the Pir Panjal, in Jammu and Kashmir union territory. Chir pine (Pinus roxburghii) is the dominant species at elevations from 2,700 to 5,400 feet (800 to 1,600 meters). In the inner valleys that species may occur even up to 6,300 feet (1,900 meters). Deodar cedar (Cedrus deodara), a highly valued endemic species, grows mainly in the western part of the range. Stands of that species occur between 6,300 and 9,000 feet (1,900 and 2,700 meters) and tend to grow at still higher elevations in the upper valleys of the Sutlej and Ganges rivers. Of the other conifers, blue pine (Pinus wallichiana) and morinda spruce (Picea smithiana) first appear between about 7,300 and 10,000 feet (2,200 and 3,000 meters).
The alpine zone begins above the tree line, between elevations of 10,500 and 11,700 feet (3,200 and 3,600 meters), and extends up to about 13,700 feet (4,200 meters) in the western Himalayas and 14,600 feet (4,500 meters) in the eastern Himalayas. In that zone can be found all the wet and moist alpine vegetation. Juniper (genus Juniperus) is widespread, especially on sunny sites, steep and rocky slopes, and drier areas. Rhododendron occurs everywhere but is more abundant in the wetter parts of the eastern Himalayas, where it grows in all sizes from trees to low shrubs. Mosses and lichens grow in shaded areas at lower levels in the alpine zone where the humidity is high; flowering plants are found at high elevations.
Animal life
The fauna of the eastern Himalayas is similar to that of the southern Chinese and Southeast Asian region. Many of those species are primarily found in tropical forests and are only secondarily adapted to the subtropical, mountain, and temperate conditions prevailing at higher elevations and in the drier western areas. The animal life of the western Himalayas, however, has more affinities with that of the Mediterranean, Ethiopian, and Turkmenian regions. The past presence in the region of some African animals, such as giraffes and the hippopotamuses, can be inferred from fossil remains in deposits found in the Siwalik Range. The animal life at elevations above the tree line consists almost exclusively of cold-tolerant endemic species that evolved from the wildlife of the steppes after the uplift of the Himalayas. Elephants and rhinoceroses are restricted to parts of the forested Tarai region—moist or marshy areas, now largely drained—at the base of the low hills in southern Nepal. Asiatic black bears, clouded leopards, langurs (a long-tailed Asian monkey), and Himalayan goat antelopes (e.g., the tahr) are some of the denizens of the Himalayan forests. The Indian rhinoceros was once abundant throughout the foothill zone of the Himalayas but is now endangered, as is the musk deer; both species are dwindling, and few live, other than those in a handful of reserves set up to protect them. The Kashmir stag, or hangul, is near extinction.
In remote sections of the Himalayas, at higher elevations, snow leopards, brown bears, lesser pandas, and Tibetan yaks have limited populations. The yak has been domesticated and is used as a beast of burden in Ladakh. Above the tree line the most numerous animals, however, are diverse types of insects, spiders, and mites, which are the only animal forms that can live as high up as 20,700 feet (6,300 meters).
Fish of the genus Glyptothorax live in most of the Himalayan streams, and the Himalayan water shrew inhabits stream banks. Lizards of the genus Japalura are widely distributed. Typhlops, a genus of blind snake, is common in the eastern Himalayas. The butterflies of the Himalayas are extremely varied and beautiful, especially those in the genus Troides.
Bird life in the Himalayas is equally rich but is more abundant in the east than in the west. In Nepal alone almost 800 species have been observed. Among some of the common Himalayan birds are different species of magpies (including the black-rumped, the blue, and the racket-tailed), titmice, choughs (related to the jackdaw), whistling thrushes, and redstarts. A few strong fliers, such as the lammergeier (bearded vulture), the black-eared kite, and the Himalayan griffon (an Old World vulture), also can be seen. Snow partridges and Cornish choughs are found at elevations of 18,600 feet (5,700 meters).
Of the four principal language families in the Indian subcontinent—Indo-European, Tibeto-Burman, Austroasiatic, and Dravidian—the first two are well represented in the Himalayas. In ancient times, peoples speaking languages from both families mixed in varying proportions in different areas. Their distribution is the result of a long history of penetrations by Central Asian and Iranian groups from the west, Indian peoples from the south, and Asian peoples from the east and north. In Nepal, which constitutes the middle third of the Himalayas, those groups overlapped and intermingled. The penetrations of the lower Himalayas were instrumental to the migrations into and through the river-plain passageways of South Asia.
Generally speaking, the Great Himalayas and the Tethys Himalayas are inhabited by Tibetans and peoples speaking other Tibeto-Burman languages, while the Lesser Himalayas are the home of Indo-European language speakers. Among the latter are the Kashmiri people of the Vale of Kashmir and the Gaddi and Gujari, who live in the hilly areas of the Lesser Himalayas. Traditionally, the Gaddi are a hill people; they possess large flocks of sheep and herds of goats and go down with them from their snowy abode in the Outer Himalayas only in winter, returning again to the highest pastures in June. The Gujari are traditionally a migrating pastoral people who live off their herds of sheep, goats, and a few cattle, for which they seek pasture at various elevations.
The Champa, Ladakhi, Balti, and Dard peoples live to the north of the Great Himalaya Range in the Kashmir Himalayas. The Dard speak Indo-European languages, while the others are Tibeto-Burman speakers. The Champa traditionally lead a nomadic pastoral life in the upper Indus valley. The Ladakhi have settled on terraces and alluvial fans that flank the Indus in the northeastern Kashmir region. The Balti have spread farther down the Indus valley and have adopted Islam.
Other Indo-European speakers are the Kanet in Himachal Pradesh and the Khasi in Uttarakhand. In Himachal Pradesh most people in the districts of Kalpa and Lahul-Spiti are the descendants of migrants from Tibet who speak Tibeto-Burman languages.
In Nepal the Pahari, speaking Indo-European languages, constitute the majority of the population, although large groups of Tibeto-Burman speakers are found throughout the country. They include the Newar, the Tamang, the Gurung, the Magar, the Sherpa and other peoples related to the Bhutia, and the Kirat. The Kirat were the earliest inhabitants of the Kathmandu Valley. The Newar are also one of the earliest groups in Nepal. The Tamang inhabit the high valleys to the northwest, north, and east of Kathmandu Valley. The Gurung live on the southern slopes of the Annapurna massif, pasturing their cattle as high as 12,000 feet (3,700 meters). The Magar inhabit western Nepal but migrate seasonally to other parts of the country. The Sherpa, who live to the south of Mount Everest, are famed mountaineers.
For some 200 years the Sikkim region (now a state in India) and the kingdom of Bhutan have been safety valves for the absorption of the excess population of eastern Nepal. More Sherpa now live in the Darjeeling area than in the Mount Everest homeland. At present the Pahari constitute the majority who come from Nepal in both Sikkim and Bhutan. Thus, the people of Sikkim belong to three distinct ethnic groups—the Lepcha, the Bhutia, and the Pahari. Generally speaking, the Nepalese and the Lepcha live in western Bhutan and the Bhutia of Tibetan origin in eastern Bhutan.
Arunachal Pradesh is the homeland of several groups—the Abor or Adi, the Aka, the Apa Tani, the Dafla, the Khampti, the Khowa, the Mishmi, the Momba, the Miri, and the Singpho. Linguistically, they are Tibeto-Burman. Each group has its homeland in a distinct river valley, and all practice shifting cultivation (i.e., they grow crops on a different tract of land each year).
Economy of the Himalayas:
Resources
Economic conditions in the Himalayas partly depend on the limited resources available in different parts of that vast region of varied ecological zones. The principal activity is animal husbandry, but forestry, trade, and tourism are also important. The Himalayas abound in economic resources. Those include pockets of rich arable land, extensive grasslands and forests, workable mineral deposits, easy-to-harness waterpower, and great natural beauty. The most productive arable lands in the western Himalayas are in the Vale of Kashmir, the Kangra valley, the Sutlej River basin, and the terraces flanking the Ganges and Yamuna rivers in Uttarakhand; those areas produce rice, corn (maize), wheat, and millet. In the central Himalayas in Nepal, two-thirds of the arable land is in the foothills and on the adjacent plains; that land yields most of the total rice production of the country. The region also produces large crops of corn, wheat, potatoes, and sugarcane.
Most of the fruit orchards of the Himalayas lie in the Vale of Kashmir and in the Kullu valley of Himachal Pradesh. Fruits such as apples, peaches, pears, and cherries—for which there is a great demand in the cities of India—are grown extensively. On the shores of Dal Lake in Kashmir, there are rich vineyards that produce grapes used to make wine and brandy. On the hills surrounding the Vale of Kashmir grow walnut and almond trees. Bhutan also has fruit orchards and exports oranges to India.
Tea is grown in plantations mainly on the hills and on the plain at the foot of the mountains in the Darjeeling district. Plantations also produce limited amounts of tea in the Kangra valley. Plantations of the spice cardamom are to be found in Sikkim, Bhutan, and the Darjeeling Hills. Medicinal herbs are grown on plantations in areas of Uttarakhand.
Transhumance (the seasonal migration of livestock) is widely practiced in the Himalayan pastures. Sheep, goats, and yaks are raised on the rough grazing lands available. During summer they graze on the pastures at higher elevations, but when the weather turns cold, shepherds migrate with their flocks to lower elevations.
The explosive population growth that has occurred in the Himalayas and elsewhere in the Indian subcontinent since the 1940s has placed great stress on the forests in many areas. Deforestation to clear land for planting and to supply firewood, paper, and construction materials has progressed up steeper and higher slopes of the Lesser Himalayas, triggering environmental degradation. Only in Sikkim and Bhutan are large areas still heavily forested.
The Himalayas are rich in minerals, although exploitation is restricted to the more accessible areas. The Kashmir region has the greatest concentration of minerals. Sapphires are found in the Zanskar Range, and alluvial gold is recovered in the nearby bed of the Indus River. There are deposits of copper ore in Baltistan, and iron ores are found in the Vale of Kashmir. Ladakh possesses borax and sulfur deposits. Coal seams are found in the Jammu Hills. Bauxite also occurs in Kashmir. Nepal, Bhutan, and Sikkim have extensive deposits of coal, mica, gypsum, and graphite and ores of iron, copper, lead, and zinc.
The Himalayan rivers have a tremendous potential for hydroelectric generation. That potential was first harnessed intensively by India beginning in the 1950s. A giant multipurpose project is located at Bhakra-Nangal on the Sutlej River in the Outer Himalayas; its reservoir was completed in 1963 and has a storage capacity of some 348 billion cubic feet (10 billion cubic meters) of water and a total installed generating capacity of 1,050 megawatts. Other Himalayan rivers—including the Kosi, the Gandak (Narayani), and the Jaldhaka—were then harnessed by India, which then supplied electric power to Nepal and Bhutan. Subsequent major projects in India included the Nathpa Jhakri dam on the Sutlej in Himachal Pradesh and, just downstream from that site, the Rampur station, which became operational in 2014. Nepal has also constructed hydropower projects in the Himalayas, as has China, which completed the Zangmu station on the Yarlung Zangbo (Brahmaputra) River in Tibet in 2015.
Tourism has become an increasingly important source of income and employment in parts of the Himalayas, especially Nepal. In addition to sightseers, there has been a dramatic rise in the number of foreign trekkers in the lower mountain elevations, as well as in mountaineers seeking to climb Everest and the other high peaks. The resultant increased traffic and tourists’ heavy consumption of the region’s limited resources, however, have further stressed the regional environment.
Transportation
Trails and footpaths long were the only means of communication in the Himalayas. Although those continue to be important, especially in the more remote locations, road transport now has made the Himalayas accessible from both north and south. In Nepal an east-west highway stretches through the Tarai lowlands, connecting roads that penetrate into many of the country’s mountain valleys. The capital, Kathmandu, is connected to Pokhara by a low Himalayan highway, and another highway through Kodari Pass gives Nepal access to Tibet. A highway running from Kathmandu through Hetaunda and Birganj to Birauni connects Nepal to Bihar state and the rest of India. To the northwest in Pakistan, the Karakoram Highway links that country with China. The Hindustan-Tibet road, which passes through Himachal Pradesh, has been considerably improved; that 300-mile (480-km) highway runs through Shimla, once the summer capital of India, and crosses the Indo-Tibetan border near Shipki Pass. From Manali in the Kullu valley, a highway now crosses not only the Great Himalayas but also the Zanskar Range and reaches Leh in the upper Indus valley. Leh is also connected to India via Srinagar in the Vale of Kashmir; the road from Srinagar to Leh passes over the 17,730-foot- (5,404-meter-) high Khardung Pass—the first of the high passes on the historic caravan trail to Central Asia from India. Many other new roads have been built since 1950.
From the Indian state of Punjab the only direct approach to the Vale of Kashmir is by the highway from Jalandhar to Srinagar (summer capital of Jammu and Kashmir union territory) through Pathankot, Jammu, Udhampur, Banihal, and Khahabal. It crosses the Pir Panjal Range through a tunnel at Banihal. The old road from Rawalpindi, Pakistan, to Srinagar lost its importance with the closing of the road at the line of control between the sectors of Kashmir administered by India and Pakistan.
The Sikkim Himalayas command the historic Kalimpang-to-Lhasa caravan trade route, which passes through Gangtok. Before the mid-1950s there was only one 30-mile (50-km) motorable highway running between Gangtok and Rangpo, on the Tista River, which then continued southward another 70 miles (110 km) to Siliguri (Shiliguri) in West Bengal state. Since then, several roads passable by four-wheel-drive vehicles have been built in the southern part of Sikkim, and the highway from Siliguri has been extended through Lachung, in northern Sikkim, to Tibet.
Only two main railroads, both of narrow gauge, penetrate into the Lesser Himalayas from the plains of India: one in the western Himalayas, between Kalka and Shimla, and the other in the eastern Himalayas, between Siliguri and Darjeeling. Another narrow-gauge line in Nepal runs some 30 miles from Raxaul in Bihar state, India, to Amlekhganj. Two other short railroads run to the Outer Himalayas—one, the railroad of the Kullu Valley, from Pathankot to Jogindarnagar and the other from Haridwar to Dehra Dun.
There are two major airstrips in the Himalayas, one at Kathmandu and the other at Srinagar; the airport at Kathmandu is served by international as well as regional flights. Besides those, there are also an increasing number of airstrips of local importance in Nepal and other countries in the region that can accommodate small aircraft. Improvements in both air and ground transportation have facilitated the growth of tourism in the Himalayas.
Study and exploration
The earliest journeys through the Himalayas were undertaken by traders, shepherds, and pilgrims. The pilgrims believed that the harder the journey was, the nearer it brought them to salvation or enlightenment; the traders and shepherds, though, accepted crossing passes as high as 18,000 to 19,000 feet (5,500 to 5,800 meters) as a way of life. For all others, however, the Himalayas constituted a formidable and fearsome barrier.
The first known Himalayan sketch map of some accuracy was drawn up in 1590 by Antonio Monserrate, a Spanish missionary to the court of the Mughal emperor Akbar. In 1733 a French geographer, Jean-Baptiste Bourguignon d’Arville, compiled the first map of Tibet and the Himalayan range based on systematic exploration. In the mid-19th century the Survey of India organized a systematic program to measure correctly the heights of the Himalayan peaks. The Nepal and Uttarakhand peaks were observed and mapped between 1849 and 1855. Nanga Parbat, as well as the peaks of the Karakoram Range to the north, were surveyed between 1855 and 1859. The surveyors did not assign individual names to the innumerable peaks observed but designated them by letters and Roman numerals. Thus, at first Mount Everest was simply labeled as “H”; that had been changed to Peak XV by 1850. In 1865 Peak XV was renamed for Sir George Everest, surveyor general of India from 1830 to 1843. Not until 1852 were the computations sufficiently advanced for it to be realized that Peak XV was higher than any other mountain in the world. By 1862 more than 40 peaks with elevations exceeding 18,000 feet (5,500 meters) had been climbed for surveying purposes.
In addition to the surveying expeditions, various scientific studies of the Himalayas were conducted in the 19th century. Between 1848 and 1849 the English botanist Joseph Dalton Hooker made a pioneering study of the plant life of the Sikkim Himalayas. He was followed by numerous others, including (in the early 20th century) the British naturalist Richard W.G. Hingston, who wrote valuable accounts of the natural history of animals living at high elevations in the Himalayas.
After World War II the Survey of India prepared some large-scale maps of the Himalayas from aerial photographs. Parts of the Himalayas were also mapped by German geographers and cartographers, with the help of ground photogrammetry. In addition, satellite reconnaissance has been employed to produce even more accurate and detailed maps. Aerial photographs have been used in conjunction with other scientific observation methods to monitor the effects of climate change on the Himalayan environment—notably the recession of glaciers.
Himalayan mountaineering began in the 1880s with the Briton W.W. Graham, who claimed to have climbed several peaks in 1883. Though his reports were received with skepticism, they did spark interest in the Himalayas among other European climbers. In the early 20th century the number of mountaineering expeditions increased markedly to the Karakoram Range and to the Kumaun and Sikkim Himalayas. Between World Wars I and II, a certain national preference developed for the various peaks: the Germans concentrated on Nanga Parbat and Kanchenjunga, the Americans on K2 (in the Karakorams), and the British on Mount Everest. Attempts at scaling Everest began in 1921, and about a dozen of them were undertaken before it was first successfully scaled in May 1953 by the New Zealand mountaineer Edmund Hillary and his Tibetan partner Tenzing Norgay. That same year an Austro-German team led by Karl Maria Herrligkoffer reached the summit of Nanga Parbat.
As the high peaks were conquered one by one, climbers began to look for greater challenges to test their skills and equipment. Some attempted to reach the summits by increasingly difficult routes, while others climbed with minimal amounts of gear or without the use of supplemental oxygen at the highest elevations. Easier access to the mountains brought increasingly large numbers of climbers and hikers into the region—hundreds alone trying to summit Everest each year. By the late 20th and early 21st centuries, the annual number of mountaineering expeditions and tourist excursions to the Himalayas was so large that in some areas the participants were threatening the delicate environmental balance of the mountains by destroying plant and animal life and by leaving behind a growing quantity of refuse. In addition, more people in such a highly dangerous environment invited disaster, as was the case in 2014, when more than 40 foreign trekkers perished in a snowstorm near Annapurna.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2694 2026-02-11 18:41:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2493) Animation
Gist
Animation is the art of creating the illusion of motion by rapidly displaying a sequence of static, slightly different images, often used in entertainment to bring characters to life. It encompasses traditional hand-drawn techniques, computer-generated imagery (CGI), and stop-motion, with 2D, 3D, and digital methods being dominant today. Key principles like timing, spacing, and squash-and-stretch ensure realistic or stylized motion, while software allows for efficient production.
The term 'animation' is derived from the Japanese word 'anime,' which can be translated as “to move” or “to give life”. The first animated “film” was made by a French cartoonist called Émile Cohl. This was the origin of animation; now, let's discuss the definition of animation.
Summary
Animation is a filmmaking technique whereby pictures are generated or manipulated to create moving images. In traditional animation, images are drawn or painted by hand on transparent celluloid sheets to be photographed and exhibited on film. Animation has been recognized as an artistic medium, specifically within the entertainment industry. Many animations are either traditional animations or computer animations made with computer-generated imagery (CGI). Stop motion animation, in particular claymation, is also prominent alongside these other forms, albeit to a lesser degree.
Animation is contrasted with live action, although the two do not exist in isolation. Many filmmakers have produced films that are a hybrid of the two. As CGI increasingly approximates photographic imagery, filmmakers can relatively easily composite 3D animated visual effects (VFX) into their film, rather than using practical effects.
General overview
Computer animation can be very detailed 3D animation, while 2D computer animation (which may have the look of traditional animation) can be used for stylistic reasons, low bandwidth, or faster real-time renderings. Other common animation methods apply a stop motion technique to two- and three-dimensional objects like paper cutouts, puppets, or clay figures.
An animated cartoon, or simply a cartoon, is an animated film, usually short, that features an exaggerated style. This style is often inspired by comic strips, gag cartoons, and other non-animated art forms. Cartoons frequently include anthropomorphic animals, superheroes, or the adventures of human protagonists. The action often revolves around exaggerated physical humor, particularly in predator/prey dynamics (e.g. cats and mice, coyotes and birds), where violent pratfalls such as falls, collisions, and explosions occur, often in ways that would be lethal in the real life.
During the late 1980s, the term "cartoon" was shortened to toon, referring to characters in animated productions, or more specifically, cartoonishly-drawn characters. This term gained popularity first in 1988 with the live-action/animated hybrid film Who Framed Roger Rabbit, which introduced ToonTown, a world inhabited by various animated cartoon characters. In 1990, Tiny Toon Adventures embraced the classic cartoon spirit, introducing a new generation of cartoon characters. Then, in 1993, Animaniacs followed, featuring the three rubber-hose-styled Warner siblings, Yakko Warner, Wakko Warner, and Dot Warner, who are trapped in the 1930s, eventually escaped and found themselves in the Warner Bros. water tower in the 1990s.
The illusion of animation—as in motion pictures in general—has traditionally been attributed to the persistence of vision and later to the phi phenomenon and beta movement, but the exact neurological causes are still uncertain. The illusion of motion caused by a rapid succession of images that minimally differ from each other, with unnoticeable interruptions, is a stroboscopic effect. While animators traditionally used to draw each part of the movements and changes of figures on transparent cels that could be moved over a separate background, computer animation is usually based on programming paths between key frames to maneuver digitally created figures throughout a digitally created environment.
Analog mechanical animation media that rely on the rapid display of sequential images include the phenakistiscope, zoetrope, flip book, praxinoscope, and film. Television and video are popular electronic animation media that originally were analog and now operate digitally. For display on computers, technology such as the animated GIF and Flash animation were developed.
In addition to short films, feature films, television series, animated GIFs (Graphics Interchange Format), and other media dedicated to the display of moving images, animation is also prevalent in video games, motion graphics, user interfaces, and visual effects.
The physical movement of image parts through simple mechanics—for instance, moving images in magic lantern shows—can also be considered animation. The mechanical manipulation of three-dimensional puppets and objects to emulate living beings has a very long history in automata. Electronic automata were popularized by Disney as animatronics.
Details
Animation is the art of making inanimate objects appear to move. Animation is an artistic impulse that long predates the movies. History’s first recorded animator is Pygmalion of Greek and Roman mythology, a sculptor who created a figure of a woman so perfect that he fell in love with her and begged Venus to bring her to life. Some of the same sense of magic, mystery, and transgression still adheres to contemporary film animation, which has made it a primary vehicle for exploring the overwhelming, often bewildering emotions of childhood—feelings once dealt with by folktales.
Early history
The theory of the animated cartoon preceded the invention of the cinema by half a century. Early experimenters, working to create conversation pieces for Victorian parlors or new sensations for the touring magic-lantern shows, which were a popular form of entertainment, discovered the principle of persistence of vision. If drawings of the stages of an action were shown in fast succession, the human eye would perceive them as a continuous movement. One of the first commercially successful devices, invented by the Belgian Joseph Plateau in 1832, was the phenakistoscope, a spinning cardboard disk that created the illusion of movement when viewed in a mirror. In 1834 William George Horner invented the zoetrope, a rotating drum lined by a band of pictures that could be changed. The Frenchman Émile Reynaud in 1876 adapted the principle into a form that could be projected before a theatrical audience. Reynaud became not only animation’s first entrepreneur but, with his gorgeously hand-painted ribbons of celluloid conveyed by a system of mirrors to a theater screen, the first artist to give personality and warmth to his animated characters.
With the invention of sprocket-driven film stock, animation was poised for a great leap forward. Although “firsts” of any kind are never easy to establish, the first film-based animator appears to be J. Stuart Blackton, whose Humorous Phases of Funny Faces in 1906 launched a successful series of animated films for New York’s pioneering Vitagraph Company. Later that year, Blackton also experimented with the stop-motion technique—in which objects are photographed, then repositioned and photographed again—for his short film Haunted Hotel.
In France, Émile Cohl was developing a form of animation similar to Blackton’s, though Cohl used relatively crude stick figures rather than Blackton’s ambitious newspaper-style cartoons. Coinciding with the rise in popularity of the Sunday comic sections of the new tabloid newspapers, the nascent animation industry recruited the talents of many of the best-known artists, including Rube Goldberg, Bud Fisher (creator of Mutt and Jeff) and George Herriman (creator of Krazy Kat), but most soon tired of the fatiguing animation process and left the actual production work to others.
The one great exception among these early illustrators-turned-animators was Winsor McCay, whose elegant, surreal Little Nemo in Slumberland and Dream of the Rarebit Fiend remain pinnacles of comic-strip art. McCay created a hand-colored short film of Little Nemo for use during his vaudeville act in 1911, but it was Gertie the Dinosaur, created for McCay’s 1914 tour, that transformed the art. McCay’s superb draftsmanship, fluid sense of movement, and great feeling for character gave viewers an animated creature who seemed to have a personality, a presence, and a life of her own. The first cartoon star had been born.
McCay made several other extraordinary films, including a re-creation of The Sinking of the Lusitania (1918), but it was left to Pat Sullivan to extend McCay’s discoveries. An Australian-born cartoonist who opened a studio in New York City, Sullivan recognized the great talent of a young animator named Otto Messmer, one of whose casually invented characters—a wily black cat named Felix—was made into the star of a series of immensely popular one-reelers. Designed by Messmer for maximum flexibility and facial expressiveness, the round-headed, big-eyed Felix quickly became the standard model for cartoon characters: a rubber ball on legs who required a minimum of effort to draw and could be kept in constant motion.
Walt Disney
This lesson did not go unremarked by the young Walt Disney, then working at his Laugh-O-gram Films studio in Kansas City, Missouri. His first major character, Oswald the Lucky Rabbit, was a straightforward appropriation of Felix; when he lost the rights to the character in a dispute with his distributor, Disney simply modified Oswald’s ears and produced Mickey Mouse.
Far more revolutionary was Disney’s decision to create a cartoon with the novelty of synchronized sound. Steamboat Willie (1928), Mickey’s third film, took the country by storm. A missing element—sound—had been added to animation, making the illusion of life that much more complete, that much more magical. Later, Disney would add carefully synchronized music (The Skeleton Dance, 1929), three-strip Technicolor (Flowers and Trees, 1932), and the illusion of depth with his multiplane camera (The Old Mill, 1937). With each step, Disney seemed to come closer to a perfect naturalism, a painterly realism that suggested academic paintings of the 19th century. Disney’s resident technical wizard was Ub Iwerks, a childhood friend who followed Disney to Hollywood and was instrumental in the creation of the multiplane camera and the synchronization techniques that made the Mickey Mouse cartoons and the Silly Symphonies series seem so robust and fully dimensional.
For Disney, the final step was, of course, Snow White and the Seven Dwarfs (1937). Although not the first animated feature, it was the first to use up-to-the-minute techniques and the first to receive a wide, Hollywood-style release. Instead of amusing his audience with talking mice and singing cows, Disney was determined to give them as profound a dramatic experience as the medium would allow; he reached into his own troubled childhood to interpret this rich fable of parental abandonment, sibling rivalry, and the onrush of adult passion.
With his increasing insistence on photographic realism in films such as Pinocchio (1940), Fantasia (1940), Dumbo (1941), and Bambi (1942), Disney perversely seemed to be trying to put himself out of business by imitating life too well. That was not the temptation followed by Disney’s chief rivals in the 1930s, all of whom came to specialize in their own kind of stylized mayhem.
The Fleischer brothers
Max and Dave Fleischer had become successful New York animators while Disney was still living in Kansas City. The Fleischers invented the rotoscoping process, still in use today, in which a strip of live-action footage can be traced and redrawn as a cartoon. The Fleischers exploited this technique in their pioneering series Out of the Inkwell (1919–29). It was this series, with its lively interaction between human and drawn figures, that Disney struggled to imitate with his early Alice cartoons.
But if Disney was Mother Goose and Norman Rockwell, the Fleischers (Max produced, Dave directed) were stride piano and red whiskey. Their extremely urban, overcrowded, sexually suggestive, and frequently nightmarish work—featuring the curvaceous torch singer Betty Boop and her two oddly infantile colleagues, Bimbo the Dog and Koko the Clown—charts a twisty route through the American subconscious of the 1920s and ’30s, before collapsing into Disneyesque cuteness with the features Gulliver’s Travels (1939) and Mr. Bug Goes to Town (1941; also released as Hoppity Goes to Town). The studio’s mainstay remained the relatively impersonal Popeye series, based on the comic strip created by Elzie Segar. The spinach-loving sailor was introduced as a supporting player in the Betty Boop cartoon Popeye the Sailor (1933) and quickly ascended to stardom, surviving through 105 episodes until the 1942 short Baby Wants a Bottleship, when the Fleischer studio collapsed and rights to the character passed to Famous Studios.
“Termite Terrace”
Less edgy than the Fleischers but every bit as anarchic were the animations produced by the Warner Bros. cartoon studio, known to its residents as “Termite Terrace.” The studio was founded by three Disney veterans, Rudolph Ising, Hugh Harmon, and Friz Freleng, but didn’t discover its identity until Tex Avery, fleeing the Walter Lantz studio at Universal, joined the team as a director. Avery was young and irreverent, and he quickly recognized the talent of staff artists such as Chuck Jones, Bob Clampett, and Bob Cannon. Together they brought a new kind of speed and snappiness to the Warners product, beginning with Gold Diggers of ’49 (1936). With the addition of director Frank Tashlin, musical director Carl W. Stalling, and voice interpreter Mel Blanc, the team was in place to create a new kind of cartoon character: cynical, wisecracking, and often violent, who, refined through a series of cartoons, finally emerged as Bugs Bunny in Tex Avery’s A Wild Hare (1940). Other characters, some invented and some reinterpreted, arrived, including Daffy Duck, Porky Pig, Tweety and Sylvester, Pepe LePew, Foghorn Leghorn, Road Runner, and Wile E. Coyote. Avery left Warner Brothers and in 1942 joined Metro-Goldwyn-Mayer’s moribund animation unit, where, if anything, his work became even wilder in films such as Red Hot Riding Hood (1943) and Bad Luck Blackie (1949).
Animation in Europe
In Europe animation had meanwhile taken a strikingly different direction. Eschewing animated line drawings, filmmakers experimented with widely different techniques: in Russia and later in France, Wladyslaw Starewicz (also billed as Ladislas Starevitch), a Polish art student and amateur entomologist, created stop-motion animation with bugs and dolls; among his most celebrated films are The Cameraman’s Revenge (1912), in which a camera-wielding grasshopper uses the tools of his trade to humiliate his unfaithful wife, and the feature-length The Tale of the Fox (1930), based on German folktales as retold by Johann Wolfgang von Goethe. A Russian working in France, Alexandre Alexeïeff, developed the pinscreen, a board perforated by some 500,000 pins that could be raised or lowered, which created patterns of light and shadow that gave the effect of an animated steel engraving. It took Alexeïeff two years to create A Night on Bald Mountain (1933), which used the music of Modest Mussorgsky; in 1963 Nikolay Gogol was the source of his most widely celebrated film, the dark fable The Nose.
Inspired by the shadow puppet theater of Thailand, Germany’s Lotte Reiniger employed animated silhouettes to create elaborately detailed scenes derived from folktales and children’s books. Her The Adventures of Prince Achmed (1926) may have been the first animated feature; it required more than two years of patient work and earned her the nickname “The Mistress of Shadows,” as bestowed on her by Jean Renoir. Her other works include Dr. Dolittle and His Animals (1928) and shorts based on musical themes by Wolfgang Amadeus Mozart (Papageno, 1935; adapted from The Magic Flute), Gaetano Donizetti (L’elisir d’amore, 1939; “The Elixir of Love”), and Igor Stravinsky (Dream Circus, 1939; adapted from Pulcinella). In the 1950s Reiniger moved to England, where she continued to produce films until her retirement in the ’70s.
Another German-born animator, Oskar Fischinger, took his work in a radically different direction. Abandoning the fairy tales and comic strips that had inspired most of his predecessors, Fischinger took his inspiration from the abstract art that dominated the 1920s. At first he worked with wax figures animated by stop motion, but his most significant films are the symphonies of shapes and sounds he called “colored rhythms,” created from shifting color fields and patterns matched to music by classical composers. He became fascinated by color photography and collaborated on a process called Gasparcolor, which, as utilized in his 1935 film Composition in Blue, won a prize at that year’s Venice Film Festival. The following year, he immigrated to Hollywood, where he worked on special effects for a number of films and was the initial designer of the Toccata and Fugue sequence in Walt Disney’s Fantasia (1940). The Disney artists modified his designs, however, and he asked that his name be removed from the finished film. Through the 1940s and ’50s he balanced his work between experimental films such as Motion Painting No. 1 (1947) and commercials, and he retired from animation in 1961 to devote himself to painting.
Fischinger’s films made a deep impression on the Scottish design student Norman McLaren, who began experimenting with cameraless films—with designs drawn directly on celluloid—as early as 1933 (Seven Till Five). A restless and brilliant researcher, he went to work for John Grierson at the celebrated General Post Office (GPO) Film Unit in London and followed Grierson to Canada in 1941, shortly after the founding of the National Film Board. Supported by government grants, he was able to play out his most radical creative impulses, using watercolors, crayons, and paper cutouts to bring abstract designs to flowing life. Attracted by the possibilities of stop-motion animation, he was able to turn inanimate objects into actors (A Chairy Tale, 1957) and actors into inanimate objects (Neighbours, 1952), a technique he called “pixellation.”
The international success of McLaren’s work (he won an Oscar for Neighbours) opened the possibilities for more personal forms of animation in America. John Hubley, an animator who worked for Disney studios on Snow White, Pinocchio, and Fantasia, left the Disney organization in 1941 and joined the independent animation company United Productions of America in 1945. Working in a radically simplified style, without the depth effects and shading of the Disney cartoons, Hubley created the nearsighted character Mister Magoo for the 1949 short Ragtime Bear. He and his wife, Faith, formed their own studio, Storyboard Productions, in 1955, and they collaborated on a series of increasingly poetic narrative films. They won Oscars for Moonbird (1959) and The Hole (1962). The Hubleys also created a much-admired series of short films based on the jazz improvisations of Dizzy Gillespie, Quincy Jones, and Benny Carter.
The evolution of animation in Eastern Europe was impeded by World War II, but several countries—in particular Poland, Hungary, and Romania—became world leaders in the field by the 1960s. Włodzimierz Haupe and Halina Bielinska were among the first important Polish animators; their Janosik (1954) was Poland’s first animated film, and their Changing of the Guard (1956) employed the stop-action gimmick of animated matchboxes. The collaborative efforts of Jan Lenica and Walerian Borowczyk foresaw the bleak themes and absurdist trends of the Polish school of the 1960s; such films as Był sobie raz… (1957; Once Upon a Time…), Nagrodzone uczucie (1957; Love Rewarded), and Dom (1958; The House) are surreal, pessimistic, plotless, and characterized by a barrage of disturbing images. Borowczyk and Lenica, each of whom went on to a successful solo career, helped launch an industry that produced as many as 120 animated films per year by the early ’60s. Animators such as Miroslaw Kijowicz, Daniel Szczechura, and Stefan Schabenbeck were among the leaders in Polish animation during the second half of the 20th century.
Nontraditional forms
Eastern Europe also became the center of puppet animation, largely because of the sweetly engaging, folkloric work of Jiří Trnka. Based on a Hans Christian Andersen story, Trnka’s The Emperor’s Nightingale became an international success when it was fitted with narration by Boris Karloff and released in 1948. His subsequent work includes ambitious adaptations of The Good Soldier Schweik (1954) and A Midsummer Night’s Dream (1959).
Born in Hungary, George Pal worked as an animator in Berlin, Prague, Paris, and the Netherlands before immigrating to the United States in 1939. There he contracted with Paramount Pictures to produce the Puppetoons series, perhaps the most popular and accomplished puppet animations to be created in the United States. A dedicated craftsman, Pal would produce up to 9,000 model figures for films such as Tulips Shall Grow, his 1942 anti-Nazi allegory. Pal abandoned animation for feature film production in 1947, though in films such as The War of the Worlds (1953) he continued to incorporate elaborate animated special-effects sequences.
Animators in Czechoslovakia and elsewhere took the puppet technique down far darker streets. Jan Švankmajer, for example, came to animation from the experimental theater movement of Prague. His work combines human figures and stop-motion animation to create disturbingly carnal meditations on sexuality and mortality, such as the short Dimensions of Dialogue (1982) and the features Alice (1988), Faust (1994), and Conspirators of Pleasure (1996). Švankmajer’s most dedicated disciples are the Quay brothers, Stephen and Timothy, identical twins born in Philadelphia who moved to London to create a series of meticulous puppet animations steeped in the atmosphere and ironic fatalism of Eastern Europe. Their Street of Crocodiles (1986), obliquely based on the stories of Bruno Schulz, is a parable of obscure import in which a puppet is freed of his strings but remains enslaved by bizarre sexual impulses.
Nick Park, the creator of the Wallace and Gromit series, is the optimist’s answer to the Quay brothers—a stop-motion animator who creates endearing characters and cozy environments that celebrate the security and complacency of provincial English life. He and his colleagues at the British firm Aardman Animations, including founders Peter Lord and Dave Sproxton, have taken the traditionally child-oriented format of clay animation to new heights of sophistication and expressiveness.
More-traditional forms of line animation have continued to be produced in Europe by filmmakers such as France’s Paul Grimault (The King and the Bird, begun in 1948 and released in 1980), Italy’s Bruno Bozzetto (whose 1976 Allegro Non Troppo broadly parodied Fantasia), and Great Britain’s John Halas and Joy Batchelor (Animal Farm, 1955) and Richard Williams (Raggedy Ann and Andy, 1977). George Dunning’s Yellow Submarine (1968) made creative use of the visual motifs of the psychedelic era, luring young adults back to a medium that had largely been relegated to children.
A victim of rising production costs, full-figure, feature-length animation appeared to be dying off until two developments gave it an unexpected boost in the 1980s. The first was the Disney company’s discovery that the moribund movie musical could be revived and made palatable to contemporary audiences by adapting it to cartoon form (The Little Mermaid, 1989); the second was the development of computer animation technology, which greatly reduced expenses while providing for new forms of expression. Although most contemporary animated films use computer techniques to a greater or lesser degree, the finest, purest achievements in the genre are the work of John Lasseter, whose Pixar Animation Studios productions have evolved from experimental shorts, such as Luxor, Jr. (1986), to lush features, such as Toy Story (1995; the first entirely computer-animated feature-length film), A Bug’s Life (1998), Finding Nemo (2003), The Incredibles (2004), WALL-E (2008), and Up (2009). Computer techniques are commonly incorporated into traditional line animations, giving films such as Disney’s Mulan (1998) and Dreamworks’s The Road to El Dorado (2000) a visual sweep and dimensionality that would otherwise require countless hours of manual labor.
Contemporary developments
A century after its birth, animation continues to evolve. The most exciting developments are found on two distinct fronts: the anime (“animation”) of Japan and the prime-time television cartoons of the United States. An offspring of the dense, novelistic style of Japanese manga comic books and the cut-rate techniques developed for television production in 1960, anime such as Miyazaki Hayao’s Princess Mononoke (1997) are the modern equivalent of the epic folk adventures once filmed by Mizoguchi Kenji (The 47 Ronin, 1941) and Kurosawa Akira (Yojimbo, 1961; “The Bodyguard”). Kon Satoshi’s Perfect Blue (1997) suggests the early Japanese New Wave films of director Oshima Nagisa with its violent exploration of a media-damaged personality.
U.S. television animation, pioneered in the 1950s by William Hanna and Joseph Barbera (Yogi Bear, The Flintstones) was for years synonymous with primitive techniques and careless writing. But with the debut of The Simpsons in 1989, TV animation became home to a kind of mordant social commentary or outright absurdism (John Kricfalusi’s Ren and Stimpy) that was too pointedly aggressive for live-action realism. When Mike Judge’s Beavis and Butt-Head debuted on the MTV network in 1993, the rock-music cable channel discovered that cartoons could push the limits of censorship in ways no live-action television productions could. Following Judge’s success in 1997 were Trey Parker and Matt Stone with South Park, a series centered on foulmouthed kids growing up in the American Rocky Mountain West and rendered in a flat, cutout animation style that would have looked primitive in 1906. The spiritual father of the new television animation is Jay Ward, whose Rocky and His Friends, first broadcast in 1959, turned the threadbare television style into a vehicle for absurdist humor and adult satire.
Despite these boundary-pushing advances, full-figure, traditionally animated films continue to be produced, most notably by Don Bluth (An American Tale, 1986), a Disney dissident who moved his operation to Ireland, and Brad Bird, a veteran of Simpsons minimalism who progressed to the spectacular full technique of The Iron Giant (1999). As digital imaging techniques continue to improve in quality and affordability, it becomes increasingly difficult to draw a clear line between live action and animation. Films such as The Matrix (1999), Star Wars: Episode One (1999), and Gladiator (2000), incorporate backgrounds, action sequences, and even major characters conceived by illustrators and brought to life by technology. Such techniques are no less creations of the animator’s art than were Gertie, Betty Boop, and Bugs Bunny.
Additional Information
Computer animation is the process used for digitally generating moving images. The more general term computer-generated imagery (CGI) encompasses both still images and moving images, while computer animation only refers to moving images. Modern computer animation usually uses 3D computer graphics.
Computer animation is a digital successor to stop motion and traditional animation. Instead of a physical model or illustration, a digital equivalent is manipulated frame-by-frame. Also, computer-generated animations allow a single graphic artist to produce such content without using actors, expensive set pieces, or props. To create the illusion of movement, an image is displayed on the computer monitor and repeatedly replaced by a new similar image but advanced slightly in time (usually at a rate of 24, 25, or 30 frames/second). This technique is identical to how the illusion of movement is achieved with television and motion pictures.
To trick the visual system into seeing a smoothly moving object, the pictures should be drawn at around 12 frames per second or faster (a frame is one complete image). At rates below 12 frames per second, most people can detect jerkiness associated with the drawing of new images that detracts from the illusion of realistic movement. Conventional hand-drawn cartoon animation often uses 15 frames per second in order to save on the number of drawings needed, but this is usually accepted because of the stylized nature of cartoons. To produce more realistic imagery, computer animation demands higher frame rates.
Films seen in theaters in the United States run at 24 frames per second, which is sufficient to create the appearance of continuous movement.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2695 2026-02-12 18:55:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2494) Metal Detector
Gist
Metal detectors use electromagnetic fields to find hidden metal, with major uses in security (airports, events for weapons), hobby/recreation (treasure hunting, coin collecting), industry (quality control in food/pharma, construction for pipes/wires), and archaeology/recovery (locating artifacts, landmines). They range from handheld wands to large walk-through arches, alerting users with sound or visual cues when metal is detected.
Metal detectors detect any conductive metal by sensing disruptions in their electromagnetic field, finding everything from weapons and coins to jewelry and tools, distinguishing between easier-to-detect ferrous (iron-based) and harder-to-detect non-ferrous (like aluminum, copper) metals, with sensitivity adjusted for different uses like security or treasure hunting.
Summary
A metal detector is an instrument that detects the nearby presence of metal. Metal detectors are useful for finding metal objects on the surface, underground, and under water. A metal detector typically consists of a control box, an adjustable shaft, and a variable-shaped pickup coil. When the coil nears metal, the control box signals its presence with a tone, numerical reading, light, or needle movement. Signal intensity typically increases with proximity or metal size and composition. A common type are stationary "walk through" metal detectors used at access points in prisons, courthouses, airports and psychiatric hospitals to detect concealed metal weapons on a person's body.
The simplest form of a metal detector consists of an oscillator producing an alternating current that passes through a coil producing an alternating magnetic field. If a piece of electrically conductive metal is close to the coil, eddy currents will be induced (inductive sensor) in the metal, and this produces a magnetic field of its own. If another coil is used to measure the magnetic field (acting as a magnetometer), the change in the magnetic field due to the metallic object can be detected.
The first industrial metal detectors came out in the 1960s, and were used for finding minerals, among other things. Metal detectors help find land mines. They also detect weapons like knives and guns, which is important for airport security. People most commonly use them to search for buried objects, like in archaeology and treasure hunting. Metal detectors are also used to detect foreign bodies in food, and in the construction industry to detect steel reinforcing bars in concrete and pipes and wires buried in walls and floors.
Details
Mention the words metal detector and you'll get completely different reactions from different people. For instance, some people think of combing a beach in search of coins or buried treasure. Other people think of airport security, or the handheld scanners at a concert or sporting event.
The fact is that all of these scenarios are valid. Metal-detector technology is a huge part of our lives, with a range of uses that spans from leisure to work to safety. The metal detectors in airports, office buildings, schools, government agencies and prisons help ensure that no one is bringing a weapon onto the premises. Consumer-oriented metal detectors provide millions of people around the world with an opportunity to discover hidden treasures (along with lots of junk).
In this article, you'll learn about metal detectors and the various technologies they use. Our focus will be on consumer metal detectors, but most of the information also applies to mounted detection systems, like the ones used in airports, as well as handheld security scanners.
Anatomy of a Metal Detector
A typical metal detector is light-weight and consists of just a few parts:
Stabilizer (optional) - used to keep the unit steady as you sweep it back and forth
Control box - contains the circuitry, controls, speaker, batteries and the microprocessor
Shaft - connects the control box and the coil; often adjustable so you can set it at a comfortable level for your height
Search coil - the part that actually senses the metal; also known as the "search head," "loop" or "antenna"
Most systems also have a jack for connecting headphones, and some have the control box below the shaft and a small display unit above.
Operating a metal detector is simple. Once you turn the unit on, you move slowly over the area you wish to search. In most cases, you sweep the coil (search head) back and forth over the ground in front of you. When you pass it over a target object, an audible signal occurs. More advanced metal detectors provide displays that pinpoint the type of metal it has detected and how deep in the ground the target object is located.
Metal detectors use one of three technologies:
* Very low frequency (VLF)
* Pulse induction (PI)
* Beat-frequency oscillation (BFO)
In the following sections, we will look at each of these technologies in detail to see how they work.
VLF Technology
Very low frequency (VLF), also known as induction balance, is probably the most popular detector technology in use today. In a VLF metal detector, there are two distinct coils:
* Transmitter coil - This is the outer coil loop. Within it is a coil of wire. Electricity is sent along this wire, first in one direction and then in the other, thousands of times each second. The number of times that the current's direction switches each second establishes the frequency of the unit.
* Receiver coil - This inner coil loop contains another coil of wire. This wire acts as an antenna to pick up and amplify frequencies coming from target objects in the ground.
The current moving through the transmitter coil creates an electromagnetic field, which is like what happens in an electric motor. The polarity of the magnetic field is perpendicular to the coil of wire. Each time the current changes direction, the polarity of the magnetic field changes. This means that if the coil of wire is parallel to the ground, the magnetic field is constantly pushing down into the ground and then pulling back out of it.
As the magnetic field pulses back and forth into the ground, it interacts with any conductive objects it encounters, causing them to generate weak magnetic fields of their own. The polarity of the object's magnetic field is directly opposite the transmitter coil's magnetic field. If the transmitter coil's field is pulsing downward, the object's field is pulsing upward.
The receiver coil is completely shielded from the magnetic field generated by the transmitter coil. However, it is not shielded from magnetic fields coming from objects in the ground. Therefore, when the receiver coil passes over an object giving off a magnetic field, a small electric current travels through the coil. This current oscillates at the same frequency as the object's magnetic field. The coil amplifies the frequency and sends it to the control box of the metal detector, where sensors analyze the signal.
The metal detector can determine approximately how deep the object is buried based on the strength of the magnetic field it generates. The closer to the surface an object is, the stronger the magnetic field picked up by the receiver coil and the stronger the electric current generated. The farther below the surface, the weaker the field. Beyond a certain depth, the object's field is so weak at the surface that it is undetectable by the receiver coil.
In the next section, we'll see how a VLF metal detector distinguishes between different types of metals.
VLF Phase Shifting
How does a VLF metal detector distinguish between different metals? It relies on a phenomenon known as phase shifting. Phase shift is the difference in timing between the transmitter coil's frequency and the frequency of the target object. This discrepancy can result from a couple of things:
* Inductance - An object that conducts electricity easily (is inductive) is slow to react to changes in the current. You can think of inductance as a deep river: Change the amount of water flowing into the river and it takes some time before you see a difference.
* Resistance - An object that does not conduct electricity easily (is resistive) is quick to react to changes in the current. Using our water analogy, resistance would be a small, shallow stream: Change the amount of water flowing into the stream and you notice a drop in the water level very quickly.
Basically, this means that an object with high inductance is going to have a larger phase shift, because it takes longer to alter its magnetic field. An object with high resistance is going to have a smaller phase shift.
Phase shift provides VLF-based metal detectors with a capability called discrimination. Since most metals vary in both inductance and resistance, a VLF metal detector examines the amount of phase shift, using a pair of electronic circuits called phase demodulators, and compares it with the average for a particular type of metal. The detector then notifies you with an audible tone or visual indicator as to what range of metals the object is likely to be in.
Many metal detectors even allow you to filter out (discriminate) objects above a certain phase-shift level. Usually, you can set the level of phase shift that is filtered, generally by adjusting a knob that increases or decreases the threshold. Another discrimination feature of VLF detectors is called notching. Essentially, a notch is a discrimination filter for a particular segment of phase shift. The detector will not only alert you to objects above this segment, as normal discrimination would, but also to objects below it.
Advanced detectors even allow you to program multiple notches. For example, you could set the detector to disregard objects that have a phase shift comparable to a soda-can tab or a small nail. The disadvantage of discrimination and notching is that many valuable items might be filtered out because their phase shift is similar to that of "junk." But, if you know that you are looking for a specific type of object, these features can be extremely useful.
PI Technology
A less common form of metal detector is based on pulse induction (PI). Unlike VLF, PI systems may use a single coil as both transmitter and receiver, or they may have two or even three coils working together. This technology sends powerful, short bursts (pulses) of current through a coil of wire. Each pulse generates a brief magnetic field. When the pulse ends, the magnetic field reverses polarity and collapses very suddenly, resulting in a sharp electrical spike. This spike lasts a few microseconds (millionths of a second) and causes another current to run through the coil. This current is called the reflected pulse and is extremely short, lasting only about 30 microseconds. Another pulse is then sent and the process repeats. A typical PI-based metal detector sends about 100 pulses per second, but the number can vary greatly based on the manufacturer and model, ranging from a couple of dozen pulses per second to over a thousand.
If the metal detector is over a metal object, the pulse creates an opposite magnetic field in the object. When the pulse's magnetic field collapses, causing the reflected pulse, the magnetic field of the object makes it take longer for the reflected pulse to completely disappear. This process works something like echoes: If you yell in a room with only a few hard surfaces, you probably hear only a very brief echo, or you may not hear one at all; but if you yell in a room with a lot of hard surfaces, the echo lasts longer. In a PI metal detector, the magnetic fields from target objects add their "echo" to the reflected pulse, making it last a fraction longer than it would without them.
A sampling circuit in the metal detector is set to monitor the length of the reflected pulse. By comparing it to the expected length, the circuit can determine if another magnetic field has caused the reflected pulse to take longer to decay. If the decay of the reflected pulse takes more than a few microseconds longer than normal, there is probably a metal object interfering with it.
The sampling circuit sends the tiny, weak signals that it monitors to a device call an integrator. The integrator reads the signals from the sampling circuit, amplifying and converting them to direct current (DC). The direct current's voltage is connected to an audio circuit, where it is changed into a tone that the metal detector uses to indicate that a target object has been found.
PI-based detectors are not very good at discrimination because the reflected pulse length of various metals are not easily separated. However, they are useful in many situations in which VLF-based metal detectors would have difficulty, such as in areas that have highly conductive material in the soil or general environment. A good example of such a situation is salt-water exploration. Also, PI-based systems can often detect metal much deeper in the ground than other systems.
BFO Technology
The most basic way to detect metal uses a technology called beat-frequency oscillator (BFO). In a BFO system, there are two coils of wire. One large coil is in the search head, and a smaller coil is located inside the control box. Each coil is connected to an oscillator that generates thousands of pulses of current per second. The frequency of these pulses is slightly offset between the two coils.
As the pulses travel through each coil, the coil generates radio waves. A tiny receiver within the control box picks up the radio waves and creates an audible series of tones (beats) based on the difference between the frequencies.
If the coil in the search head passes over a metal object, the magnetic field caused by the current flowing through the coil creates a magnetic field around the object. The object's magnetic field interferes with the frequency of the radio waves generated by the search-head coil. As the frequency deviates from the frequency of the coil in the control box, the audible beats change in duration and tone.
BFO Technology
The simplicity of BFO-based systems allows them to be manufactured and sold for a very low cost. But these detectors do not provide the level of control and accuracy provided by VLF or PI systems.
Buried Treasure
Metal detectors are great for finding buried objects. But typically, the object must be within a foot or so of the surface for the detector to find it. Most detectors have a normal maximum depth somewhere between 8 and 12 inches (20 and 30 centimeters). The exact depth varies based on a number of factors:
The type of metal detector - The technology used for detection is a major factor in the capability of the detector. Also, there are variations and additional features that differentiate detectors that use the same technology. For example, some VLF detectors use higher frequencies than others, while some provide larger or smaller coils. Plus, the sensor and amplification technology can vary between manufacturers and even between models offered by the same manufacturer.
* The type of metal in the object - Some metals, such as iron, create stronger magnetic fields than others.
* The size of the object - A dime is much harder to detect at deep levels than a quarter.
* The makeup of the soil - Certain minerals are natural conductors and can seriously interfere with the metal detector.
* The object's halo - When certain types of metal objects have been in the ground for a long time, they can actually increase the conductivity of the soil around them.
* Interference from other objects - This can be items in the ground, such as pipes or cables, or items above ground, like power lines.
Hobbyist metal detecting is a fascinating world with several sub-groups. Here are some of the more popular activities:
* Coin shooting - looking for coins after a major event, such as a ball game or concert, or just searching for old coins in general
* Prospecting - searching for valuable metals, such as gold nuggets
* Relic hunting - searching for items of historical value, such as weapons used in the U.S. Civil War
* Treasure hunting - researching and trying to find caches of gold, silver or anything else rumored to have been hidden somewhere
* Many metal-detector enthusiasts join local or national clubs that provide tips and tricks for hunting. Some of these clubs even sponsor organized treasure hunts or other outings for their members.
Detective Work
In addition to recreational use, metal detectors serve a wide range of utilitarian functions. Mounted detectors usually use some variation of PI technology, while many of the basic handheld scanners are BFO-based.
Some nonrecreational applications for metal detectors are:
* Airport security - screen people before allowing access to the boarding area and the plane (see How Airport Security Works)
* Building security - screen people entering a particular building, such as a school, office or prison
* Event security - screen people entering a sporting event, concert or other large gathering of people
* Item recovery - help someone search for a lost item, such as a piece of jewelry
* Archaeological exploration - find metallic items of historical significance
* Geological research - detect the metallic composition of soil or rock formations
Manufacturers of metal detectors are constantly tuning the process to make their products more accurate, more sensitive and more versatile. On the next page, you will find links to the manufacturers, as well as clubs and more information on metal detecting as a hobby.
Additional Information
A metal detector is an electronic device that finds metal objects nearby. These devices are very useful for discovering metal pieces hidden inside other objects. They can also find metal items buried underground. Most metal detectors have a handheld part with a special sensor. You can sweep this sensor over the ground or other things. If the sensor gets close to metal, you will hear a changing sound in earphones. Sometimes, a needle on a display will move. The closer the metal is, the louder the sound or the higher the needle goes.
Another common type of metal detector is a "walk-through" scanner. These are used for security checks at places like airports. They help find hidden metal weapons on a person's body.
The basic idea behind a metal detector is simple. It uses an electronic circuit to create an alternating current. This current goes through a coil, which then makes an invisible magnetic field. If a piece of metal that conducts electricity comes close to this coil, tiny electric currents called eddy currents are created in the metal. These eddy currents then make their own magnetic field. The metal detector has another coil that measures these magnetic fields. When the magnetic field changes because of a metal object, the detector senses it.
History and Uses of Metal Detectors
The first industrial metal detectors were made in the 1960s. They quickly became popular for finding minerals and for other industrial jobs.
Finding Hidden Treasures and More
Metal detectors have many interesting uses today:
* Finding landmines: They help locate dangerous land mines left behind after wars.
* Security: They are used to find weapons like knives and guns, especially at airports and other secure locations.
* Exploring the Earth: Scientists use them for geophysical prospecting, which means exploring the Earth's surface for minerals.
* Archaeology: Archaeologists use them to find old artifacts buried underground.
* Treasure hunting: Many people use metal detectors as a hobby to search for lost coins, jewelry, and other treasures.
Metal Detectors in Everyday Life
Metal detectors are also used in other important ways:
* Food safety: They help find foreign objects, like small pieces of metal, in food products. This keeps our food safe to eat.
* Construction: In the construction industry, they find steel reinforcing bars inside concrete. They can also locate pipes and wires hidden in walls and floors.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2696 2026-02-13 18:32:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2495) Adhesive
Gist
An adhesive is a substance—such as glue, cement, or paste—that binds materials together through surface attachment, resisting separation. Primarily divided into natural (e.g., starch) and synthetic (e.g., epoxy, acrylic) types, they are used for bonding, sealing, and coating in industrial and consumer applications.
Adhesives are used to bond materials together across virtually every industry, from construction, automotive, and aerospace to everyday household tasks, by creating strong, durable connections that distribute stress evenly, join dissimilar materials (like metal to plastic), and offer aesthetic advantages over mechanical fasteners like nails or screws, by avoiding holes and creating smoother finishes. They're essential for product assembly, packaging (like sealing boxes), labeling (stickers, bottle labels), and repairs, enabling lighter, stronger, and more complex designs in everything from smartphones to spacecraft.
Summary
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advantages over other binding techniques such as sewing, mechanical fastenings, and welding. These include the ability to bind different materials together, the more efficient distribution of stress across a joint, the cost-effectiveness of an easily mechanized process, and greater flexibility in design. Disadvantages of adhesive use include decreased stability at high temperatures, relative weakness in bonding large objects with a small bonding surface area, and greater difficulty in separating objects during testing. Adhesives are typically organized by the method of adhesion followed by reactive or non-reactive, a term which refers to whether the adhesive chemically reacts in order to harden. Alternatively, they can be organized either by their starting physical phase or whether their raw stock is of natural or synthetic origin.
Adhesives may be found naturally or produced synthetically. The earliest human use of adhesive-like substances was approximately 200,000 years ago, when Neanderthals produced tar from the dry distillation of birch bark for use in binding stone tools to wooden handles. The first references to adhesives in literature appeared approximately 2000 BC. The Greeks and Romans made great contributions to the development of adhesives. In Europe, glue was not widely used until the period AD 1500–1700. From then until the 1900s increases in adhesive use and discovery were relatively gradual. Only since the 20th century has the development of synthetic adhesives accelerated rapidly, and innovation in the field continues to the present.
Details
An adhesive is any substance that is capable of holding materials together in a functional manner by surface attachment that resists separation. “Adhesive” as a general term includes cement, mucilage, glue, and paste—terms that are often used interchangeably for any organic material that forms an adhesive bond. Inorganic substances such as portland cement also can be considered adhesives, in the sense that they hold objects such as bricks and beams together through surface attachment, but this article is limited to a discussion of organic adhesives, both natural and synthetic.
Natural adhesives have been known since antiquity. Egyptian carvings dating back 3,300 years depict the gluing of a thin piece of veneer to what appears to be a plank of sycamore. Papyrus, an early nonwoven fabric, contained fibres of reedlike plants bonded together with flour paste. Bitumen, tree pitches, and beeswax were used as sealants (protective coatings) and adhesives in ancient and medieval times. The gold leaf of illuminated manuscripts was bonded to paper by egg white, and wooden objects were bonded with glues from fish, horn, and cheese. The technology of animal and fish glues advanced during the 18th century, and in the 19th century rubber- and nitrocellulose-based cements were introduced. Decisive advances in adhesives technology, however, awaited the 20th century, during which time natural adhesives were improved and many synthetics came out of the laboratory to replace natural adhesives in the marketplace. The rapid growth of the aircraft and aerospace industries during the second half of the 20th century had a profound impact on adhesives technology. The demand for adhesives that had a high degree of structural strength and were resistant to both fatigue and severe environmental conditions led to the development of high-performance materials, which eventually found their way into many industrial and domestic applications.
This article begins with a brief explanation of the principles of adhesion and then proceeds to a review of the major classes of natural and synthetic adhesives.
Adhesion
In the performance of adhesive joints, the physical and chemical properties of the adhesive are the most important factors. Also important in determining whether the adhesive joint will perform adequately are the types of adherend (that is, the components being joined—e.g., metal alloy, plastic, composite material) and the nature of the surface pretreatment or primer. These three factors—adhesive, adherend, and surface—have an impact on the service life of the bonded structure. The mechanical behaviour of the bonded structure in turn is influenced by the details of the joint design and by the way in which the applied loads are transferred from one adherend to the other.
Implicit in the formation of an acceptable adhesive bond is the ability of the adhesive to wet and spread on the adherends being joined. Attainment of such interfacial molecular contact is a necessary first step in the formation of strong and stable adhesive joints. Once wetting is achieved, intrinsic adhesive forces are generated across the interface through a number of mechanisms. The precise nature of these mechanisms have been the object of physical and chemical study since at least the 1960s, with the result that a number of theories of adhesion exist. The main mechanism of adhesion is explained by the adsorption theory, which states that substances stick primarily because of intimate intermolecular contact. In adhesive joints this contact is attained by intermolecular or valence forces exerted by molecules in the surface layers of the adhesive and adherend.
In addition to adsorption, four other mechanisms of adhesion have been proposed. The first, mechanical interlocking, occurs when adhesive flows into pores in the adherend surface or around projections on the surface. The second, interdiffusion, results when liquid adhesive dissolves and diffuses into adherend materials. In the third mechanism, adsorption and surface reaction, bonding occurs when adhesive molecules adsorb onto a solid surface and chemically react with it. Because of the chemical reaction, this process differs in some degree from simple adsorption, described above, although some researchers consider chemical reaction to be part of a total adsorption process and not a separate adhesion mechanism. Finally, the electronic, or electrostatic, attraction theory suggests that electrostatic forces develop at an interface between materials with differing electronic band structures. In general, more than one of these mechanisms play a role in achieving the desired level of adhesion for various types of adhesive and adherend.
In the formation of an adhesive bond, a transitional zone arises in the interface between adherend and adhesive. In this zone, called the interphase, the chemical and physical properties of the adhesive may be considerably different from those in the noncontact portions. It is generally believed that the interphase composition controls the durability and strength of an adhesive joint and is primarily responsible for the transference of stress from one adherend to another. The interphase region is frequently the site of environmental attack, leading to joint failure.
The strength of adhesive bonds is usually determined by destructive tests, which measure the stresses set up at the point or line of fracture of the test piece. Various test methods are employed, including peel, tensile lap shear, cleavage, and fatigue tests. These tests are carried out over a wide range of temperatures and under various environmental conditions. An alternate method of characterizing an adhesive joint is by determining the energy expended in cleaving apart a unit area of the interphase. The conclusions derived from such energy calculations are, in principle, completely equivalent to those derived from stress analysis.
Adhesive materials
Virtually all synthetic adhesives and certain natural adhesives are composed of polymers, which are giant molecules, or macromolecules, formed by the linking of thousands of simpler molecules known as monomers. The formation of the polymer (a chemical reaction known as polymerization) can occur during a “cure” step, in which polymerization takes place simultaneously with adhesive-bond formation (as is the case with epoxy resins and cyanoacrylates), or the polymer may be formed before the material is applied as an adhesive, as with thermoplastic elastomers such as styrene-isoprene-styrene block copolymers. Polymers impart strength, flexibility, and the ability to spread and interact on an adherend surface—properties that are required for the formation of acceptable adhesion levels.
Natural adhesives
Natural adhesives are primarily of animal or vegetable origin. Though the demand for natural products has declined since the mid-20th century, certain of them continue to be used with wood and paper products, particularly in corrugated board, envelopes, bottle labels, book bindings, cartons, furniture, and laminated film and foils. In addition, owing to various environmental regulations, natural adhesives derived from renewable resources are receiving renewed attention. The most important natural products are described below.
Animal glue
The term animal glue usually is confined to glues prepared from mammalian collagen, the principal protein constituent of skin, bone, and muscle. When treated with acids, alkalies, or hot water, the normally insoluble collagen slowly becomes soluble. If the original protein is pure and the conversion process is mild, the high-molecular-weight product is called gelatin and may be used for food or photographic products. The lower-molecular-weight material produced by more vigorous processing is normally less pure and darker in colour and is called animal glue.
Animal glue traditionally has been used in wood joining, book bindery, sandpaper manufacture, heavy gummed tapes, and similar applications. In spite of its advantage of high initial tack (stickiness), much animal glue has been modified or entirely replaced by synthetic adhesives.
Casein glue
This product is made by dissolving casein, a protein obtained from milk, in an aqueous alkaline solvent. The degree and type of alkali influences product behaviour. In wood bonding, casein glues generally are superior to true animal glues in moisture resistance and aging characteristics. Casein also is used to improve the adhering characteristics of paints and coatings.
Blood albumen glue
Glue of this type is made from serum albumen, a blood component obtainable from either fresh animal blood or dried soluble blood powder to which water has been added. Addition of alkali to albumen-water mixtures improves adhesive properties. A considerable quantity of glue products from blood is used in the plywood industry.
Starch and dextrin
Starch and dextrin are extracted from corn, wheat, potatoes, or rice. They constitute the principal types of vegetable adhesives, which are soluble or dispersible in water and are obtained from plant sources throughout the world. Starch and dextrin glues are used in corrugated board and packaging and as a wallpaper adhesive.
Natural gums
Substances known as natural gums, which are extracted from their natural sources, also are used as adhesives. Agar, a marine-plant colloid (suspension of extremely minute particles), is extracted by hot water and subsequently frozen for purification. Algin is obtained by digesting seaweed in alkali and precipitating either the calcium salt or alginic acid. Gum arabic is harvested from acacia trees that are artificially wounded to cause the gum to exude. Another exudate is natural rubber latex, which is harvested from Hevea trees. Most gums are used chiefly in water-remoistenable products.
Synthetic adhesives
Although natural adhesives are less expensive to produce, most important adhesives are synthetic. Adhesives based on synthetic resins and rubbers excel in versatility and performance. Synthetics can be produced in a constant supply and at constantly uniform properties. In addition, they can be modified in many ways and are often combined to obtain the best characteristics for a particular application.
The polymers used in synthetic adhesives fall into two general categories—thermoplastics and thermosets. Thermoplastics provide strong, durable adhesion at normal temperatures, and they can be softened for application by heating without undergoing degradation. Thermoplastic resins employed in adhesives include nitrocellulose, polyvinyl acetate, vinyl acetate-ethylene copolymer, polyethylene, polypropylene, polyamides, polyesters, acrylics, and cyanoacrylics.
Thermosetting systems, unlike thermoplastics, form permanent, heat-resistant, insoluble bonds that cannot be modified without degradation. Adhesives based on thermosetting polymers are widely used in the aerospace industry. Thermosets include phenol formaldehyde, urea formaldehyde, unsaturated polyesters, epoxies, and polyurethanes. Elastomer-based adhesives can function as either thermoplastic or thermosetting types, depending on whether cross-linking is necessary for the adhesive to perform its function. The characteristics of elastomeric adhesives include quick assembly, flexibility, variety of type, economy, high peel strength, ease of modification, and versatility. The major elastomers employed as adhesives are natural rubber, butyl rubber, butadiene rubber, styrene-butadiene rubber, nitrile rubber, silicone, and neoprene.
An important challenge facing adhesive manufacturers and users is the replacement of adhesive systems based on organic solvents with systems based on water. This trend has been driven by restrictions on the use of volatile organic compounds (VOC), which include solvents that are released into the atmosphere and contribute to the depletion of ozone. In response to environmental regulation, adhesives based on aqueous emulsions and dispersions are being developed, and solvent-based adhesives are being phased out.
The polymer types noted above are employed in a number of functional types of adhesives. These functional types are described below.
Contact cements
Contact adhesives or cements are usually based on solvent solutions of neoprene. They are so named because they are usually applied to both surfaces to be bonded. Following evaporation of the solvent, the two surfaces may be joined to form a strong bond with high resistance to shearing forces. Contact cements are used extensively in the assembly of automotive parts, furniture, leather goods, and decorative laminates. They are effective in the bonding of plastics.
Structural adhesives
Structural adhesives are adhesives that generally exhibit good load-carrying capability, long-term durability, and resistance to heat, solvents, and fatigue. Ninety-five percent of all structural adhesives employed in original equipment manufacture fall into six structural-adhesive families: (1) epoxies, which exhibit high strength and good temperature and solvent resistance, (2) polyurethanes, which are flexible, have good peeling characteristics, and are resistant to shock and fatigue, (3) acrylics, a versatile adhesive family that bonds to oily parts, cures quickly, and has good overall properties, (4) anaerobics, or surface-activated acrylics, which are good for bonding threaded metal parts and cylindrical shapes, (5) cyanoacrylates, which bond quickly to plastic and rubber but have limited temperature and moisture resistance, and (6) silicones, which are flexible, weather well out-of-doors, and provide good sealing properties. Each of these families can be modified to provide adhesives that have a range of physical and mechanical properties, cure systems, and application techniques.
Polyesters, polyvinyls, and phenolic resins are also used in industrial applications but have processing or performance limitations. High-temperature adhesives, such as polyimides, have a limited market.
Hot-melt adhesives
Hot-melt adhesives are employed in many nonstructural applications. Based on thermoplastic resins, which melt at elevated temperatures without degrading, these adhesives are applied as hot liquids to the adherend. Commonly used polymers include polyamides, polyesters, ethylene-vinyl acetate, polyurethanes, and a variety of block copolymers and elastomers such as butyl rubber, ethylene-propylene copolymer, and styrene-butadiene rubber.
Hot-melts find wide application in the automotive and home-appliance fields. Their utility, however, is limited by their lack of high-temperature strength, the upper use temperature for most hot-melts being in the range of 40–65 °C (approximately 100–150 °F). In order to improve performance at higher temperatures, so-called structural hot-melts—thermoplastics modified with reactive urethanes, moisture-curable urethanes, or silane-modified polyethylene—have been developed. Such modifications can lead to enhanced peel adhesion, higher heat capability (in the range of 70–95 °C [160–200 °F]), and improved resistance to ultraviolet radiation.
Pressure-sensitive adhesives
Pressure-sensitive adhesives, or PSAs, represent a large industrial and commercial market in the form of adhesive tapes and films directed toward packaging, mounting and fastening, masking, and electrical and surgical applications. PSAs are capable of holding adherends together when the surfaces are mated under briefly applied pressure at room temperature. (The difference between these adhesives and contact cements is that the latter require no pressure to bond.)
Materials used to formulate PSA systems include natural and synthetic rubbers, thermoplastic elastomers, polyacrylates, polyvinylalkyl ethers, and silicones. These polymers, in both solvent-based and hot-melt formulations, are applied as a coating onto a substrate of paper, cellophane, plastic film, fabric, or metal foil. As solvent-based adhesive formulations are phased out in response to environmental regulations, water-based PSAs will find greater use.
Ultraviolet-cured adhesives
Ultraviolet-cured adhesives became available in the early 1960s but developed rapidly with advances in chemical and equipment technology during the 1980s. These types of adhesive normally consist of a monomer (which also can serve as the solvent) and a low-molecular-weight prepolymer combined with a photoinitiator. Photoinitiators are compounds that break down into free radicals upon exposure to ultraviolet radiation. The radicals induce polymerization of the monomer and prepolymer, thus completing the chain extension and cross-linking required for the adhesive to form. Because of the low process temperatures and very rapid polymerization (from 2 to 60 seconds), ultraviolet-cured adhesives are making rapid advances in the electronic, automotive, and medical areas. They consist mainly of acrylated formulations of silicones, urethanes, and methacrylates. Combined ultraviolet–heat-curing formulations also exist.
Additional Information
An adhesive is a type of substance that holds two or more materials together with cohesive forces and surface attachment in a practical way. Adhesives can be made from and be composed of a variety of substances such as tree sap, bee wax, cement, and epoxy.Ideally, there are two broad adhesive categories, natural and synthetic adhesives. Most commercially found adhesives are synthetic adhesives as they provide better consistency, bond strength, and adaptability compared to natural adhesives. Synthetic adhesives are further classified as consumer adhesives and Industrial adhesives based on their application.
An adhesive’s chemical composition determines its application methods, usage, and bonding strength. Therefore, adhesive manufacturers need to custom-engineer modern synthetic based on the needs of different industries and applications.
Adhesive Applications In Various Industries
1. Bonding:
Bonding is a process in which two surfaces are practically joined together with the help of a suitable adhesive, such as epoxy adhesives. Adhesives are used for bonding materials in various industries, such as electronics, medical, food, optical, chemical and oil and gas industries to bond a range of metals, ceramics, glass, plastics, rubbers and composites.
2. Sealing:
Unlike bonding which sees two surfaces fused together, sealants are ideal for closing gaps and cavities to block fluids, dust, and dirt from either entering or getting out. Sealants are widely used in aerospace, oil and gas, chemical, electronic, optical, automotive and specialty OEM industries.
3. Coating:
Coatings are predominantly used in aerospace, electronic conformal coating, along with some other uses in OEM and oil & chemical industries. Industrial adhesive coatings can provide superior protection against chemicals, dust and moisture, reduce friction, improve abrasion resistance and provide EMI/RFI shielding.
4. Potting:
Potting is an encapsulation method used in the electronics industry to cover small or large electrical components placed inside a housing with a suitable potting material that can withstand high temperatures, protect the circuits from moisture, dirt, dust and other harsh conditions. Potting and encapsulation are used for electronic and microelectronic components, such as sensors, motors, coils, transformers, capacitors, switches, connectors, power supplies, and cable harnesses.
5. Impregnation:
Impregnation is a method used to wet various fibres, such as glass, carbon, kevlar, aramid among others. Once the fibres are completely saturated with the resin, the resin is allowed to fully cure in place forming a composite substrate. Such impregnated composite surfaces are widely used in the aerospace, windmill and electronics and electrical industries.
How Do Adhesives Work?
The working of adhesive depends on the types of bonding process used to attach the surfaces to each other. Mechanical adhesion and chemical adhesion are two types of bondings that can be used to stick one surface to another with adhesives.
Usually, surfaces that need to be attached with the help of adhesives, have a lot of micropores. These pores when filled with adhesives act as grips to keep another surface attached to them. This is called mechanical adhesion. With mechanical adhesion, the adhesives are in liquid form. The liquid adhesives will gradually penetrate the pores during the drying and curing process. You should also keep in mind that mechanical bonding is dependent on the surface roughness and surface energy of the substrates to be bonded. The higher the surface energy and roughness of a substance, the stronger is the bond.
On the other hand, chemical bonding is completely different which sees the surface of a material completely bond with another material on a molecular level. It is a complex process but very effective at the same time. Chemical bonding is further categorised into two types; adsorption and chemisorption depending on the type of bond between the adhesive’s molecules and the surface. Although chemical adhesives are easily available, they are not a common form of adhesive used in Industries.
Types of Adhesives
1. Hot Melt:
Hot melt is a type of thermoplastic polymer adhesive. Thermoplastic polymer adhesives are in a solid state at room temperature. During the application process , they are liquified by heating to be applied as an adhesive. Hot melt adhesives are used for manufacturing and packaging purposes in a wide array of industries due to their superior bonding strength, versatility and setting time. They are also eco-friendly, safe and have a longer shelf life.
Different hot melt adhesives might have different softening points and hardening times as per their applications. Some of the common types of hot melt adhesives are polyurethane, metallocene, EVA hot melt, and polyethene hot melt adhesives.
Let’s talk about reactive hot melt adhesives, which are different from hot melt adhesives. Reactive hot melt adhesives, once applied to a surface and cured, will not be able to melt again as they generate additional chemical bonds during the curing process. This makes reactive hot melt adhesives a better choice than simple hot melt adhesives as they have stronger adherence. Reactive hot melt or RHM are high-quality adhesives also known for their heightened resistance to moisture, and other chemicals with higher thermal stability.
2. Thermosetting:
Thermosetting adhesives are materials which cannot be re-melted after they have cured. Thermosetting adhesives are usually made of two parts, namely, the resin and hardener. However one-part forms can also be found.
There are various types of thermosetting grades such as:
* Phenolics
* Epoxies
* Polyesters
* Polyurethanes
* Silicones
Out of these, epoxy thermosetting resins are the most commonly used in various industries such as electronics and electrical, oil and chemical, automotive, aerospace, optical etc. This is due to their excellent resistance to heat and harsh chemicals and superior mechanical bonding properties.
3. Pressure Sensitive:
Pressure-sensitive adhesives are low-modulus elastomers which means they can be easily taken apart, but are the best choice for light usage. Pressure-sensitive adhesives can be easily found in tapes, bandages, sticky notes, etc. Pressure sensitive adhesives are non-structural adhesives which are not suitable for high-pressure industrial applications. However, they can be used for lighter and thinner material surfaces for which strong adhesives are not suitable. Pressure sensitive adhesives are also cheaper as compared to other adhesive materials and can be found more easily.
4. Contact Adhesive:
Contact adhesives are generally used to create strong mechanical bonds by applying adhesive to both surfaces that are supposed to be bonded together. Contact adhesives are also elastomeric which means the polymers used in the adhesives have rubber-like properties which helps them stay in shape. This gives contact adhesives excellent flexibility and mechanical strength. These adhesives are commonly used in the automotive industry, construction, aerospace and OEM for sealing and coating. They can also be found in rubber cement or countertop laminates. Contact adhesives are ideal for applications which require stability and durability.
Adhesive Application Methods
1. Manual:
As the name suggests, in this method, the applicator uses handheld devices and tools to apply adhesives to the surfaces. Manual adhesive application methods can include spraying, web coating, using a brush and a roller, curtain coating etc. Manual application is cost-effective and is recommended for smaller applications.
2. Glue Applicator:
Glue applicators are handheld devices that assist you to apply adhesives uniformly and at a faster rate than manually. These applicators contain a gun fitted with a cartridge containing the adhesive. A mixing tip is attached to the front of the cartridge to eliminate the need for any manual mixing. These semi-automatic devices enable higher speed, precision and efficiency. Glue applicators are ideal for medium to large-scale applications and are commonly used in the aerospace, electronics and optical industry to fuse small and detailed pieces of equipment.
3. Automatic Dispensing:
Automatic dispensing is ideal for fast-paced and high-volume environments where consistency and quality finish is crucial. This method is more costly as compared to the above two, however, automatic dispensing can increase efficiency, reduce waste and complete the task at a large scale. Metre-mix-dispense systems are used for two component adhesives and robotic dispensing is used for single component adhesives.
Conclusion
Adhesives are used in almost every manufacturing and packaging industry and are an important part of their process. As we have seen, there are different types of adhesives with varying properties suitable for numerous industries.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2697 2026-02-14 17:07:16
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2496) Oceanography
Gist
Oceanography, or marine science, is the interdisciplinary study of the world's oceans, covering 70% of Earth's surface. It integrates biology, chemistry, physics, and geology to examine marine life, ocean currents, seafloor structure, and chemical properties. Key branches include physical, chemical, geological, and biological oceanography, which are vital for understanding climate regulation, marine ecosystems, and resources.
Oceanography is the interdisciplinary study of the ocean, covering its physical, chemical, biological, and geological aspects, from marine life and currents to seafloor geology and its role in climate. It combines fields like biology, chemistry, physics, and geology to understand ocean processes, ecosystems, and the impact of human activities, using tools like research vessels, underwater vehicles, and satellites to explore the 70% of Earth covered by water.
Summary
Oceanography is a scientific discipline concerned with all aspects of the world’s oceans and seas, including their physical and chemical properties, their origin and geologic framework, and the life forms that inhabit the marine environment.
A brief treatment of oceanography follows.
Traditionally, oceanography has been divided into four separate but related branches: physical oceanography, chemical oceanography, marine geology, and marine ecology. Physical oceanography deals with the properties of seawater (temperature, density, pressure, and so on), its movement (waves, currents, and tides), and the interactions between the ocean waters and the atmosphere. Chemical oceanography has to do with the composition of seawater and the biogeochemical cycles that affect it. Marine geology focuses on the structure, features, and evolution of the ocean basins. Marine ecology, also called biological oceanography, involves the study of the plants and animals of the sea, including life cycles and food production.
Oceanography is the sum of these several branches. Oceanographic research entails the sampling of seawater and marine life for close study, the remote sensing of oceanic processes with aircraft and Earth-orbiting satellites, and the exploration of the seafloor by means of deep-sea drilling and seismic profiling of the terrestrial crust below the ocean bottom. Greater knowledge of the world’s oceans enables scientists to more accurately predict, for example, long-term weather and climatic changes and also leads to more efficient exploitation of the Earth’s resources. Oceanography also is vital to understanding the effect of pollutants on ocean waters and to the preservation of the quality of the oceans’ waters in the face of increasing human demands made on them.
Details
Oceanography, also known as oceanology, sea science, ocean science, and marine science, is the scientific study of the ocean, including its physics, chemistry, biology, and geology.
It is an Earth science, which covers a wide range of topics, including ocean currents, waves, and geophysical fluid dynamics; fluxes of various chemical substances and physical properties within the ocean and across its boundaries; ecosystem dynamics; and plate tectonics and seabed geology.
Oceanographers draw upon a wide range of disciplines to deepen their understanding of the world’s oceans, incorporating insights from astronomy, biology, chemistry, geography, geology, hydrology, meteorology and physics.
Modern oceanography
Knowledge of the oceans remained confined to the topmost few fathoms of the water and a small amount of the bottom, mainly in shallow areas. Almost nothing was known of the ocean depths. The British Royal Navy's efforts to chart all of the world's coastlines in the mid-19th century reinforced the vague idea that most of the ocean was very deep, although little more was known. As exploration ignited both popular and scientific interest in the polar regions and Africa, so too did the mysteries of the unexplored oceans.
The seminal event in the founding of the modern science of oceanography was the 1872–1876 Challenger expedition. As the first true oceanographic cruise, this expedition laid the groundwork for an entire academic and research discipline. In response to a recommendation from the Royal Society, the British Government announced in 1871 an expedition to explore world's oceans and conduct appropriate scientific investigation. Charles Wyville Thomson and Sir John Murray launched the Challenger expedition. Challenger, leased from the Royal Navy, was modified for scientific work and equipped with separate laboratories for natural history and chemistry. Under the scientific supervision of Thomson, Challenger travelled nearly 70,000 nautical miles (130,000 km) surveying and exploring. On her journey circumnavigating the globe, 492 deep sea soundings, 133 bottom dredges, 151 open water trawls and 263 serial water temperature observations were taken. Around 4,700 new species of marine life were discovered. The result was the Report Of The Scientific Results of the Exploring Voyage of H.M.S. Challenger during the years 1873–76. Murray, who supervised the publication, described the report as "the greatest advance in the knowledge of our planet since the celebrated discoveries of the fifteenth and sixteenth centuries". He went on to found the academic discipline of oceanography at the University of Edinburgh, which remained the centre for oceanographic research well into the 20th century. Murray was the first to study marine trenches and in particular the Mid-Atlantic Ridge, and map the sedimentary deposits in the oceans. He tried to map out the world's ocean currents based on salinity and temperature observations, and was the first to correctly understand the nature of coral reef development.
In the late 19th century, other Western nations also sent out scientific expeditions (as did private individuals and institutions). The first purpose-built oceanographic ship, Albatros, was built in 1882. In 1893, Fridtjof Nansen allowed his ship, Fram, to be frozen in the Arctic ice. This enabled him to obtain oceanographic, meteorological and astronomical data at a stationary spot over an extended period.
In 1881 the geographer John Francon Williams published a seminal book, Geography of the Oceans. Between 1907 and 1911 Otto Krümmel published the Handbuch der Ozeanographie, which became influential in awakening public interest in oceanography. The four-month 1910 North Atlantic expedition headed by John Murray and Johan Hjort was the most ambitious research oceanographic and marine zoological project ever mounted until then, and led to the classic 1912 book The Depths of the Ocean.
The first acoustic measurement of sea depth was made in 1914. Between 1925 and 1927 the "Meteor" expedition gathered 70,000 ocean depth measurements using an echo sounder, surveying the Mid-Atlantic Ridge.
In 1934, Easter Ellen Cupp, the first woman to have earned a PhD (at Scripps) in the United States, completed a major work on diatoms that remained the standard taxonomy in the field until well after her death in 1999. In 1940, Cupp was let go from her position at Scripps. Sverdrup specifically commended Cupp as a conscientious and industrious worker and commented that his decision was no reflection on her ability as a scientist. Sverdrup used the instructor billet vacated by Cupp to employ Marston Sargent, a biologist studying marine algae, which was not a new research program at Scripps. Financial pressures did not prevent Sverdrup from retaining the services of two other young post-doctoral students, Walter Munk and Roger Revelle. Cupp's partner, Dorothy Rosenbury, found her a position teaching high school, where she remained for the rest of her career.
Sverdrup, Johnson and Fleming published The Oceans in 1942, which was a major landmark. The Sea (in three volumes, covering physical oceanography, seawater and geology) edited by M.N. Hill was published in 1962, while Rhodes Fairbridge's Encyclopedia of Oceanography was published in 1966.
The Great Global Rift, running along the Mid Atlantic Ridge, was discovered by Maurice Ewing and Bruce Heezen in 1953 and mapped by Heezen and Marie Tharp using bathymetric data; in 1954 a mountain range under the Arctic Ocean was found by the Arctic Institute of the USSR. The theory of seafloor spreading was developed in 1960 by Harry Hammond Hess. The Ocean Drilling Program started in 1966. Deep-sea vents were discovered in 1977 by Jack Corliss and Robert Ballard in the submersible DSV Alvin.
In the 1950s, Auguste Piccard invented the bathyscaphe and used the bathyscaphe Trieste to investigate the ocean's depths. The United States nuclear submarine Nautilus made the first journey under the ice to the North Pole in 1958. In 1962 the FLIP (Floating Instrument Platform), a 355-foot (108 m) spar buoy, was first deployed.
In 1968, Tanya Atwater led the first all-woman oceanographic expedition. Until that time, gender policies restricted women oceanographers from participating in voyages to a significant extent.
From the 1970s, there has been much emphasis on the application of large scale computers to oceanography to allow numerical predictions of ocean conditions and as a part of overall environmental change prediction. Early techniques included analog computers (such as the Ishiguro Storm Surge Computer) generally now replaced by numerical methods (e.g. SLOSH.) An oceanographic buoy array was established in the Pacific to allow prediction of El Niño events.
1990 saw the start of the World Ocean Circulation Experiment (WOCE) which continued until 2002. Geosat seafloor mapping data became available in 1995.
Study of the oceans is critical to understanding shifts in Earth's energy balance along with related global and regional changes in climate, the biosphere and biogeochemistry. The atmosphere and ocean are linked because of evaporation and precipitation as well as thermal flux (and solar insolation). Recent studies have advanced knowledge on ocean acidification, ocean heat content, ocean currents, sea level rise, the oceanic carbon cycle, the water cycle, Arctic sea ice decline, coral bleaching, marine heatwaves, extreme weather, coastal erosion and many other phenomena in regards to ongoing climate change and climate feedbacks.
In general, understanding the world ocean through further scientific study enables better stewardship and sustainable utilization of Earth's resources. The Intergovernmental Oceanographic Commission reports that 1.7% of the total national research expenditure of its members is focused on ocean science.
Additional Information
Oceanography is an interdisciplinary science where math, physics, chemistry, biology and geology intersect.
Traditionally, we discuss oceanography in terms of four separate but related branches: physical oceanography, chemical oceanography, biological oceanography and geological oceanography.
Physical oceanography involves the study of the properties (temperature, density, etc.) and movement (waves, currents, and tides) of seawater and the interaction between the ocean and the atmosphere.
Chemical oceanography involves the study of the composition of seawater and the biogeochemical cycles that affect it.
Biological oceanography involves the study of the biological organisms in the ocean (including life cycles and food production) such as bacteria, phytoplankton, zooplankton and extending to the more traditional marine biology focus of fish and marine mammals.
Geological oceanography focuses on the structure, features, and evolution of the ocean basins.
Oceanography is greater than the sum of these specific branches. Oceanographers use a variety of tools to study the ocean, and many of these studies involve more than one branch. Oceanographers collect discrete water, sediment and biological samples using ships (Research Vessels). They deploy autonomous sampling systems such as buoys and gliders to collect data over time and space scales that cannot be done with a ship. Remote sensing from aircraft and satellites allows oceanographers to get a global view of some parameters. Modeling allows oceanographers to look at the past and predict the future state of the ocean (e.g circulation, air-sea interactions, sustainability of fisheries, quality of water, etc.).
The knowledge gained from all of these types of measurements allows oceanographers to do many things including, but not limited to:
* better predict (using models) changes in weather and climate improve the forecast for hazards; natural (e.g. hurricanes) or man-made (e.g. oil spills)
* assess the impact of pollutants on the quality of water in the ocean
* protect the quality of the water in the ocean in the face of increasing human demands (e.g. fisheries, tourism, shipping, offshore oil & gas, offshore wind farms, etc.).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2698 2026-02-15 18:43:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2497) Jaguar
Gist
Jaguars rarely attack humans and will almost never do so without provocation. They will, however, attack and kill livestock that local farmers rely on for their livelihoods. This prompts local communities to hunt and kill jaguars in retaliation and to protect their herds.
There is a high risk associated with keeping this species as a pet. This is not a suitable pet.
Summary
The jaguar (Panthera onca) is a large cat species and the only living member of the genus Panthera that is native to the Americas. Its distinctively marked coat features pale yellow to tan colored fur covered by spots that transition to rosettes on the sides, although a melanistic black coat appears in some individuals. With a body length of up to 1.85 m (6 ft 1 in) and a weight of up to 158 kg (348 lb), it is the biggest cat species in the Americas and the third largest in the world. The jaguar's powerful bite allows it to pierce the carapaces of turtles and tortoises, and to employ an unusual killing method: it bites directly through the skull of mammalian prey between the ears to deliver a fatal blow to the brain.
The modern jaguar's ancestors probably entered the Americas from Eurasia during the Early Pleistocene via the land bridge that once spanned the Bering Strait. The oldest jaguar fossils found in North America date to between 0.85 to 0.82 million years ago. Today, the jaguar's range extends from the Southwestern United States across Mexico and much of Central America, the Amazon rainforest and south to Paraguay and northern Argentina. It inhabits a variety of forested and open terrains, but its preferred habitat is tropical and subtropical moist broadleaf forest, wetlands and wooded regions. It is adept at swimming and is largely a solitary, opportunistic, stalk-and-ambush apex predator. As a keystone species, it plays an important role in stabilizing ecosystems and in regulating prey populations.
The jaguar is threatened by habitat loss, habitat fragmentation, poaching for trade with its body parts and killings in human–wildlife conflict situations, particularly with ranchers in Central and South America. It has been listed as Near Threatened on the IUCN Red List since 2002. The wild population is thought to have declined since the late 1990s. Priority areas for jaguar conservation comprise 51 large areas inhabited by at least 50 breeding individuals, called Jaguar Conservation Units. They are located in 36 geographic regions from Mexico to Argentina.
The jaguar has featured prominently in the mythology of indigenous peoples of the Americas, including those of the Aztec and Maya civilizations.
Details:
What is the jaguar?
Jaguars are the only big cat in the Americas and the third biggest in the world after tigers and lions. They look a lot like leopards, which live in Africa and Asia, but jaguars’ spots are more complex and often have a dot in the center.
These powerful cats were worshipped as gods in many ancient South American cultures, and representations of the jaguar show up in the art and archaeology of pre-Columbian cultures across the jaguar’s range.
Diet and behavior
Unlike many other cats, jaguars do not avoid water. In fact, they are quite good swimmers. They hunt fish, turtles, and even caimans, using their incredibly powerful jaws to pierce the animals’ skulls. Jaguars also eat deer, peccaries, capybaras, tapirs, and a number of other land animals, which they prefer to ambush at night.
Jaguars live alone, and they’re territorial—they define their area by marking with their waste or clawing trees.
Females have litters of one to four cubs, which are blind and helpless at birth. The mother stays with them and defends them fiercely from any animal that may approach—even their own father. Young jaguars learn to hunt by living with their mothers for two years or more.
Range and habitat
Jaguars once roamed broadly from central Argentina all the way up to the southwestern United States. Since the 1880s, they’ve lost more than half their territory. Their main stronghold today is the Amazon Basin, though they still exist in smaller numbers through Central America as well.
They’re typically found in tropical rainforests but also live in savannas and grasslands.
Threats to survival
Jaguars face a number of threats, including habitat fragmentation and illegal killing. South and Central America’s high rates of deforestation—for grazing land, agriculture, and other uses—have not only destroyed jaguars’ habitat but also broken it up. Fragmented forests mean that cats get boxed into patches of forest and can’t travel far to find new mates. That kind of isolation can lead to inbreeding and local extinctions.
Another threat jaguars face is retaliatory killings from ranchers. As grazing land replaces forests, jaguars are more likely to hunt cattle. In response—and sometimes in anticipation—cattle owners kill jaguars.
Poaching is another growing problem for jaguars. They’ve long been hunted for their pelts, and now there’s a growing illegal, international trade in jaguar teeth and jaguar bone products going to China.
Conservation
Jaguars are classified as near-threatened by the International Union for the Conservation of Nature. The species has national protections in almost every country it’s found, and trade in its parts is banned by CITES, a global treaty that regulates the cross-border wildlife trade. Still, poaching and the illegal trade continues so strengthening law enforcement is important.
There are major efforts to support and develop jaguar corridors to connect isolated populations as well as to work with ranchers to reduce human-jaguar conflict. Workshops help ranchers learn better husbandry practices, and a growing number of programs compensate ranchers when they lose cattle to jaguars, so that they’re less motivated to kill the cat in retaliation.
Fighting deforestation, which a number of international NGOs and indigenous groups are involved in, is critical.
Additional Information
Big cats are some of the most captivating wild animals. Elusive, powerful, and graceful. Lions and tigers are easily identifiable and greatly loved, but there’s far more to the world of big cats than these icons.
Jaguars are some of the most beautiful and mysterious big cats in the world, but few people know much about them. World Animal Protection wants to put that right.
We’ve produced a short documentary Jaguar Spirit, to highlight these incredible animals and the challenges they face. To get you ready for the documentary, let’s whet your appetite with some amazing jaguar facts.
1. Jaguars are the third largest cats in the world
Jaguars are “big” cats packed into a smaller form factor. They’re the largest cat in the Americas and are the third largest cat in the world, behind tigers and lions. Male Jaguars weigh up to 120kg, while the smaller females reach a maximum of 100kg. To put this in context, jaguars are about half the weight of a lion, but more than double that of a cheetah.
However, jaguars are no taller than a meter, making them powerful but relatively compact compared to lions and tigers.
2. Jaguars were revered by Mesoamerican civilizations
In Mesoamerican indigenous communities, jaguars were one of the most revered animals. Every major Mesoamerican civilization had a jaguar god, many of which were important parts of their religion. These communities viewed jaguars as symbols of strength and power.
For example, the Aztecs named their most elite warriors “cuāuhocēlōtl”, a combination of the Aztec words for eagle and jaguar. They wore costumes that made them resemble these majestic cats and lead their communities both on the battlefield and at home. In Aztec culture, many believed that shamans could transform into jaguars at will and would become jaguars after they died.
3. Jaguar spots are unique
Learning to tell jaguars apart from other big cats is easy. They have beautiful tan and orange fur covered with black spots known as rosettes.
These rosettes are different from the spots on any other cat. Each rosette is a jagged circle that makes up an outer ring with a single black spot in the middle.
Other spotted cats have the ring that characterises the rosettes, but none have the central spot of the jaguar.
4. Jaguars love swimming
Unlike most domestic cats, jaguars are excellent swimmers and love the water. They can fully submerge and dive in pursuit of prey if needed and won’t hesitate to attack prey animals in the water.
5. Jaguars look after their cubs
Jaguars don’t have big litters. They typically have two cubs at one time (though they can have up to four). The cubs are born completely helpless and blind. They remain with their mother for two years as they grow and learn to hunt and take care of themselves.
Jaguars are sentient beings, meaning they think and feel and have personalities and needs of their own. The breakthrough research and studies of animals' feelings and emotions show how the life of a wild animal, like a jaguar, is mentally and physically better in the wild with its cubs.
6. Jaguars are exploited for commercial purposes
Jaguars aren’t some of the most endangered animals in the world, but that doesn’t mean that the species is thriving. There are only 173,000 jaguars left in the wild, the population of jaguars is “near threatened”. Regardless of their status, jaguars should not be exploited for any commercial purposes. They are wildlife and deserve a life in the wild.
7. Jaguars live primarily in the rainforest
As opportunistic ambush predators, jaguars aren’t well-suited to large open spaces. Instead, the natural habitat of jaguars is rainforests and wetlands. Approximately half of the world’s population of jaguars live in Brazil.
The rest can be found in Mexico and Central and South America, including northern Argentina.
8. Jaguars are fast
Cheetahs are the first to come to mind when you think of fast runners in the animal kingdom. Jaguars can’t quite reach the 70 miles per hour of the cheetah, but they reach a very respectable 50mph.
This makes them the second-fastest big cats in the world. They also have an impressive ability to jump and climb when necessary.
9. Jaguars aren’t fussy eaters
Jaguars are carnivores, like all other cats. On land, they will commonly hunt deer, capybaras, and tapirs. And they’re just as comfortable in the water, seeking out fish, turtles, and caiman to meet their appetite.
As opportunistic hunters, however, they will prey on almost any animal they come across.
These techniques and their incredible bite power allows them to dispatch prey as large as cows.
10. Jaguars are crepuscular
Most of us think of animals as being either diurnal or nocturnal, but jaguars don’t fit into this pattern. They can be active either during the day or at night.
In fact, jaguars are considered crepuscular and nocturnal, meaning they’re most active during dusk and dawn. These are the times when they’re most likely to be hunting. That said, it’s also common to find them hunting during the day.
11. Jaguars travel long distances
Jaguars can regularly travel over six miles per day in search of food. They’re solitary animals, marking their territory and only getting together with others to mate. Males do not remain with females to avoid the risk of infanticide.
Where space allows, jaguars can have territories of up to 54 square miles.
12. Jaguars are struggling with the loss of their habitats
Jaguars are being threatened because their habitats are being lost to deforestation and other human activities. Much of this land has been appropriated for agricultural activities such as cattle ranching and crops used to produce animal feed.
Any reduction in habitat makes it harder for these solitary cats to maintain sufficient territory to feed themselves. It also increases the risk of conflict between individual animals.
13. Jaguar prey have also reduced in number
The loss of habitat has also impacted the animals that jaguars rely on for food. When prey becomes scarce, jaguars start looking for alternative food sources, including agricultural livestock.
The people living in areas frequented by jaguars attempt to protect their animals, which unfortunately often leads to killing the big cats threatening their livelihood.
14. Jaguars are facing increased risk from fire
Jaguar territories are increasingly at risk from fire, threatening both jaguars and the prey animals they rely on, further exacerbating the problems they already face.
Factory farming methods are becoming increasingly common throughout the Amazon, with catastrophic consequences. Fires are often deliberately set to clear large swathes of land quickly.
Once rainforests have been removed, they are replaced with monoculture crops, which are more vulnerable to wildfires than the original ecosystems.
15. Jaguars are popular targets for poaching
Despite their impressive camouflage, speed, and agility, jaguars are popular with poachers. Their beautiful fur means that they command high prices for their pelts and skins, and their sharp teeth and claws are seen as valuable trophies.
Beyond poaching for trophies, jaguars are often captured and sold as exotic pets, to private wildlife collectors, or as tourist attractions.
16. Jaguars are used in traditional Asian medicine
Jaguars are also often killed for use in traditional Asian medicine. They are typically boiled whole until they reduce down into a paste used for various purposes, from treating arthritis to improving sexual performance.
None of these animal-derived products are scientifically validated or effective.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2699 2026-02-16 18:14:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2498) Food Chain
Gist
A food chain is a linear sequence illustrating how energy and nutrients transfer between organisms in an ecosystem, starting from producers and moving through various consumers to decomposers. It typically begins with plants (producers) capturing sunlight, followed by herbivores (primary consumers), carnivores (secondary/tertiary consumers), and finally decomposers.
A food chain is a linear sequence showing how energy and nutrients move through an ecosystem when one organism eats another, starting with producers (like plants) and moving up through consumers (herbivores, carnivores, omnivores) to decomposers, illustrating dependence for survival. Arrows in a food chain represent the flow of energy, pointing from the organism being eaten to the organism that eats it (e.g., Grass → Grasshopper → Frog).
Summary
A food chain outlines who eats whom. A food web is all of the food chains in an ecosystem. Each organism in an ecosystem occupies a specific trophic level or position in the food chain or web. Producers, who make their own food using photosynthesis or chemosynthesis, make up the bottom of the trophic pyramid. Primary consumers, mostly herbivores, exist at the next level, and secondary and tertiary consumers, omnivores and carnivores, follow. At the top of the system are the apex predators: animals who have no predators other than humans.
Details
A food chain is a linear network of links in a food web, often beginning with an autotroph (such as grass or algae), also called a producer, and typically ending at an apex predator (such as grizzly bears or killer whales), detritivore (such as earthworms and woodlice), or decomposer (such as fungi or bacteria). A food web is distinct from a food chain. A food chain illustrates the associations between organisms according to the energy sources they consume in trophic levels, and the most common way to quantify them is in length: the number of links between a trophic consumer and the base of the chain.
Studies of food chains are essential to many biological studies.
Stability of the food chain is crucial for survival of most species. Removing even one component from the food chain could result in extinction or significant decreases in a species' probability of surviving. Many food chains and food webs contain a keystone species, a species that could directly affect the food chain and has a significant impact on the environment. The absence of a keystone species could destroy the balance of the entire food chain.
The efficiency of a food chain depends on the energy first consumed by the primary producers. This energy then moves through the trophic levels.
History
Food chains were first discussed by al-Jahiz, a 10th century Arab philosopher. The modern concepts of food chains and food webs were introduced by Charles Elton.
Food chain versus food web
A food chain differs from a food web as a food chain follows a direct linear pathway of consumption and energy transfer. Natural interconnections between food chains make a food web, which are non-linear and depict interconnecting pathways of consumption and energy transfer.
Trophic levels
Food chain models typically predict that communities are controlled by predators at the top and plants (autotrophs or producers) at the bottom.
Thus, the foundation of the food chain typically consists of primary producers. Primary producers, or autotrophs, utilize energy derived from either sunlight or inorganic chemical compounds to create complex organic compounds, such as starch, for energy. Because the sun's light is necessary for photosynthesis, most life could not exist if the sun disappeared. Even so, it has recently been discovered that there are some forms of life, chemotrophs, that appear to gain all their metabolic energy from chemosynthesis driven by hydrothermal vents, thus showing that some life may not require solar energy to thrive. Chemosynthetic bacteria and archaea use hydrogen sulfide and methane from hydrothermal vents and cold seeps as an energy source (just as plants use sunlight) to produce carbohydrates; they form the base of the food chain in regions with little to no sunlight. Regardless of where the energy is obtained, a species that produces its own energy lies at the base of the food chain model, and is a critically important part of an ecosystem.
Higher trophic levels cannot produce their own energy and so must consume producers or other life that itself consumes producers. In the higher trophic levels lies consumers (secondary consumers, tertiary consumers, etc.). Consumers are organisms that eat other organisms. All organisms in a food chain, except the first organism, are consumers. Secondary consumers eat and obtain energy from primary consumers, tertiary consumers eat and obtain energy from secondary consumers, etc.
At the highest trophic level is typically an apex predator, a consumer with no natural predators in the food chain model.
When any trophic level dies, detritivores and decomposers consume their organic material for energy and expel nutrients into the environment in their waste. Decomposers and detritivores break down the organic compounds into simple nutrients that are returned to the soil. These are the simple nutrients that plants require to create organic compounds. It is estimated that there are more than 100,000 different decomposers in existence.
Models of trophic levels also often model energy transfer between trophic levels. Primary consumers get energy from the producer and pass it to the secondary and tertiary consumers.
Studies
Food chains are vital in ecotoxicology studies, which trace the pathways and biomagnification of environmental contaminants. It is also necessary to consider interactions amongst different trophic levels to predict community dynamics; food chains are often the base level for theory development of trophic levels and community/ecosystem investigations.
Additional Information
Food chain, in ecology, is the sequence of transfers of matter and energy in the form of food from organism to organism. Food chains intertwine locally into a food web because most organisms consume more than one type of animal or plant. Plants, which convert solar energy to food by photosynthesis, are the primary food source. In a predator chain, a plant-eating animal is eaten by a flesh-eating animal. In a parasite chain, a smaller organism consumes part of a larger host and may itself be parasitized by even smaller organisms. In a saprophytic chain, microorganisms live on dead organic matter.
Because energy, in the form of heat, is lost at each step, or trophic level, chains do not normally encompass more than four or five trophic levels. People can increase the total food supply by cutting out one step in the food chain: instead of consuming animals that eat cereal grains, the people themselves consume the grains. Because the food chain is made shorter, the total amount of energy available to the final consumers is increased.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2700 2026-02-17 18:18:13
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,435
Re: Miscellany
2499) Induction Coil
Gist
An induction coil is an electrical transformer that converts low-voltage direct current (DC) into high-voltage pulses using a primary coil, a secondary coil with many more turns, and an iron core. It operates by interrupting DC with a magnetic vibrator, causing a rapidly collapsing magnetic field that induces high voltage, creating sparks.
An induction coil is defined as a component used in induction heating that generates eddy currents and heat through a varying magnetic field, and can be designed in various forms such as single turn, multi-turn, pancake, hairpin, or split coils, depending on the application and substrate geometry.
Summary
An induction coil or "spark coil" is a type of electrical transformer. It is used to produce high-voltage pulses from a low-voltage direct current (DC) supply. To create the flux changes necessary to induce voltage in the secondary coil, the direct current in the primary coil is repeatedly interrupted by a vibrating mechanical contact called an interrupter.
The induction coil was the first type of transformer. It was widely used in X-ray machines, spark-gap radio transmitters, arc lighting and quack medical devices from the 1880s to the 1920s. Today its only common use is for ignition coils in internal combustion engines and in physics education to demonstrate induction.
Details
An induction coil or "spark coil" (archaically known as an inductorium or Ruhmkorff coil after Heinrich Rühmkorff) is a type of transformer used to produce high-voltage pulses from a low-voltage direct current (DC) supply. To create the flux changes necessary to induce voltage in the secondary coil, the direct current in the primary coil is repeatedly interrupted by a vibrating mechanical contact called an interrupter. Invented in 1836 by the Irish-Catholic priest Nicholas Callan, also independently by American inventor Charles Grafton Page, the induction coil was the first type of transformer. It was widely used in x-ray machines, spark-gap radio transmitters, arc lighting and quack medical electrotherapy devices from the 1880s to the 1920s. Today its only common use is as the ignition coils in internal combustion engines and in physics education to demonstrate induction.
Construction and function
An induction coil consists of two coils of insulated wire wound around a common iron core (M). One coil, called the primary winding (P), is made from relatively few (tens or hundreds) turns of coarse wire. The other coil, the secondary winding, (S) typically consists of up to a million turns of fine wire (up to 40 gauge).
An electric current is passed through the primary, creating a magnetic field. Because of the common core, most of the primary's magnetic field couples with the secondary winding. The primary behaves as an inductor, storing energy in the associated magnetic field. When the primary current is suddenly interrupted, the magnetic field rapidly collapses. This causes a high voltage pulse to be developed across the secondary terminals through electromagnetic induction. Because of the large number of turns in the secondary coil, the secondary voltage pulse is typically many thousands of volts. This voltage is often sufficient to cause an electric spark, to jump across an air gap (G) separating the secondary's output terminals. For this reason, induction coils were called spark coils.
An induction coil is traditionally characterised by the length of spark it can produce; a '4 inch' (10 cm) induction coil could produce a 4 inch spark. Until the development of the cathode ray oscilloscope, this was the most reliable measurement of peak voltage of such asymmetric waveforms. The relationship between spark length and voltage is linear within a wide range:
4 inches (10 cm) = 110kV; 8 inches (20 cm) = 150kV; 12 inches (30 cm) = 190kV; 16 inches (41 cm) = 230kV
Curves supplied by a 1984 reference agree closely with those values.
Interrupter
To operate the coil continually, the direct current must be repeatedly connected and disconnected to create the magnetic field changes needed for induction. To do that, induction coils use a magnetically activated vibrating arm called an interrupter or break (A) to rapidly connect and break the current flowing into the primary coil. The interrupter is mounted on the end of the coil next to the iron core. When the power is turned on, the increasing current in the primary coil produces an increasing magnetic field, the magnetic field attracts the interrupter's iron armature (A). After a time, the magnetic attraction overcomes the armature's spring force, and the armature begins to move. When the armature has moved far enough, the pair of contacts (K) in the primary circuit open and disconnect the primary current. Disconnecting the current causes the magnetic field to collapse and create the spark. Also, the collapsed field no longer attracts the armature, so the spring force accelerates the armature toward its initial position. A short time later the contacts reconnect, and the current starts building the magnetic field again. The whole process starts over and repeats many times per second.
Opposite potentials are induced in the secondary when the interrupter breaks the circuit and closes the circuit. However, the current change in the primary is much more abrupt when the interrupter breaks. When the contacts close, the current builds up slowly in the primary because the supply voltage has a limited ability to force current through the coil's inductance. In contrast, when the interrupter contacts open, the current falls to zero suddenly. So the pulse of voltage induced in the secondary at break is much larger than the pulse induced at close, it is the break that generates the coil's high-voltage output.
Capacitor
An arc forms at the interrupter contacts on break which has undesirable effects: the arc consumes energy stored in the magnetic field, reduces the output voltage, and damages the contacts. To prevent this, a quenching capacitor (C) of 0.5 to 15 μF is connected across the primary coil to slow the rise in the voltage after a break. The capacitor and primary winding together form a tuned circuit, so on break, a damped sinusoidal wave of current flows in the primary and likewise induces a damped wave in the secondary. As a result, the high-voltage output consists of a series of damped waves.
Construction details
To prevent the high voltages generated in the coil from breaking down the thin insulation and arcing between the secondary wires, the secondary coil uses special construction so as to avoid having wires carrying large voltage differences lying next to each other. In one widely used technique, the secondary coil is wound in many thin flat pancake-shaped sections (called "pies"), connected in series.
The primary coil is first wound on the iron core and insulated from the secondary with a thick paper or rubber coating. Then each secondary subcoil is connected to the coil next to it and slid onto the iron core, insulated from adjoining coils with waxed cardboard disks. The voltage developed in each subcoil isn't large enough to jump between the wires in the subcoil. Large voltages are only developed across many subcoils in series, which are too widely separated to arc over. To give the entire coil a final insulating coating, it is immersed in melted paraffin wax or rosin; the air evacuated to ensure there are no air bubbles left inside and the paraffin allowed to solidify, so the entire coil is encased in wax.
To prevent eddy currents, which cause energy losses, the iron core is made of a bundle of parallel iron wires, individually coated with shellac to insulate them electrically. The eddy currents, which flow in loops in the core perpendicular to the magnetic axis, are blocked by the layers of insulation. The ends of the insulated primary coil often protruded several inches from either end of the secondary coil, to prevent arcs from the secondary to the primary or the core.
Additional Information
An induction heating system consists of an induction power supply for converting line power to an alternating current and delivering it to a workhead, and a work coil for generating an electromagnetic field within the coil. The work piece is positioned in the coil such that this field induces a current in the work piece, which in turn produces heat.
The water-cooled coil is positioned around or bordering the work piece. It does not contact the work piece, and the heat is only produced by the induced current transmitted through the work piece. The material used to make the work piece can be a metal such as copper, aluminum, steel, or brass. It can also be a semiconductor such as graphite, carbon or silicon carbide.
For heating non-conductive materials such as plastics or glass, induction can be used to heat an electrically-conductive susceptor e.g., graphite, which then passes the heat to the non-conducting material.
Induction heating finds applications in processes where temperatures are as low as 100ºC (212°F) and as high as 3000°C (5432°F). It is also used in short heating processes lasting for less than half a second and in heating processes that extend over several months.
Induction heating is used both domestic and commercial cooking, in several applications such as heat treating, soldering, preheating for welding, melting, shrink fitting in industry, sealing, brazing, curing, and in research and development.
How Does Induction Heating Work?
Induction produces an electromagnetic field in a coil to transfer energy to a work piece to be heated. When the electrical current passes along a wire, a magnetic field is produced around that wire.
Key Benefits of Induction
The benefits of induction are:
* Efficient and quick heating
* Accurate, repeatable heating
* Safe heating as there is no flame
* Prolonged life of fixturing due to accurate heating
Methods of Induction Heating
Induction heating is done using two methods:
The first method is referred to as eddy current heating from the I²R losses caused from the resistivity of a work piece’s material. The second is referred to as hysteretic heating, in which energy is produced within a part by the alternating magnetic field generated by the coil modifying the component’s magnetic polarity.
Hysteretic heating occurs in a component up to the Curie temperature when the material’s magnetic permeability decreases to 1 and hysteretic heating is reduced. Eddy current heating constitutes the remaining induction heating effect.
When there is a change in the direction of electrical current (AC) the magnetic field generated fails, and is produced in the reverse direction, as the direction of the current is reversed. When a second wire is positioned in that alternating magnetic field, an alternating current is produced in the second wire.
The current transmitted through the second wire and that through the first wire are proportional to each other and also to the inverse of the square of the distance between them.
When the wire in this model is substituted with a coil, the alternating current on the coil generates an electromagnetic field and while the work piece to be heated is in the field, the work piece matches to the second wire and an alternating current is produced in the work piece. The I²R losses of the material resistivity of the work piece causes heat to be created in the work piece of the work piece’s material resistivity. This is called eddy current heating.
Working of an Induction Coil
With the help of an alternating electric field, energy is transmitted to the work piece with a work coil.
The alternating current passing via the coil produces the electromagnetic field which induces a current passing in the work piece as a mirror image to the current passing in the work coil. The work coil/inductor is a part of the induction heating system that displays the effectiveness and efficiency of the work piece when it is heated. Work coils are of numerous types ranging from complex to simple.
The helical wound (or solenoid) coil is an example of simple coil, which consists of many turns of copper tube wound around a mandrel. A coil precision-machined from solid copper and brazed together is an example of complex coil.
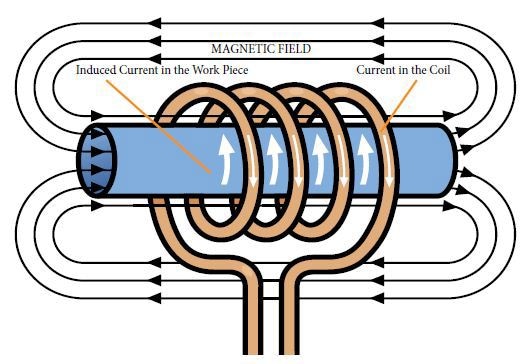
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline