Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2526 2025-06-03 20:36:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 50,890
Re: Miscellany
2326) Plastics/Plastic Tecnology
Gist
Plastics are polymers, which means they are made by linking chains of the molecules (called monomers) together to create a large molecule (a polymer). An example of this is polystyrene. These links make polymers strong and durable. That's why poly- appears in common names for plastic, like polyethylene.
Plastic technology encompasses the science, engineering, and practices involved in creating, processing, and utilizing plastic materials. It involves understanding the properties of polymers, developing new materials, and designing and manufacturing plastic products for various applications.
Plastic is defined as a material that contains an essential ingredient an organic substance of large molecular weight. It is also defined as polymers of long carbon chains. Carbon atoms are linked in chains and are produced in long-chain molecules
Summary
Plastics are a wide range of synthetic or semisynthetic materials composed primarily of polymers. Their defining characteristic, plasticity, allows them to be molded, extruded, or pressed into a diverse range of solid forms. This adaptability, combined with a wide range of other properties such as low weight, durability, flexibility, chemical resistance, low toxicity, and low-cost production, has led to their widespread use around the world. While most plastics are produced from natural gas and petroleum, a growing minority are produced from renewable resources like polylactic acid.
Between 1950 and 2017, 9.2 billion metric tons of plastic are estimated to have been made, with more than half of this amount being produced since 2004. In 2023 alone, preliminary figures indicate that over 400 million metric tons of plastic were produced worldwide. If global trends in plastic demand continue, it is projected that annual global plastic production will exceed 1.3 billion tons by 2060. The primary uses for plastic include packaging, which makes up about 40% of its usage, and building and construction, which makes up about 20% of its usage.
The success and dominance of plastics since the early 20th century has had major benefits for mankind, ranging from medical devices to light-weight construction materials. The sewage systems in many countries relies on the resiliency and adaptability of polyvinyl chloride. It is also true that plastics are the basis of widespread environmental concerns, due to their slow decomposition rate in natural ecosystems. Most plastic produced has not been reused. Some is unsuitable for reuse. Much is captured in landfills or as plastic pollution. Particular concern focuses on microplastics. Marine plastic pollution, for example, creates garbage patches. Of all the plastic discarded so far, some 14% has been incinerated and less than 10% has been recycled.
In developed economies, about a third of plastic is used in packaging and roughly the same in buildings in applications such as piping, plumbing or vinyl siding. Other uses include automobiles (up to 20% plastics), furniture, and toys. In the developing world, the applications of plastic may differ; 42% of India's consumption is used in packaging. Worldwide, about 50 kg of plastic is produced annually per person, with production doubling every ten years.
The world's first fully synthetic plastic was Bakelite, invented in New York in 1907, by Leo Baekeland, who coined the term "plastics". Dozens of different types of plastics are produced today, such as polyethylene, which is widely used in product packaging, and polyvinyl chloride (PVC), used in construction and pipes because of its strength and durability. Many chemists have contributed to the materials science of plastics, including Nobel laureate Hermann Staudinger, who has been called "the father of polymer chemistry", and Herman Mark, known as "the father of polymer physics".
Structure
Most plastics contain organic polymers. The vast majority of these polymers are formed from chains of carbon atoms, with or without the attachment of oxygen, nitrogen or sulfur atoms. These chains comprise many repeating units formed from monomers. Each polymer chain consists of several thousand repeating units. The backbone is the part of the chain that is on the main path, linking together a large number of repeat units. To customize the properties of a plastic, different molecular groups called side chains hang from this backbone; they are usually attached to the monomers before the monomers themselves are linked together to form the polymer chain. The structure of these side chains influences the properties of the polymer.
Details
A plastic is a polymeric material that has the capability of being molded or shaped, usually by the application of heat and pressure. This property of plasticity, often found in combination with other special properties such as low density, low electrical conductivity, transparency, and toughness, allows plastics to be made into a great variety of products. These include tough and lightweight beverage bottles made of polyethylene terephthalate (PET), flexible garden hoses made of polyvinyl chloride (PVC), insulating food containers made of foamed polystyrene, and shatterproof windows made of polymethyl methacrylate.
The composition, structure, and properties of plastics
Many of the chemical names of the polymers employed as plastics have become familiar to consumers, although some are better known by their abbreviations or trade names. Thus, polyethylene terephthalate and polyvinyl chloride are commonly referred to as PET and PVC, while foamed polystyrene and polymethyl methacrylate are known by their trademarked names, Styrofoam and Plexiglas (or Perspex).
Industrial fabricators of plastic products tend to think of plastics as either “commodity” resins or “specialty” resins. (The term resin dates from the early years of the plastics industry; it originally referred to naturally occurring amorphous solids such as shellac and rosin.) Commodity resins are plastics that are produced at high volume and low cost for the most common disposable items and durable goods. They are represented chiefly by polyethylene, polypropylene, polyvinyl chloride, and polystyrene. Specialty resins are plastics whose properties are tailored to specific applications and that are produced at low volume and higher cost. Among this group are the so-called engineering plastics, or engineering resins, which are plastics that can compete with die-cast metals in plumbing, hardware, and automotive applications. Important engineering plastics, less familiar to consumers than the commodity plastics listed above, are polyacetal, polyamide (particularly those known by the trade name nylon), polytetrafluoroethylene (trademark Teflon), polycarbonate, polyphenylene sulfide, epoxy, and polyetheretherketone. Another member of the specialty resins is thermoplastic elastomers, polymers that have the elastic properties of rubber yet can be molded repeatedly upon heating. Thermoplastic elastomers are described in the article elastomer.
Plastics also can be divided into two distinct categories on the basis of their chemical composition. One category is plastics that are made up of polymers having only aliphatic (linear) carbon atoms in their backbone chains. All the commodity plastics listed above fall into this category. The structure of polypropylene can serve as an example; here attached to every other carbon atom is a pendant methyl group (CH3):
The other category of plastics is made up of heterochain polymers. These compounds contain atoms such as oxygen, nitrogen, or sulfur in their backbone chains, in addition to carbon. Most of the engineering plastics listed above are composed of heterochain polymers. An example would be polycarbonate, whose molecules contain two aromatic (benzene) rings.
Additional Information:
Introduction
A plastic is a kind of material that is made by people and can be formed into almost any shape. Most plastics are strong, long-lasting, and lightweight. They resist damage by water, heat, chemicals, and electricity. In addition, plastics can be made in many colors.
There are about 50 main types of plastic. They have countless uses. Manufacturers often use plastics in place of more expensive materials. In nylon stockings, for example, plastic takes the place of silk. In vinyl house siding, plastic takes the place of wood. In many automobile body parts, plastic takes the place of metal.
Making Plastics
Most plastics are made from chemicals that come from petroleum (oil), natural gas, or coal. Heating these chemicals causes them to break down into molecules. (Molecules are groups of two or more atoms, which are the tiny building blocks of everything.) Scientists then join these molecules into chains. These chains make up plastics. Different combinations of molecules form different kinds of plastic.
Plastics can be made into almost any shape by heating them at a high temperature. The heat softens the plastic, which can then be poured into a mold. As the softened plastic cools, it hardens. When reheated, some types of plastic will soften again. The plastic can then be made into new shapes. Other types of plastic will stay hard even when reheated.
History
In 1869 John Wesley Hyatt, a U.S. inventor, made the first plastic. He called it celluloid because he made it from a plant material called cellulose. In 1909 a U.S. chemist named Leo H. Baekeland developed the first plastic made completely from synthetic (human-made) materials. Baekeland named the new material Bakelite. Scientists developed many more plastics from the 1920s through the 1940s. Later scientists invented stronger plastics and blended plastics with other materials.
Plastics and the Environment
Plastics are very useful, but they can also cause many problems for the environment. Items made out of plastic do not break down. When they are thrown out they take up room in landfills. A great deal of plastic waste winds up in the oceans, where it can hurt animals. Because they do not break down, things like plastic bags, bottles, and fishing lines collect in large areas of the ocean. Sea turtles and other animals may eat the plastic. The animals can also be hurt when plastic fishing lines get wrapped around their bodies. People who are concerned about the environment try to encourage people to recycle plastics instead of throwing them away. Recycled plastic can be turned into clothing, outdoor furniture, playground equipment, and more bottles.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2527 2025-06-30 23:15:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 50,890
Re: Miscellany
2327) Bimetallic Strip
Gist
Bimetal strips are used in miniature circuit breakers to protect circuits from excess current. A coil of wire is used to heat a bimetal strip, which bends and operates a linkage that unlatches a spring-operated contact. This interrupts the circuit and can be reset when the bimetal strip has cooled down.
Summary:
Bimetallic Strip Method
Bimetallic strips are used for temperature measurement and control. They operate based on the differential expansion of two metals with different coefficients of thermal expansion. While not highly precise, their durability and cost-effectiveness make them ideal for ON/OFF temperature control applications.
How Do Bimetallic Strips Work?
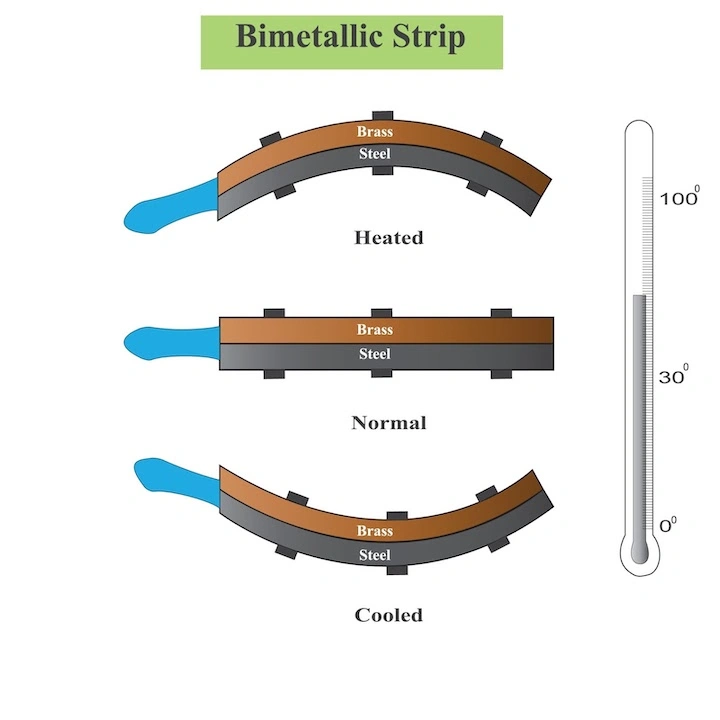
* A bimetallic strip consists of two bonded metal strips, each expanding at different rates when exposed to heat
* As temperature changes, the strip bends due to the difference in expansion rates.
* This bending movement is used to activate a switch or move a pointer in thermometers.
Temperature Range and Utility
* Bimetallic strips can operate in a temperature range from -180°C to 430°C.
* They find usage in various applications, from oven thermometers to home and industrial control thermostats.
These features make bimetallic strips suitable for specific temperature measurement applications where ruggedness and low cost are more important than high accuracy.
Details
What is a Bimetallic Strip?
A bimetallic strip is composed of two dissimilar metals joined together, usually in the form of two strips or two ribbons. The two metals are specifically dissimilar in terms of their electrical conductivity, thermal conductivity, and mechanical properties. When the strip is exposed to heat, the two dissimilar metals expand at different rates, and the resulting bending is utilized to determine the value of the temperature change. This simplicity makes the bimetallic strip an ideal component for a wide variety of applications.
How is a Bimetallic Strip Constructed?
A bimetallic strip consists of two thin strips of metal that are typically brass, copper, or steel. The strips are layered on top of each other, with one end joined and the other end free, allowing the assembly to bend and respond to temperature changes. Typically the two strips or ribbons that compose a bimetallic strip are secured together by welding or soldering.
How Does a Bimetallic Strip Work?
The two dissimilar metals in bimetallic strips are chosen based on the application and the desired properties. The operating mechanism that allows for movement of the bimetallic strip is from Thermodynamics, and the equation for linear thermal expansion helps to understand it:
Now, if you have two metal strips exposed to the same change in temperature and the same original length, but they have different coefficients of linear thermal expansion, you will see varying changes in length.
For an increase in temperature, the metal with the higher coefficient of thermal expansion will grow more than the other strip, causing the strip to bend towards the metal with the lower thermal coefficient.
For a decrease in temperature, the strip with the higher coefficient of linear thermal expansion decreases in length more, causing the strip to bend towards the metal with the higher thermal coefficient.
Now, the only operating mechanism for the movement of a bimetallic strip is the application of heat — the two metal strips are joined together, and the bending is caused by the difference in thermal expansion coefficients of the two metals. Basically, the thermal energy is converted to mechanical energy and a resulting displacement. In other words, when one metal expands more than the other due to a change in temperature, the strip bends or flexes to the contact point. That contact point is a bimetallic strip sensor that activates a switch and sends a signal to a control device or circuit.
Important Physical Properties of Bimetallic Strip Materials
When designing a bimetallic strip for any application, it is important to evaluate certain physical properties of the proposed materials:
* Coefficient of thermal expansion (how the material responds to changes in temperature)
* Modulus of elasticity (the ratio of stress to strain for a material undergoing elastic deformation)
* Maximum operating temperature (the maximum temperature the material can operate at without losing significant mechanical properties or permanently deforming)
* Electrical conductivity (especially for electrical applications)
* Stiffness and ductility
Common Bimetallic Strip Designs
Evaluating material properties is an important starting point for bimetallic strip design. Understanding the most prevalent designs is also a benefit at the outset because best practice is to have knowledge of previous designs in your area before creating something new — knowing what others have explored before you explore it yourself can save a lot of time and struggle.
The most common bimetallic strip designs are disc, ribbon, and coil designs. Of those, the disc design is the most prevalent, which consists of two metal discs stacked together. Ribbon designs consist of two metal strips joined together, and coils consist of two metal strips that are wound together into a coil. Each of these designs has its advantages and disadvantages, so the best design for a particular application should be chosen based on your requirements.
Common Applications for a Bimetallic Strip
The most common use for a bimetallic strip is as a temperature-sensitive switch. This type of switch is used in a wide range of applications, including thermostats, refrigerators, and other household appliances. But bimetallic strips are also used in a variety of mechanical applications. They can be used as a spring to provide tension or adjust the tension on a part. They can also be used to make a variety of mechanical linkages, such as a bell crank, which changes the direction of a force.
Bimetallic strips are also used in electrical applications, often as relays, which are electrical switches that control circuits. They can also be used as a current-limiting device, a type of resistor that limits the amount of current flowing through a circuit. Bimetallic strips are also used in a variety of other applications, including heat-sensitive switches, thermal fuses, and temperature-sensitive resistance elements.
In all of these applications, the two metals are carefully chosen based on their properties in order to achieve the desired result.
Additional Information
A bimetallic strip or bimetal strip is a strip that consists of two strips of different metals which expand at different rates as they are heated. The different expansion rates cause the strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. Thus, a bimetal strip converts a temperature change into mechanical displacement. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled. Common applications include temperature sensing (thermometer) and regulation (thermostat).
The invention of the bimetallic strip is generally credited to John Harrison, an eighteenth-century clockmaker who made it for his third marine chronometer (H3) of 1759 to compensate for temperature-induced changes in the balance spring. Harrison's invention is recognized in the memorial to him in Westminster Abbey, England.
Characteristics
The strip consists of two strips of different metals which expand at different rates as they are heated, usually steel and copper, or in some cases steel and brass. The strips are joined together throughout their length by riveting, brazing or welding. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled. The sideways displacement of the strip is much larger than the small lengthways expansion in either of the two metals.
In some applications, the bimetal strip is used in the flat form. In others, it is wrapped into a coil for compactness. The greater length of the coiled version gives improved sensitivity.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2528 2025-07-01 21:20:57
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 50,890
Re: Miscellany
2328) Geothermal Energy
Gist
Geothermal energy is heat derived from the Earth's interior. It's a renewable energy source, continuously replenished by the Earth's core, and can be harnessed for electricity generation, heating, and cooling.
Geothermal energy refers to the heat within the Earth's interior, which can be harnessed for various purposes. This heat originates from the planet's formation and the radioactive decay of elements within the Earth's core and mantle. It's a renewable energy source that can be used for heating buildings, generating electricity, and other applications.
Geothermal energy is generated by harnessing the heat from the Earth's interior. This heat, primarily from the slow decay of radioactive particles in the Earth's core, is used to produce steam, which then drives turbines connected to electricity generators. The process involves drilling into geothermal reservoirs to access steam or hot water, which is then used to generate power.
Summary
Geothermal energy, a natural resource of heat energy from within Earth that can be captured and harnessed for cooking, bathing, space heating, electrical power generation, and other uses. The total amount of geothermal energy incident on Earth is vastly in excess of the world’s current energy requirements, but it can be difficult to harness for electricity production. Despite its challenges, geothermal energy stands in stark contrast to the combustion of greenhouse gas-emitting fossil fuels (namely coal, petroleum, and natural gas) driving much of the climate crisis, and it has become increasingly attractive as a renewable energy source.
Mechanism and potential
Temperatures increase below Earth’s surface at a rate of about 30 °C per km in the first 10 km (roughly 90 °F per mile in the first 6 miles) below the surface. This internal heat of Earth is an immense store of energy and can manifest aboveground in phenomena such as volcanoes, lava flows, geysers, fumaroles, hot springs, and mud pots. The heat is produced mainly by the radioactive decay of potassium, thorium, and uranium in Earth’s crust and mantle and also by friction generated along the margins of continental plates.
Worldwide, the annual low-grade heat flow to the surface of Earth averages between 50 and 70 milliwatts (mW) per square meter. In contrast, incoming solar radiation striking Earth’s surface provides 342 watts per square meter annually (see solar energy). In the upper 10 km of rock beneath the contiguous United States alone, geothermal energy amounts to 3.3 × {10}^{25} joules, or about 6,000 times the energy contained in the world’s oil reserves. The estimated energy that can be recovered and utilized on the surface is 4.5 × {10}^{6} exajoules, or about 1.4 × {10}^{6} terawatt-years, which equates to roughly three times the world’s annual consumption of all types of energy.
Although geothermal energy is plentiful, geothermal power is not. The amount of usable energy from geothermal sources varies with depth and by extraction method. Normally, heat extraction requires a fluid (or steam) to bring the energy to the surface. Locating and developing geothermal resources can be challenging. This is especially true for the high-temperature resources needed for generating electricity. Such resources are typically limited to parts of the world characterized by recent volcanic activity or located along plate boundaries (such as along the Pacific Ring of Fire) or within crustal hot spots (such as Yellowstone National Park and the Hawaiian Islands). Geothermal reservoirs associated with those regions must have a heat source, adequate water recharge, adequate permeability or faults that allow fluids to rise close to the surface, and an impermeable caprock to prevent the escape of the heat. In addition, such reservoirs must be economically accessible (that is, within the range of drills). The most economically efficient facilities are located close to the geothermal resource to minimize the expense of constructing long pipelines. In the case of electric power generation, costs can be kept down by locating the facility near electrical transmission lines to transmit the electricity to market. Even though there is a continuous source of heat within Earth, the extraction rate of the heated fluids and steam can exceed the replenishment rate, and, thus, use of the resource must be managed sustainably.
Uses and history
Geothermal energy use can be divided into three categories: direct-use applications, geothermal heat pumps (GHPs), and electric power generation.
Details
Geothermal energy is heat that is generated within Earth. (Geo means “earth,” and thermal means “heat” in Greek.) It is a renewable resource that can be harvested for human use.
About 2,900 kilometers (1,800 miles) below Earth’s crust, or surface, is the hottest part of our planet: the core. A small portion of the core’s heat comes from the friction and gravitational pull formed when Earth was created more than four billion years ago. However, the vast majority of Earth’s heat is constantly generated by the decay of radioactive isotopes, such as potassium-40 and thorium-232.
Isotopes are forms of an element that have a different number of neutrons than the most common versions of the element’s atom.
Potassium, for instance, has 20 neutrons in its nucleus. Potassium-40, however, has 21 neutrons. As potassium-40 decays, its nucleus changes, emitting enormous amounts of energy (radiation). Potassium-40 most often decays to isotopes of calcium (calcium-40) and argon (argon-40).
Radioactive decay is a continual process in the core. Temperatures there rise to more than 5,000° Celsius (about 9,000° Fahrenheit). Heat from the core is constantly radiating outward and warming rocks, water, gas, and other geological material.
Earth’s temperature rises with depth from the surface to the core. This gradual change in temperature is known as the geothermal gradient. In most parts of the world, the geothermal gradient is about 25° C per 1 kilometer of depth (1° F per 77 feet of depth).
If underground rock formations are heated to about 700-1,300° C (1,300-2,400° F), they can become magma. Magma is molten (partly melted) rock permeated by gas and gas bubbles. Magma exists in the mantle and lower crust, and sometimes bubbles to the surface as lava.
Magma heats nearby rocks and underground aquifers. Hot water can be released through geysers, hot springs, steam vents, underwater hydrothermal vents, and mud pots.
These are all sources of geothermal energy. Their heat can be captured and used directly for heat, or their steam can be used to generate electricity. Geothermal energy can be used to heat structures such as buildings, parking lots, and sidewalks.
Most of the Earth’s geothermal energy does not bubble out as magma, water, or steam. It remains in the mantle, emanating outward at a slow pace and collecting as pockets of high heat. This dry geothermal heat can be accessed by drilling, and enhanced with injected water to create steam.
Many countries have developed methods of tapping into geothermal energy. Different types of geothermal energy are available in different parts of the world. In Iceland, abundant sources of hot, easily accessible underground water make it possible for most people to rely on geothermal sources as a safe, dependable, and inexpensive source of energy. Other countries, such as the U.S., must drill for geothermal energy at greater cost.
Harvesting Geothermal Energy: Heating and Cooling:
Low-Temperature Geothermal Energy
Almost anywhere in the world, geothermal heat can be accessed and used immediately as a source of heat. This heat energy is called low-temperature geothermal energy. Low-temperature geothermal energy is obtained from pockets of heat about 150° C (302° F). Most pockets of low-temperature geothermal energy are found just a few meters below ground.
Low-temperature geothermal energy can be used for heating greenhouses, homes, fisheries, and industrial processes. Low-temperature energy is most efficient when used for heating, although it can sometimes be used to generate electricity.
People have long used this type of geothermal energy for engineering, comfort, healing, and cooking. Archaeological evidence shows that 10,000 years ago, groups of Native Americans gathered around naturally occurring hot springs to recuperate or take refuge from conflict. In the third century BCE, scholars and leaders warmed themselves in a hot spring fed by a stone pool near Lishan, a mountain in central China. One of the most famous hot spring spas is in the appropriately named town of Bath, England. Starting construction in about 60 CE, Roman conquerors built an elaborate system of steam rooms and pools using heat from the region’s shallow pockets of low-temperature geothermal energy.
The hot springs of Chaudes Aigues, France, have provided a source of income and energy for the town since the 1300s. Tourists flock to the town for its elite spas. The low-temperature geothermal energy also supplies heat to homes and businesses.
The United States opened its first geothermal district heating system in 1892 in Boise, Idaho. This system still provides heat to about 450 homes.
Co-Produced Geothermal Energy
Co-produced geothermal energy technology relies on other energy sources. This form of geothermal energy uses water that has been heated as a byproduct in oil and gas wells.
In the United States, about 25 billion barrels of hot water are produced every year as a byproduct. In the past, this hot water was simply discarded. Recently, it has been recognized as a potential source of even more energy: Its steam can be used to generate electricity to be used immediately or sold to the grid.
One of the first co-produced geothermal energy projects was initiated at the Rocky Mountain Oilfield Testing Center in the U.S. state of Wyoming.
Newer technology has allowed co-produced geothermal energy facilities to be portable. Although still in experimental stages, mobile power plants hold tremendous potential for isolated or impoverished communities.
Geothermal Heat Pumps
Geothermal heat pumps (GHPs) take advantage of Earth’s heat, and can be used almost anywhere in the world. GHPs are drilled about three to 90 meters (10 to 300 feet) deep, much shallower than most oil and natural gas wells. GHPs do not require fracturing bedrock to reach their energy source.
A pipe connected to a GHP is arranged in a continuous loop—called a "slinky loop"—that circles underground and above ground, usually throughout a building. The loop can also be contained entirely underground, to heat a parking lot or landscaped area.
In this system, water or other liquids (such as glycerol, similar to a car’s antifreeze) move through the pipe. During the cold season, the liquid absorbs underground geothermal heat. It carries the heat upward through the building and gives off warmth through a duct system. These heated pipes can also run through hot water tanks and offset water-heating costs.
During the summer, the GHP system works the opposite way: The liquid in the pipes is warmed from the heat in the building or parking lot, and carries the heat to be cooled underground.
The U.S. Environmental Protection Agency has called geothermal heating the most energy-efficient and environmentally safe heating and cooling system. The largest GHP system was completed in 2012 at Ball State University in Indiana. The system replaced a coal-fired boiler system, and experts estimate the university will save about two million dollars a year in heating costs.
Harvesting Geothermal Energy: Electricity
In order to obtain enough energy to generate electricity, geothermal power plants rely on heat that exists a few kilometers below the surface of Earth. In some areas, the heat can naturally exist underground as pockets steam or hot water. However, most areas need to be “enhanced” with injected water to create steam.
Dry-Steam Power Plants
Dry-steam power plants take advantage of natural underground sources of steam. The steam is piped directly to a power plant, where it is used to fuel turbines and generate electricity.
Dry steam is the oldest type of power plant to generate electricity using geothermal energy. The first dry-steam power plant was constructed in Larderello, Italy, in 1911. Today, the dry-steam power plants at Larderello continue to supply electricity to more than a million residents of the area.
There are only two known sources of underground steam in the United States: Yellowstone National Park in Wyoming and The Geysers in California. Since Yellowstone is a protected area, The Geysers is the only place where a dry-steam power plant is in use. It is one of the largest geothermal energy complexes in the world, and provides about a fifth of all renewable energy in the U.S. state of California.
Flash-Steam Power Plant
Flash-steam power plants use naturally occurring sources of underground hot water and steam. Water that is hotter than 182° C (360° F) is pumped into a low-pressure area. Some of the water “flashes,” or evaporates rapidly into steam, and is funneled out to power a turbine and generate electricity. Any remaining water can be flashed in a separate tank to extract more energy.
Flash-steam power plants are the most common type of geothermal power plants. The volcanically active island nation of Iceland supplies nearly all its electrical needs through a series of flash-steam geothermal power plants. The steam and excess warm water produced by the flash-steam process heat icy sidewalks and parking lots in the frigid Arctic winter.
The islands of the Philippines also sit over a tectonically active area, the "Ring of Fire" that rims the Pacific Ocean. Government and industry in the Philippines have invested in flash-steam power plants, and today the nation is second only to the United States in its use of geothermal energy. In fact, the largest single geothermal power plant is a flash-steam facility in Malitbog, Philippines.
Binary Cycle Power Plants
Binary cycle power plants use a unique process to conserve water and generate heat. Water is heated underground to about 107°-182° C (225°-360° F). The hot water is contained in a pipe, which cycles above ground. The hot water heats a liquid organic compound that has a lower boiling point than water. The organic liquid creates steam, which flows through a turbine and powers a generator to create electricity. The only emission in this process is steam. The water in the pipe is recycled back to the ground, to be reheated by Earth and provide heat for the organic compound again.
The Beowawe Geothermal Facility in the U.S. state of Nevada uses the binary cycle to generate electricity. The organic compound used at the facility is an industrial refrigerant (tetrafluoroethane, a greenhouse gas). This refrigerant has a much lower boiling point than water, meaning it is converted into gas at low temperatures. The gas fuels the turbines, which are connected to electrical generators.
Enhanced Geothermal Systems
Earth has virtually endless amounts of energy and heat beneath its surface. However, it is not possible to use it as energy unless the underground areas are "hydrothermal." This means the underground areas are not only hot, but also contain liquid and are permeable. Many areas do not have all three of these components. An enhanced geothermal system (EGS) uses drilling, fracturing, and injection to provide fluid and permeability in areas that have hot—but dry—underground rock.
To develop an EGS, an “injection well” is drilled vertically into the ground. Depending on the type of rock, this can be as shallow as one kilometer (0.6 mile) to as deep as 4.5 kilometers (2.8 miles). High-pressure cold water is injected into the drilled space, which forces the rock to create new fractures, expand existing fractures, or dissolve. This creates a reservoir of underground fluid.
Water is pumped through the injection well and absorbs the rocks’ heat as it flows through the reservoir. This hot water, called brine, is then piped back up to Earth’s surface through a “production well.” The heated brine is contained in a pipe. It warms a secondary fluid that has a low boiling point, which evaporates to steam and powers a turbine. The brine cools off, and cycles back down through the injection well to absorb underground heat again. There are no gaseous emissions besides the water vapor from the evaporated liquid.
Pumping water into the ground for EGSs can cause seismic activity, or small earthquakes. In Basel, Switzerland, the injection process caused hundreds of tiny earthquakes that grew to more significant seismic activity even after the water injection was halted. This led to the geothermal project being canceled in 2009.
Geothermal Energy and the Environment
Geothermal energy is a renewable resource. Earth has been emitting heat for about 4.5 billion years, and will continue to emit heat for billions of years into the future because of the ongoing radioactive decay in Earth’s core.
However, most wells that extract the heat will eventually cool, especially if heat is extracted more quickly than it is given time to replenish. Larderello, Italy, site of the world’s first electrical plant supplied by geothermal energy, has seen its steam pressure fall by more than 25 percent since the 1950s.
Reinjecting water can sometimes help a cooling geothermal site last longer. However, this process can cause “micro-earthquakes.” Although most of these are too small to be felt by people or register on a scale of magnitude, sometimes the ground can quake at more threatening levels and cause the geothermal project to shut down, as it did in Basel, Switzerland.
Geothermal systems do not require enormous amounts of freshwater. In binary systems, water is only used as a heating agent, and is not exposed or evaporated. It can be recycled, used for other purposes, or released into the atmosphere as nontoxic steam. However, if the geothermal fluid is not contained and recycled in a pipe, it can absorb harmful substances such as math, boron, and fluoride. These toxic substances can be carried to the surface and released when the water evaporates. In addition, if the fluid leaks to other underground water systems, it can contaminate clean sources of drinking water and aquatic habitats.
Advantages
There are many advantages to using geothermal energy either directly or indirectly:
* Geothermal energy is renewable; it is not a fossil fuel that will be eventually used up. Earth is continuously radiating heat out from its core, and will continue to do so for billions of years.
* Some form of geothermal energy can be accessed and harvested anywhere in the world.
* Using geothermal energy is relatively clean. Most systems only emit water vapor, although some emit very small amounts of sulfur dioxide, nitrous oxides, and particulates.
* Geothermal power plants can last for decades and possibly centuries. If a reservoir is managed properly, the amount of extracted energy can be balanced with the rock’s rate of renewing its heat.
* Unlike other renewable energy sources, geothermal systems are “baseload.” This means they can work in the summer or winter, and are not dependent on changing factors such as the presence of wind or sun. Geothermal power plants produce electricity or heat 24 hours a day, seven days a week.
The space it takes to build a geothermal facility is much more compact than other power plants. To produce a GWh (a gigawatt hour, or one million kilowatts of energy for one hour, an enormous amount of energy), a geothermal plant uses the equivalent of about 1,046 square kilometers (404 square miles) of land. To produce the same GWh, wind energy requires 3,458 square kilometers (1,335 square miles), a solar photovoltaic center requires 8,384 square kilometers (3,237 square miles), and coal plants use about 9,433 square kilometers (3,642 square miles).
Geothermal energy systems are adaptable to many different conditions.
They can be used to heat, cool, or power individual homes, whole districts, or industrial processes.
Disadvantages
Harvesting geothermal energy still poses many challenges:
* The process of injecting high-pressure streams of water into the planet can result in minor seismic activity, or small earthquakes.
* Geothermal plants have been linked to subsidence, or the slow sinking of land. This happens as the underground fractures collapse upon themselves. This can lead to damaged pipelines, roadways, buildings, and natural drainage systems.
* Geothermal plants can release small amounts of greenhouse gases such as hydrogen sulfide and carbon dioxide.
* Water that flows through underground reservoirs can pick up trace amounts of toxic elements such as math, mercury, and selenium. These harmful substances can be leaked to water sources if the geothermal system is not properly insulated.
Although the process requires almost no fuel to run, the initial cost of installing geothermal technology is expensive. Developing countries may not have the sophisticated infrastructure or start-up costs to invest in a geothermal power plant. Several facilities in the Philippines, for example, were made possible by investments from U.S. industry and government agencies. Today, the plants are Philippine-owned and operated.
Geothermal Energy and People
Geothermal energy exists in different forms all over Earth (by steam vents, lava, geysers, or simply dry heat), and there are different possibilities for extracting and using this heat.
In New Zealand, natural geysers and steam vents heat swimming pools, homes, greenhouses, and prawn farms. New Zealanders also use dry geothermal heat to dry timber and feedstock.
Other countries, such as Iceland, have taken advantage of molten rock and magma resources from volcanic activity to provide heat for homes and buildings. In Iceland, almost 90 percent of the country’s people use geothermal heating resources. Iceland also relies on its natural geysers to melt snow, warm fisheries, and heat greenhouses.
The United States generates the most amount of geothermal energy of any other country. Every year, the U.S. generates at least 15 billion kilowatt-hours, or the equivalent of burning about 25 million barrels of oil. Industrial geothermal technologies have been concentrated in the western U.S. In 2012, Nevada had 59 geothermal projects either operational or in development, followed by California with 31 projects, and Oregon with 16 projects.
The cost of geothermal energy technology has gone down in the last decade, and is becoming more economically possible for individuals and companies.
Additional Information
Geothermal energy is thermal energy extracted from the crust. It combines energy from the formation of the planet and from radioactive decay. Geothermal energy has been exploited as a source of heat and/or electric power for millennia.
Geothermal heating, using water from hot springs, for example, has been used for bathing since Paleolithic times and for space heating since Roman times. Geothermal power (generation of electricity from geothermal energy), has been used since the 20th century. Unlike wind and solar energy, geothermal plants produce power at a constant rate, without regard to weather conditions. Geothermal resources are theoretically more than adequate to supply humanity's energy needs. Most extraction occurs in areas near tectonic plate boundaries.
The cost of generating geothermal power decreased by 25% during the 1980s and 1990s. Technological advances continued to reduce costs and thereby expand the amount of viable resources. In 2021, the US Department of Energy estimated that power from a plant "built today" costs about $0.05/kWh.
In 2019, 13,900 megawatts (MW) of geothermal power was available worldwide. An additional 28 gigawatts provided heat for district heating, space heating, spas, industrial processes, desalination, and agricultural applications as of 2010. As of 2019 the industry employed about one hundred thousand people.
The adjective geothermal originates from the Greek roots γῆ (gê), meaning Earth, and θερμός (thermós), meaning hot.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2529 2025-07-02 22:56:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 50,890
Re: Miscellany
2329) Quasar
Gist
A quasar is an extremely luminous active galactic nucleus (AGN). It is sometimes known as a quasi-stellar object, abbreviated QSO.
A quasar is a very luminous galactic core, or active galactic nucleus (AGN), powered by a supermassive black hole actively accreting matter. As gas and dust spiral into the black hole, they form a swirling accretion disk that heats up and emits intense radiation across the electromagnetic spectrum. This makes quasars exceptionally bright, sometimes outshining the entire galaxy they reside in.
Summary
A quasar is an extremely luminous active galactic nucleus (AGN). It is sometimes known as a quasi-stellar object, abbreviated QSO. The emission from an AGN is powered by accretion onto a supermassive black hole with a mass ranging from millions to tens of billions of solar masses, surrounded by a gaseous accretion disc. Gas in the disc falling towards the black hole heats up and releases energy in the form of electromagnetic radiation. The radiant energy of quasars is enormous; the most powerful quasars have luminosities thousands of times greater than that of a galaxy such as the Milky Way. Quasars are usually categorized as a subclass of the more general category of AGN. The redshifts of quasars are of cosmological origin.
The term quasar originated as a contraction of "quasi-stellar [star-like] radio source"—because they were first identified during the 1950s as sources of radio-wave emission of unknown physical origin—and when identified in photographic images at visible wavelengths, they resembled faint, star-like points of light. High-resolution images of quasars, particularly from the Hubble Space Telescope, have shown that quasars occur in the centers of galaxies, and that some host galaxies are strongly interacting or merging galaxies. As with other categories of AGN, the observed properties of a quasar depend on many factors, including the mass of the black hole, the rate of gas accretion, the orientation of the accretion disc relative to the observer, the presence or absence of a jet, and the degree of obscuration by gas and dust within the host galaxy.
About a million quasars have been identified with reliable spectroscopic redshifts, and between 2-3 million identified in photometric catalogs. The nearest known quasar is about 600 million light-years from Earth, while the record for the most distant known AGN is at a redshift of 10.1, corresponding to a comoving distance of 31.6 billion light-years, or a look-back time of 13.2 billion years.
Quasar discovery surveys have shown that quasar activity was more common in the distant past; the peak epoch was approximately 10 billion years ago. Concentrations of multiple quasars are known as large quasar groups and may constitute some of the largest known structures in the universe if the observed groups are good tracers of mass distribution.
Details
Quasar, an astronomical object of very high luminosity found in the centres of some galaxies and powered by gas spiraling at high velocity into an extremely large black hole. The brightest quasars can outshine all of the stars in the galaxies in which they reside, which makes them visible even at distances of billions of light-years. Quasars are among the most distant and luminous objects known.
Discovery of quasars
The term quasar derives from how these objects were originally discovered in the earliest radio surveys of the sky in the 1950s. Away from the plane of the Milky Way Galaxy, most radio sources were identified with otherwise normal-looking galaxies. Some radio sources, however, coincided with objects that appeared to be unusually blue stars, although photographs of some of these objects showed them to be embedded in faint, fuzzy halos. Because of their almost starlike appearance, they were dubbed “quasi-stellar radio sources,” which by 1964 had been shortened to “quasar.”
The optical spectra of the quasars presented a new mystery. Photographs taken of their spectra showed locations for emission lines at wavelengths that were at odds with all celestial sources then familiar to astronomers. The puzzle was solved by the Dutch American astronomer Maarten Schmidt, who in 1963 recognized that the pattern of emission lines in 3C 273, the brightest known quasar, could be understood as coming from hydrogen atoms that had a redshift (i.e., had their emission lines shifted toward longer, redder wavelengths by the expansion of the universe) of 0.158. In other words, the wavelength of each line was 1.158 times longer than the wavelength measured in the laboratory, where the source is at rest with respect to the observer. At a redshift of this magnitude, 3C 273 was placed by Hubble’s law at a distance of slightly more than two billion light-years. This was a large, though not unprecedented, distance (bright clusters of galaxies had been identified at similar distances), but 3C 273 is about 100 times more luminous than the brightest individual galaxies in those clusters, and nothing so bright had been seen so far away.
An even bigger surprise was that continuing observations of quasars revealed that their brightness can vary significantly on timescales as short as a few days, meaning that the total size of the quasar cannot be more than a few light-days across. Since the quasar is so compact and so luminous, the radiation pressure inside the quasar must be huge; indeed, the only way a quasar can keep from blowing itself up with its own radiation is if it is very massive, at least a million solar masses if it is not to exceed the Eddington limit—the minimum mass at which the outward radiation pressure is balanced by the inward pull of gravity (named after English astronomer Arthur Eddington). Astronomers were faced with a conundrum: how could an object about the size of the solar system have a mass of about a million stars and outshine by 100 times a galaxy of a hundred billion stars?
The right answer—accretion by gravity onto supermassive black holes—was proposed shortly after Schmidt’s discovery independently by Russian astronomers Yakov Zel’dovich and Igor Novikov and Austrian American astronomer Edwin Salpeter. The combination of high luminosities and small sizes was sufficiently unpalatable to some astronomers that alternative explanations were posited that did not require the quasars to be at the large distances implied by their redshifts. These alternative interpretations have been discredited, although a few adherents remain. For most astronomers, the redshift controversy was settled definitively in the early 1980s when American astronomer Todd Boroson and Canadian American astronomer John Beverly Oke showed that the fuzzy halos surrounding some quasars are actually starlight from the galaxy hosting the quasar and that these galaxies are at high redshifts.
By 1965 it was recognized that quasars are part of a much larger population of unusually blue sources and that most of these are much weaker radio sources too faint to have been detected in the early radio surveys. This larger population, sharing all quasar properties except extreme radio luminosity, became known as “quasi-stellar objects” or simply QSOs. Since the early 1980s most astronomers have regarded QSOs as the high-luminosity variety of an even larger population of “active galactic nuclei,” or AGNs. (The lower-luminosity AGNs are known as “Seyfert galaxies,” named after the American astronomer Carl K. Seyfert, who first identified them in 1943.)
Finding quasars
Although the first quasars known were discovered as radio sources, it was quickly realized that quasars could be found more efficiently by looking for objects bluer than normal stars. This can be done with relatively high efficiency by photographing large areas of the sky through two or three different-coloured filters. The photographs are then compared to locate the unusually blue objects, whose nature is verified through subsequent spectroscopy. This remains the primary technique for finding quasars, although it has evolved over the years with the replacement of film by electronic charge-coupled devices (CCDs), the extension of the surveys to longer wavelengths in the infrared, and the addition of multiple filters that, in various combinations, are effective at isolating quasars at different redshifts. Quasars have also been discovered through other techniques, including searches for starlike sources whose brightness varies irregularly and X-ray surveys from space; indeed, a high level of X-ray emission is regarded by astronomers as a sure indicator of an accreting black-hole system.
Physical structure of quasars
Quasars and other AGNs are apparently powered by gravitational accretion onto supermassive black holes, where “supermassive” means from roughly a million to a few billion times the mass of the Sun. Supermassive black holes reside at the centres of many large galaxies. In about 5–10 percent of these galaxies, gas tumbles into the deep gravitational well of the black hole and is heated to incandescence as the gas particles pick up speed and pile up in a rapidly rotating “accretion disk” close to the horizon of the black hole. There is a maximum rate set by the Eddington limit at which a black hole can accrete matter before the heating of the infalling gas results in so much outward pressure from radiation that the accretion stops. What distinguishes an “active” galactic nucleus from other galactic nuclei (the 90–95 percent of large galaxies that are currently not quasars) is that the black hole in an active nucleus accretes a few solar masses of matter per year, which, if it is accreting at around 1 percent or more of the Eddington rate, is sufficient to account for a typical quasar with a total luminosity of about {10}^{39} watts. (The Sun’s luminosity is about 4 × {10}^{26} watts.)
In addition to black holes and accretion disks, quasars have other remarkable features. Just beyond the accretion disk are clouds of gas that move at high velocities around the inner structure, absorbing high-energy radiation from the accretion disk and reprocessing it into the broad emission lines of hydrogen and ions of other atoms that are the signatures of quasar spectra. Farther from the black hole but still largely in the accretion disk plane are dust-laden gas clouds that can obscure the quasar itself. Some quasars are also observed to have radio jets, which are highly collimated beams of plasma propelled out along the rotation axis of the accretion disk at speeds often approaching that of light. These jets emit beams of radiation that can be observed at X-ray and radio wavelengths (and less often at optical wavelengths).
Because of this complex structure, the appearance of a quasar depends on the orientation of the rotation axis of the accretion disk relative to the observer’s line of sight. Depending on this angle, different quasar components—the accretion disk, emission-line clouds, jets—appear to be more or less prominent. This results in a wide variety of observed phenomena from what are, in reality, physically similar sources.
Evolution of quasars
The number density of quasars increases dramatically with redshift, which translates through Hubble’s law to more quasars at larger distances. Because of the finite speed of light, when quasars are observed at great distances, they are observed as they were in the distant past. Thus, the increasing density of quasars with distance means that they were more common in the past than they are now. This trend increases until “look-back times” that correspond to around three billion years after the big bang, which occurred approximately 13.5 billion years ago. At earlier ages, the number density of quasars decreases sharply, corresponding to an era when the quasar population was still building up. The most distant, and thus earliest, quasars known were formed less than a billion years after the big bang.
Individual quasars appear as their central black holes begin to accrete gas at a high rate, possibly triggered by a merger with another galaxy, building up the mass of the central black hole. The current best estimate is that quasar activity is episodic, with individual episodes lasting around a million years and the total quasar lifetime lasting around 10 million years. At some point, quasar activity ceases completely, leaving behind the dormant massive black holes found in most massive galaxies. This “life cycle” appears to proceed most rapidly with the most-massive black holes, which become dormant earlier than less-massive black holes. Indeed, in the current universe the remaining AGN population is made up predominantly of lower-luminosity Seyfert galaxies with relatively small supermassive black holes.
In the present-day universe there is a close relationship between the mass of a black hole and the mass of its host galaxy. This is quite remarkable, since the central black hole accounts for only about 0.1 percent of the mass of the galaxy. It is believed that the intense radiation, mass outflows, and jets from the black hole during its active quasar phase are responsible. The radiation, outflows, and jets heat up and can even remove entirely the interstellar medium from the host galaxy. This loss of gas in the galaxy simultaneously shuts down star formation and chokes off the quasar’s fuel supply, thus freezing both the mass in stars and the mass of the black hole.
Additional Information
A quasar is an extremely active and luminous type of active galactic nucleus (AGN). All quasars are AGNs, but not all AGNs are quasars.
Quasars are a subclass of active galactic nuclei (AGNs), extremely luminous galactic cores where gas and dust falling into a supermassive black hole emit electromagnetic radiation across the entire electromagnetic spectrum. The gas and dust become luminous as a result of the extreme gravitational and frictional forces exerted on them as they fall into the black hole. Quasars are amongst the most luminous objects in the known Universe, typically emitting thousands of times more light than the entire Milky Way. They are distinguished from other AGNs by their enormous luminosity, and their enormous distances from Earth. As the speed of light is finite, objects observed from Earth are seen as they were when the light we see left them. The nearest quasars to Earth are still several hundred million light-years away, meaning that they are observed now as they were 600 million years ago. The absence of quasars closer to Earth does not mean that there were never quasars in our region of the Universe, but instead means that quasars existed when the universe was younger. The study of quasars provides fascinating insights into the evolution of the Universe.
In 1996 Hubble’s 100 000th exposure was celebrated by capturing an image of a quasar located 9 billion light-years from Earth.
In 2019 it was announced that Hubble had observed the brightest quasar in the early Universe. After 20 years of searching, astronomers identified the ancient quasar with the help of strong gravitational lensing. A dim galaxy is located right between the quasar and Earth, bending the light from the quasar and making it appear three times as large and 50 times as bright as it would be without the effect of gravitational lensing. Even still, the lensed quasar is extremely compact and unresolved in images from optical ground-based telescopes. Only Hubble’s sharp vision allowed it to resolve the system, and this unique object provides an insight into the birth of galaxies when the Universe was less than a billion years old. Hubble’s study of gravitationally lensed quasars has also contributed to our understanding of the rate of expansion of the Universe.
Hubble has also imaged quasar ghosts — ethereal green objects which mark the graves of these objects that flickered to life and then faded. These unusual structures orbit their host galaxies and glow in a bright and eerie green hue, and offer insights into the pasts of these galaxies.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2530 2025-07-06 23:36:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 50,890
Re: Miscellany
2330) Continent
Gist
A continent is one of the Earth's seven main landmasses. They are, in approximate order of size: Asia, Africa, North America, South America, Antarctica, Europe, and Australia. These are conventionally defined by convention rather than strict criteria.
The seven continents in order from largest to smallest by land area are: Asia, Africa, North America, South America, Antarctica, Europe, and Australia (sometimes called Oceania or Australasia).
What defines a Continent?
By convention, continents "are understood to be large, continuous, discrete masses of land, ideally separated by expanses of water". By this definition, all continents have to be an island of some metric.
Summary
A continent is any of several large geographical regions. Continents are generally identified by convention rather than any strict criteria. A continent could be a single large landmass, a part of a very large landmass, as in the case of Asia or Europe within Eurasia, or a landmass and nearby islands within its continental shelf. Due to these varying definitions, the number of continents varies; up to seven or as few as four geographical regions are commonly regarded as continents. Most English-speaking countries recognize seven regions as continents. In order from largest to smallest in area, these seven regions are Asia, Africa, North America, South America, Antarctica, Europe, and Australia (sometimes called Oceania or Australasia). Different variations with fewer continents merge some of these regions; examples of this are merging Asia and Europe into Eurasia, North America and South America into the Americas (or simply America), and Africa, Asia, and Europe into Afro-Eurasia.
Oceanic islands are occasionally grouped with a nearby continent to divide all the world's land into geographical regions. Under this scheme, most of the island countries and territories in the Pacific Ocean are grouped together with the continent of Australia to form the geographical region of Oceania.
In geology, a continent is defined as "one of Earth's major landmasses, including both dry land and continental shelves". The geological continents correspond to seven large areas of continental crust that are found on the tectonic plates, but exclude small continental fragments such as Madagascar that are generally referred to as microcontinents. Continental crust is only known to exist on Earth.
The idea of continental drift gained recognition in the 20th century. It postulates that the current continents formed from the breaking up of a supercontinent (Pangaea) that formed hundreds of millions of years ago.
Details
When geographers identify a continent, they usually include all the islands associated with it. Japan, for instance, is part of the continent of Asia. Likewise, Greenland and all the islands in the Caribbean Sea are usually considered part of North America.
Together, the continents add up to about 148 million square kilometers (57 million square miles) of land. Continents make up most—but not all—of Earth’s land surface. A very small portion of the total land area is made of islands that are not considered physical parts of continents. The ocean covers almost three-fourths of Earth. The area of the ocean is more than double the area of all the continents combined. All continents border at least one ocean. Asia, the largest continent, has the longest series of coastlines.
Coastlines, however, do not indicate the actual boundaries of the continents. Continents are defined by their continental shelves. A continental shelf is a gently sloping area that extends outward from the beach far into the ocean. A continental shelf is part of the ocean, but also part of the continent.
To human geographers, continents are also culturally distinct. The continents of Europe and Asia, for example, are actually part of a single, enormous piece of land called Eurasia. But historically, the areas of Asia and Europe have been separated because of people’s perceptions about their different cultures. Because of this, most geographers continue to divide Eurasia into Europe and Asia. An imaginary line, running from the northern Ural Mountains in Russia south to the Caspian and Black Seas, separates Europe, to the west, from Asia, to the east.
Building the Continents
Earth formed 4.6 billion years ago from a great, swirling cloud of cosmic dust and gas. The continuous smashing of space debris and the pull of gravity made the inside of Earth heat up. As the heat increased, some of Earth’s rocky materials melted and rose to the surface, where they cooled and formed a crust. Heavier material sank toward Earth’s center. Eventually, Earth came to have three main layers: the core, the mantle and the crust.
The crust and the top portion of the mantle form a rigid shell around Earth that is broken into huge sections called tectonic plates. The heat from inside Earth causes the plates to slide around on the molten mantle. Today, tectonic plates continue to slowly slide around the surface, just as they have for hundreds of millions of years. Geologists believe the interaction of the plates, a process called plate tectonics, contributed to the creation of continents.
Studies of rocks found in ancient areas of North America have revealed that the oldest known pieces of the continents began to form nearly 4 billion years ago, soon after Earth formed. At that time, a primitive ocean covered Earth. Only a small fraction of the crust was made of continental material. Scientists theorize that this material built up along the boundaries of tectonic plates during a process called subduction. During subduction, plates collide and the edge of one plate slides beneath the edge of another.
When heavy oceanic crust subducted toward the mantle, it melted in the mantle’s intense heat. Once it melted, the rock became lighter. Now in the form of magma, it rose through the overlying plate and burst out as lava. When the lava cooled, it hardened into igneous rock.
Gradually, the igneous rock built up into small volcanic islands above the surface of the ocean. Over time, these islands grew bigger, partly as the result of more lava flows and partly from the buildup of material scraped off descending plates. When plates carrying islands subducted, the islands themselves did not descend into the mantle. Their material fused with that of islands on the neighboring plate. This made even larger landmasses in the form of the first continents.
The building of volcanic islands and continental material through plate tectonics is a process that continues today. Continental crust is much lighter than oceanic crust. In subduction zones, where tectonic plates interact with each other, oceanic crust always subducts beneath continental crust. Oceanic crust is constantly being recycled in the mantle. For this reason, continental crust is much, much older than oceanic crust.
Wandering Continents
If you could visit Earth as it was millions of years ago, it would look very different. The continents have not always been where they are today. About 480 million years ago, most continents were scattered chunks of land lying along or below the Equator. Millions of years of continuous tectonic activity changed their positions, and by 240 million years ago, almost all of the world’s land was joined in a single, huge continent.
By about 200 million years ago, the forces that helped form the supercontinent caused it to begin to break apart. The pieces of the supercontinent that began to move apart were the beginnings of the continents that we know today.
A giant landmass that would become Europe, Asia, and North America separated from another mass that would split up into other continents. In time, Antarctica and Australia, still joined together, broke away and drifted south. The small piece of land that would become the peninsula of India broke away and for millions of years moved north as a large island. It eventually collided with Asia. Gradually, the different landmasses moved to their present locations.
The positions of the continents are always changing. North America and Europe are moving away from each other at the rate of about 2.5 centimeters (1 inch) per year. If you could visit the planet in the future, you might find that part of the U.S. state of California had separated from North America and become an island. Africa might have split in two along the Great Rift Valley. It is even possible that another supercontinent may form someday.
Continental Features
The surface of the continents has changed many times because of mountain building, weathering, erosion and buildup of sediment. Continuous, slow movement of tectonic plates also changes surface features.
The rocks that form the continents have been shaped and reshaped many times. Great mountain ranges have risen and then have been worn away. Ocean waters have flooded huge areas and then gradually dried up. Massive ice sheets have come and gone, sculpting the landscape in the process.
Today, all continents have great mountain ranges, vast plains, extensive plateaus, and complex river systems. The landmasses’ average elevation above sea level is about 838 meters (2,750 feet).
Although each is unique, all the continents share two basic features: old, geologically stable regions, and younger, somewhat more active regions. In the younger regions, the process of mountain building has happened recently and often continues to happen.
The power for mountain building, or orogeny, comes from plate tectonics. One way mountains form is through the collision of two tectonic plates. The impact creates wrinkles in the crust, just as a rug wrinkles when you push against one end of it. Such a collision created Asia’s Himalaya mountain range several million years ago. The plate carrying India slowly and forcefully shoved the landmass of India into Asia, which was riding on another plate. The collision continues today, causing the Himalayas to continually grow taller.
Recently formed mountains, called coastal ranges, rise near the western coasts of North and South America. Older, more stable mountain ranges are found in the interior of continents. The Appalachians of North America and the Urals, on the border between Europe and Asia, are older mountain ranges that are not geologically active.
Even older than these ancient, eroded mountain ranges are flatter, more stable areas of the continents called cratons. A craton is an area of ancient crust that formed during the Earth’s early history. Every continent has a craton. Microcontinents, like New Zealand, lack cratons.
Cratons have two forms: shields and platforms. Shields are bare rocks that may be the roots or cores of ancient mountain ranges that have completely eroded away. Platforms are cratons with sediment and sedimentary rock lying on top.
The Canadian Shield makes up about a quarter of North America. For hundreds of thousands of years, sheets of ice up to 3.2 kilometers (2 miles) thick coated the Canadian Shield. The moving ice wore away material on top of ancient rock layers, exposing some of the oldest formations on Earth. When you stand on the oldest part of the Canadian Shield, you stand directly on rocks that formed more than 3.5 billion years ago.
North America
North America, the third-largest continent, extends from the tiny Aleutian Islands in the northwest to the Isthmus of Panama in the south. The continent includes the enormous island of Greenland (an autonomous territory of Denmark) in the northeast. In the far north, the continent stretches halfway around the world, from Greenland to the Aleutians. But at Panama’s narrowest part, the continent is just 50 kilometers (31 miles) across.
Young mountains—including the Rockies, North America’s largest chain—rise in the West. Some of Earth’s youngest mountains are found in the Cascade Range of the U.S. states of Washington, Oregon and California. Some peaks there began to form only about a million years ago—a wink of an eye in Earth’s long history. North America’s older mountain ranges rise near the East Coast of the United States and Canada.
In between the mountain systems lie wide plains that contain deep, rich soil. Much of the soil was formed from material deposited during the most recent glacial period. This ice age reached its peak about 18,000 years ago. As glaciers retreated, streams of melted ice dropped sediment on the land, building layers of fertile soil in the plains region. Grain grown in this region, called the “breadbasket of North America,” feeds a large part of the world.
North America contains a variety of natural wonders. Landforms and all types of vegetation can be found within its boundaries. North America has deep canyons, such as Copper Canyon in the Mexican state of Chihuahua. Yellowstone National Park, in the U.S. state of Wyoming, has some of the world’s most active geysers. Canada’s Bay of Fundy has the greatest variation of tide levels in the world. The Great Lakes form the planet’s largest area of fresh water. In the U.S. state of California, giant sequoias—the largest tree species in the world—grow more than 76 meters (250 feet) tall and nearly 31 meters (100 feet) around.
Greenland, off the east coast of Canada, is the world’s largest island. Despite its name, Greenland is mostly covered with ice. Its ice is a remnant of the great ice sheets that once blanketed much of the North American continent. Greenland is the only place besides Antarctica that still has an ice sheet.
From the freezing Arctic to the tropical jungles of Central America, North America has more climate variation than any other continent. Almost every type of ecosystem is represented somewhere on the continent, from coral reefs in the Caribbean to Greenland’s ice sheet to the Great Plains in the United States and Canada. North America has two overarching types of ecology, both of which support a wide variety of flora and fauna. One is the Nearctic region, which spans Canada, most of the United States and northern Mexico. Animals native to this region include bison (Bison bison), moose (Alces alces) and the California condor (Gymnogyps californianus). The other is the Neotropical region, which covers southern Mexico and extends south. Animals native to this region include llamas (Lama glama), tapirs and vipers.
Today, North America is made of Canada, the United States, Greenland, Mexico, Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Panama, and the island countries and territories that dot the Caribbean Sea and the western North Atlantic. People have migrated to North America for thousands of years and continue to immigrate to North America today. Since the United States became a country in 1783, more than 86 million people have immigrated to the country, adding to the Indigenous people, colonizers and formerly enslaved people (mostly Africans) already in the country. Despite their lives, cultures and customs being threatened or destroyed by European colonizers, more than 500 Indigenous nations continue to live in North America, including the Inuit of Arctic Canada and Alaska, the Iroquois of the United States and the Nahua of Mexico.
Most of North America sits on the North American Plate. Parts of the Canadian province of British Columbia and the U.S. states of Washington, Oregon, and California sit on the tiny Juan de Fuca Plate. Parts of California and the Mexican state of Baja California sit on the enormous Pacific Plate. Parts of Baja California and the Mexican states of Baja California Sur, Sonora, Sinaloa, and Jalisco sit on the Cocos Plate. The Caribbean Plate carries most of the small islands of the Caribbean Sea (south of the island of Cuba) as well as Central America from Honduras to Panama. The Hawaiian Islands, in the middle of the Pacific Ocean on the Pacific Plate, are usually considered part of North America.
South America
South America is connected to North America by the narrow Isthmus of Panama. These two continents were no’t always connected; they came together only 3 million years ago. South America is the fourth-largest continent and extends from the sunny beaches of the Caribbean Sea to the frigid waters near the Antarctic Circle.
South America’s southernmost islands, called Tierra del Fuego, are less than 1,120 kilometers (700 miles) from Antarctica. These islands even host some Antarctic birds, such as penguins, albatrosses and terns. Though its name comes from Spanish colonizers, the islands were home to many Indigenous groups, including the Yaghan. During colonization, many Yaghan died from European diseases and colonial violence, but there remains a sizable population on the islands, which now belong to Argentina and Chile.
The Andes, Earth’s longest terrestrial mountain range, stretch the entire length of South America. Many active volcanoes dot the range. These volcanic areas are fueled by heat generated as a large oceanic plate, called the Nazca Plate, grinds beneath the plate carrying South America.
The central-southern area of South America has pampas, or plains. These rich areas are ideal for agriculture. Growing wheat is a major industry in the pampas. Grazing animals, such as cattle and sheep, are also raised in the pampas region.
In northern South America, the Amazon River and its tributaries flow through the world’s largest tropical rainforest. In volume, the Amazon is the largest river in the world. More water flows from it than from the next six largest rivers combined.
South America is also home to the world’s tallest waterfall, Angel Falls, in the country of Venezuela. Water flows more than 979 meters (3,212 feet)—almost a mile. The falls are so high that most of the water evaporates into mist or is blown away by wind before it reaches the ground.
South American rainforests contain an enormous wealth of animal and plant life. More than 15,000 species of plants and animals are found only in the Amazon Basin, including the Amazon River dolphin (Inia geoffrensis) and the blue-throated macaw (Ara glaucogularis). Many Amazonian plant species are sources of food and medicine for the rest of the world. Scientists are trying to find ways to preserve this precious and fragile environment as people move into the Amazon Basin and clear land for settlements and agriculture.
Twelve independent countries make up South America: Brazil, Colombia, Argentina, Peru, Venezuela, Chile, Ecuador, Bolivia, Paraguay, Uruguay, Guyana, and Suriname. The territories of French Guiana, once colonized by and now an incorporated part of France, and the Falkland Islands, part of the United Kingdom, are also part of South America. The colonization of South America by Spain and Portugal destroyed many Indigenous cultures, but others, such as the Guarani in Brazil, have successfully fought against their oppressors to maintain their ways of life.
Almost all of South America sits on top of the South American Plate.
Europe
Europe, the sixth-largest continent, contains just 7% of the world’s land. In total area, the continent of Europe is only slightly larger than the country of Canada. However, the population of Europe is more than twice that of South America. Europe has more than 40 countries and many well-known cities, including London, England; Paris, France; Berlin, Germany; Rome, Italy; Madrid, Spain; and Moscow, Russia.
Most European countries have access to the ocean. The continent is bordered by the Arctic Ocean in the north, the Atlantic Ocean in the west, the Caspian Sea in the southeast, and the Mediterranean and Black Seas in the south. The nearness of these bodies of water and the navigation of many of Europe’s rivers played a major role in the continent’s history. Early Europeans learned the river systems of the Volga, Danube, Don, Rhine and Po, and could successfully travel the length and width of the small continent for trade, communication or conquest.
Navigation and colonization outside of Europe was an important part of the development of the continent’s economic, social, linguistic and political legacy. Europeans colonized land on every continent except Antarctica, leading to huge, and often catastrophic, changes for the Indigenous people. European countries extracted natural resources from the countries they colonized, leading to increased wealth for the colonizers at the expense of the people in their colonies, who continue to face wealth disparities and political instability to this day.
In the east, the Ural Mountains separate Europe from Asia. The nations of Russia and Kazakhstan straddle both continents. Another range, the Kjølen Mountains, extends along the northern part of the border between Sweden and Norway. To the south, the Alps form an arc stretching from Albania to Austria, then across Switzerland and northern Italy into France. As the youngest and steepest of Europe’s mountains, the Alps geologically resemble the Rockies of North America, another young range.
A large area of gently rolling plains extends from northern France eastward to the Urals. A climate of warm summers, cold winters and plentiful rain helps make much of this European farmland very productive.
Human development in Europe led to the extinction or near extinction of many wild animals indigenous to the continent. Some indigenous animals that have survived include the Eurasian lynx (Lynx lynx), the Golden eagle (Aquila chrysaetos) and Mediterranean tortoises (Testudo graeca).
Almost all of Europe sits on the massive Eurasian Plate.
Africa
Africa, the second-largest continent, covers an area more than three times that of the United States. From north to south, Africa stretches about 8,000 kilometers (5,000 miles). It is connected to Asia by the Isthmus of Suez in Egypt.
The Sahara, which covers much of North Africa, is the world’s largest hot desert. The world’s longest river, the Nile, flows more than 6,560 kilometers (4,100 miles) from its most remote headwaters in Lake Victoria to the Mediterranean Sea in the north. A series of falls and rapids along the southern part of the river makes navigation difficult. The Nile has played an important role in the history of Africa. In ancient Egyptian civilization, it was a source of life for food, water, and transportation.
The top half of Africa is mostly dry, hot desert. The middle area has savannas, or flat, grassy plains. This region is home to wild animals such as lions (Panthera leo), giraffes, elephants, hyenas, cheetahs (Acinonyx jubatus) and wildebeests. The central and southern areas of Africa are dominated by rainforests. Many of these forests thrive around Africa’s other great rivers, the Zambezi, the Congo and the Niger. These rivers were also home to Great Zimbabwe, the Kingdom of Kongo and the Ghana Empire, respectively. However, trees from the rainforests fed by these rivers are being cut down for many of the same reasons deforestation is taking place in the rainforests of South America and Asia: development for businesses, homes and agriculture.
Much of Africa is a high plateau surrounded by narrow strips of coastal lowlands. Hilly uplands and mountains rise in some areas of the interior. Glaciers on Mount Kilimanjaro in Tanzania sit just miles from the tropical jungles below. Even though Kilimanjaro is not far from the Equator, snow covers its summit all year long.
In eastern Africa, a giant depression called the Great Rift Valley runs from the Red Sea to the country of Mozambique. (The rift valley actually starts in southwestern Asia.) The Great Rift Valley is a site of major tectonic activity, where the continent of Africa is splitting into two. Geologists have already named the two parts of the African Plate. The Nubian Plate carries most of the continent to the west of the rift, while the Somali Plate carries the far eastern part of the continent, including the so-called “Horn of Africa.” The Horn of Africa is a peninsula that resembles the upturned horn of a rhinoceros. The countries of Eritrea, Ethiopia, Djibouti and Somalia sit on the Horn of Africa and the Somali Plate.
Africa is home to 56 countries but only 18.3% of the world’s total population. The area of central-eastern Africa is important to scientists who study evolution and the earliest origins of humanity. This area is thought to be the place where hominins began to evolve. During the era of colonization, more than 12.5 million Africans were kidnapped from Africa and enslaved as part of the transatlantic slave trade. Later, nearly all of Africa was colonized by Europe, until the beginning in the mid-20th century when African leaders began to break free. Today, many indigenous groups in Africa continue to fight for their autonomy. The Maasai in Tanzania, for example, who raise cattle as part of their traditional culture, are fighting for land that is being taken away to use for other agricultural purposes or game reserves.
The entire continent of Africa sits on the African Plate.
Asia
Asia, the largest continent, stretches from the eastern Mediterranean Sea to the western Pacific Ocean. There are more than 40 countries in Asia. Some are among the most-populated countries in the world, including China, India and Indonesia. About 60 percent of Earth’s population lives in Asia. More than a third of the world’s people live in China and India alone. Asia has the world’s highest population of Indigenous people of any continent, but some, like the Hmong of Southeast Asia, have faced systemic persecution. Asia includes many islands, some of them countries. The Philippines, Indonesia, Japan and Taiwan are major island nations in Asia.
Most of Asia’s people live in cities or fertile farming areas near river valleys, plains and coasts. The plateaus in Central Asia are largely unsuitable for farming and are thinly populated.
Asia has some of the world’s most desired natural resources, which European countries sought to exploit through colonization. Through the years, European colonizers have gained wealth through the control of spices, opium, rubber and oil. Today, foreign powers compete for political influence and access to natural resources, such as rare earth materials, lithium, timber, oil and natural gas.
Asia accounts for almost a third of the world’s land. The continent has a wide range of climate regions, from polar in the Siberian Arctic to tropical in equatorial Indonesia. Parts of Central Asia, including the Gobi Desert in China and Mongolia, are dry year-round. Southeast Asia, on the other hand, depends on the annual monsoons, which bring rain and make agriculture possible. Asia’s climate and topography vary greatly, providing ecosystems for a wide variety of animals, including King cobras, Asian elephants (Elephas maximus) and Oriental scops owls (Otus sunia).Monsoon rains and snowmelt feed Asian rivers, such as the Ganges, the Yellow, the Mekong, the Indus and the Yangtze. The rich valley between the Tigris and Euphrates Rivers in western Asia is called the “Fertile Crescent” for its place in the development of agriculture and human civilization.Asia is the most mountainous of all the continents. More than 50 of the highest peaks in the world are in Asia. Mount Everest, which reaches more than 8,700 meters (29,000 feet) high in the Himalayan range, is the highest point on Earth. These mountains have become major destination spots for adventurous travelers.Plate tectonics continuously push the mountains higher. As the landmass of India pushes northward into the landmass of Eurasia, parts of the Himalayas rise at a rate of about 2.5 centimeters (1 inch) every five years. Asia contains not only Earth’s highest elevation, but also its lowest place on land: the shores of the Dead Sea in Israel, Jordan and the Palestinian West Bank (under Israeli control). The land there lies more than 390 meters (1,300 feet) below sea level.
Although the Eurasian Plate carries most of Asia, it is not the only one supporting major parts of the large continent. The Arabian Peninsula, in the continent’s southwest, is carried by the Arabian Plate. The Indian Plate supports the Indian peninsula, sometimes called the South Asian subcontinent. The Australian Plate carries some islands in Indonesia. The North American Plate carries eastern Siberia and the northern islands of Japan.
Australia
In addition to being the smallest continent, Australia is the flattest and the second-driest, after Antarctica. The continent is sometimes called Oceania, to include the thousands of tiny islands of the Central and South Pacific, most notably Melanesia, Micronesia and Polynesia (including the U.S. state of Hawai‘i). However, the continent of Australia itself includes only the nation of Australia, the eastern portion of the island of New Guinea (part of the nation of Papua New Guinea) and the island nation of New Zealand.Australia covers just fewer than 8.5 million square kilometers (about 3.5 million square miles). Its population is about 31 million. It is the most sparsely populated continent, after Antarctica.A plateau in the middle of mainland Australia makes up most of the continent’s total area. Rainfall is light on the plateau, and not many people have settled there. The Great Dividing Range, a long mountain range, rises near the east coast and extends from the northern part of the territory of Queensland through the territories of New South Wales and Victoria. Mainland Australia is known for the Outback, a desert area in the interior. This area is so dry, hot, and barren that few people live there.In addition to the hot plateaus and deserts in mainland Australia, the continent also features lush equatorial rain forests on the island of New Guinea, tropical beaches, and high mountain peaks and glaciers in New Zealand. Most of Australia’s people live in cities along the southern and eastern coasts of the mainland. Major cities include Perth, Sydney, Brisbane, Melbourne and Adelaide, all in Australia. The country of Australia has two main Indigenous groups: Aboriginal and Torres Strait Islander. European colonization of Australia threatened and harmed the culture and health of both Indigenous groups, but they were able to resist and protect their way of life, which they continue to do to this day.
Biologists who study animals consider Australia a living laboratory. When the continent began to break away from Antarctica more than 60 million years ago, it carried a cargo of animals with it. Isolated from life on other continents, the animals developed into creatures unique to Australia, such as the koala (Phascolarctos cinereus), the platypus (Ornithorhynchus anatinus) and the Tasmanian devil (Sarcophilus harrisii).The Great Barrier Reef, off mainland Australia’s northeast coast, is another living laboratory. The world’s largest coral reef ecosystem, it is home to thousands of species of fish, sponges, marine mammals, corals, and crustaceans. The reef itself is 1,920 kilometers (1,200 miles) of living coral communities. By some estimates, it is the world’s largest living organism. Most of Australia sits on the Australian Plate. The southern part of the South Island of New Zealand sits on the Pacific Plate.
Antarctica
Antarctica is the windiest, driest and iciest place on Earth. Antarctica is larger than Europe or Australia, but unlike those continents, it has no permanent human population. People who live and work there are scientific researchers and support staff, such as pilots and cooks. The large animals that live in Antarctica, such as penguins, albatrosses and seals, generally rely on the sea to survive. The climate of Antarctica makes it impossible to support agriculture or a permanent civilization. Temperatures in Antarctica, much lower than Arctic temperatures, plunge lower than -73 degrees Celsius (-100 degrees Fahrenheit).Scientific bases and laboratories have been established in Antarctica for studies in fields like geology, oceanography and meteorology. The freezing temperatures of Antarctica make it an excellent place to study the history of Earth’s atmosphere and climate. Ice cores from the massive Antarctic ice sheet have recorded changes in Earth’s temperature and atmospheric gases for thousands of years. Antarctica is also an ideal place for discovering meteorites, or stony objects that have impacted Earth from outer space. The dark meteorites, often made of metals like iron, stand out from the white landscape of most of the continent.Antarctica is almost completely covered with ice, sometimes as thick as 3.2 kilometers (2 miles). In winter, Antarctica’s surface area may double as pack ice builds up in the ocean around the continent. Like all other continents, Antarctica has volcanic activity. The most active volcano is Mount Erebus, which is less than 1,392 kilometers (870 miles) from the South Pole. Evidence of its frequent eruptions can be found in the hot, molten rock beneath the continent’s icy surface.Antarctica does not have any countries. However, scientific groups from different countries inhabit the research stations. A multinational treaty negotiated in 1959 and reviewed in 1991 states that research in Antarctica can only be used for peaceful purposes. McMurdo Station, the largest community in Antarctica, is operated by the United States. Vostok Station, where the coldest temperature on Earth was recorded, is operated by Russia. All of Antarctica sits on the Antarctic Plate.
Additional Information
A continent is one of the larger continuous masses of land, namely, Asia, Africa, North America, South America, Antarctica, Europe, and Australia, listed in order of size. (Europe and Asia are sometimes considered a single continent, Eurasia.)
There is great variation in the sizes of continents; Asia is more than five times as large as Australia. The largest island in the world, Greenland, is only about one-fourth the size of Australia. The continents differ sharply in their degree of compactness. Africa has the most regular coastline and, consequently, the lowest ratio of coastline to total area. Europe is the most irregular and indented and has by far the highest ratio of coastline to total area.
The continents are not distributed evenly over the surface of the globe. If a hemisphere map centred in northwestern Europe is drawn, most of the world’s land area can be seen to lie within that hemisphere. More than two-thirds of the Earth’s land surface lies north of the Equator, and all the continents except Antarctica are wedge shaped, wider in the north than they are in the south.
The distribution of the continental platforms and ocean basins on the surface of the globe and the distribution of the major landform features have long been among the most intriguing problems for scientific investigation and theorizing. Among the many hypotheses that have been offered as explanation are: (1) the tetrahedral (four-faced) theory, in which a cooling earth assumes the shape of a tetrahedron by spherical collapse; (2) the accretion theory, in which younger rocks attached to older shield areas became buckled to form the landforms; (3) the continental-drift theory, in which an ancient floating continent drifted apart; and (4) the convection-current theory, in which convection currents in the Earth’s interior dragged the crust to cause folding and mountain making.
Geological and seismological evidence accumulated in the 20th century indicates that the continental platforms do “float” on a crust of heavier material that forms a layer completely enveloping the Earth. Each continent has one of the so-called shield areas that formed 2 billion to 4 billion years ago and is the core of the continent to which the remainder (most of the continent) has been added. Even the rocks of the extremely old shield areas are older in the centre and younger toward the margins, indicating that this process of accumulation started early. In North America the whole northeast quarter of the continent, called the Canadian, or Laurentian, Shield, is characterized by the ancient rocks of what might be called the original continent. In Europe the shield area underlies the eastern Scandinavian peninsula and Finland. The Guiana Highlands of South America are the core of that continent. Much of eastern Siberia is underlain by the ancient rocks, as are western Australia and southern Africa.
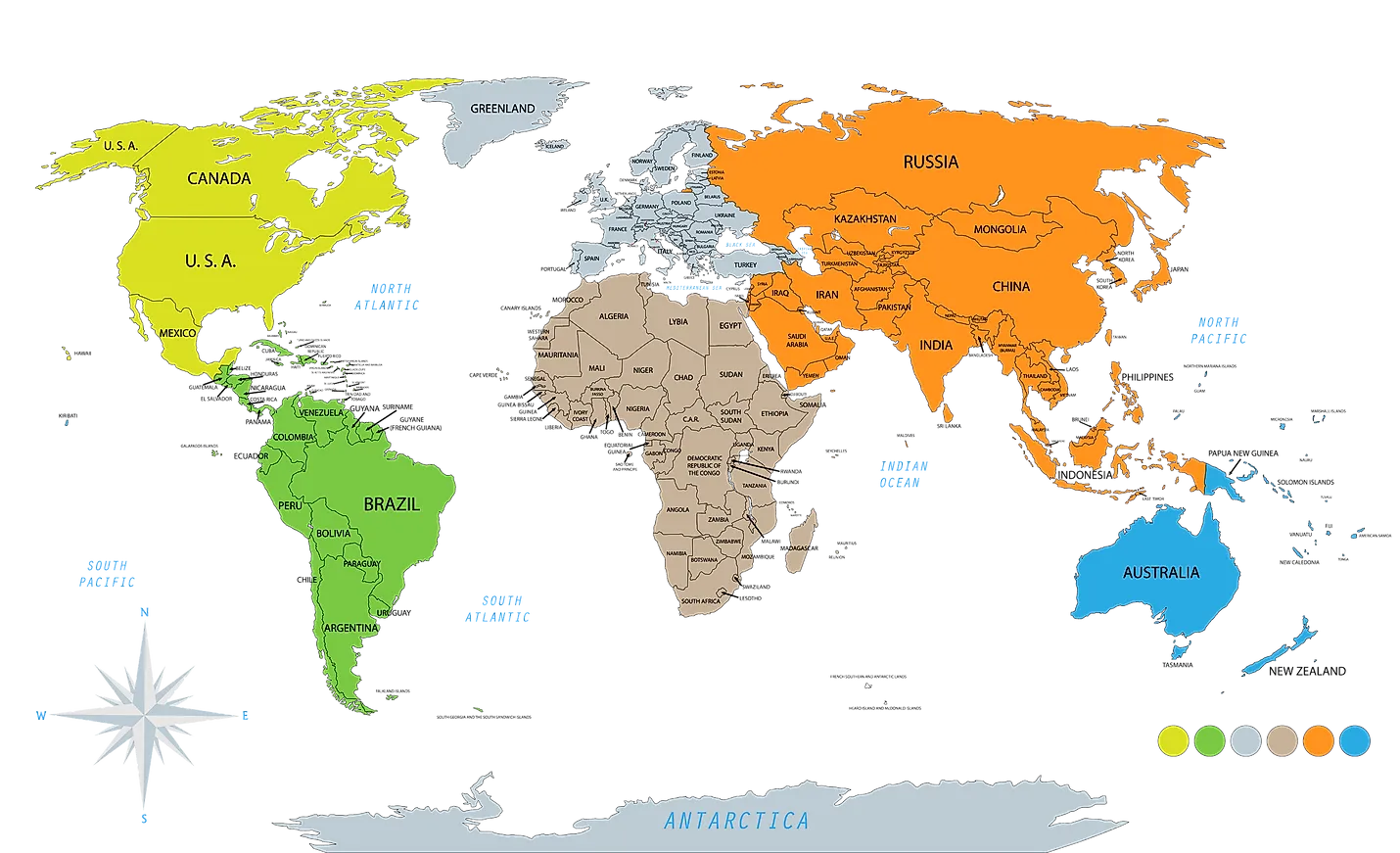
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline